Development of a Temperature Distribution Measurement System for Transmission Oil for Transportation Equipment
Abstract
1. Introduction
2. Measurement Methods
2.1. Characteristics of Phosphor Material as Sensors for Temperature Measurement
- (1)
- pH
- (2)
- Solution polarity
- (3)
- Concentration quenching
- (4)
- Temperature quenching
- (5)
- Photo-quenching
- (1)
- High phosphor concentrations (concentration quenching).
- (2)
- The presence of oxygen and ions (chlorine and various metal ions) in the solution (chemical quenching).
- (3)
- Excessive excitation of light intensity (quenching caused by excessive excitation light intensity).
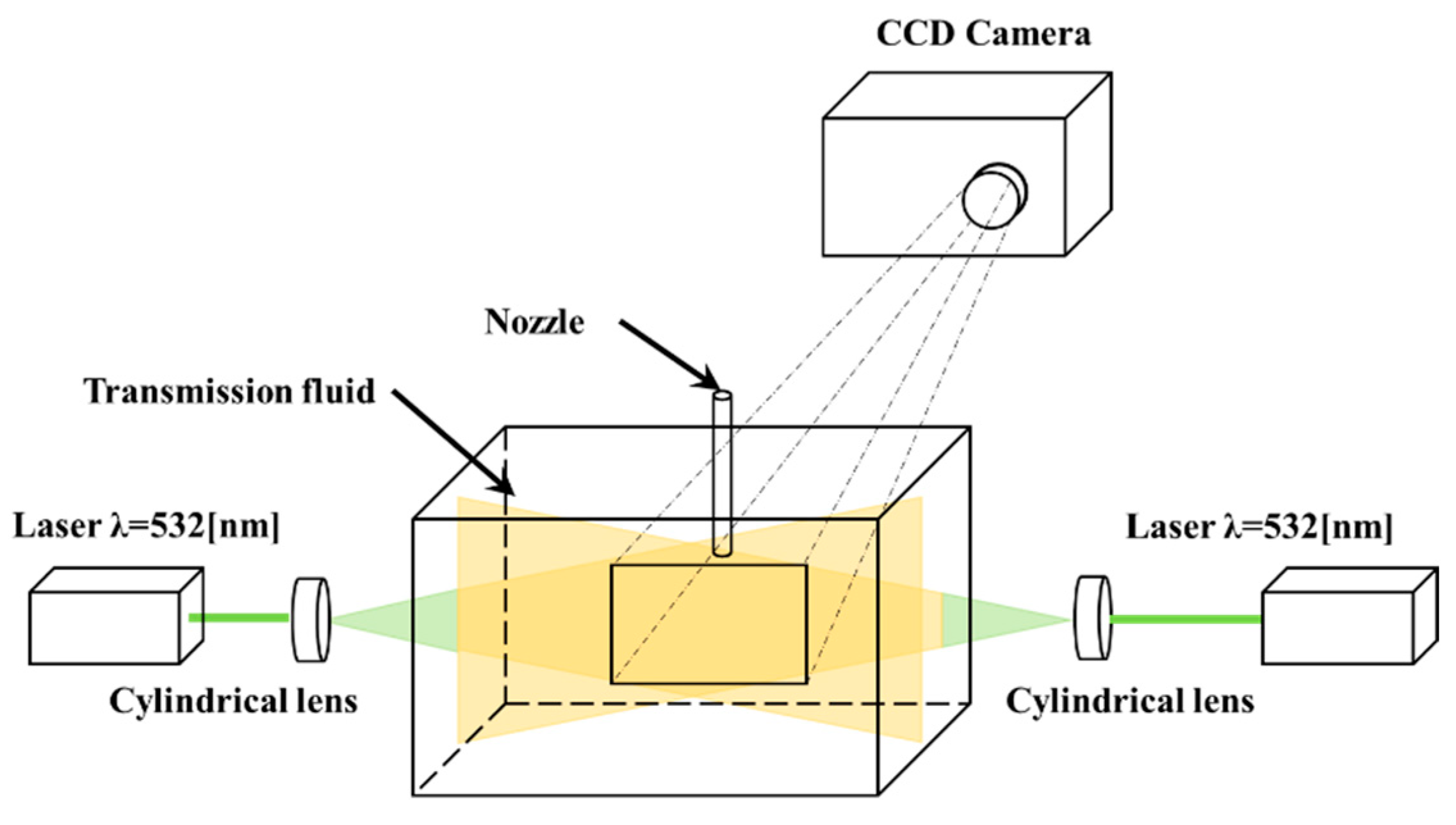
2.2. Measurement of Temperature Using the LIF Method
3. Results
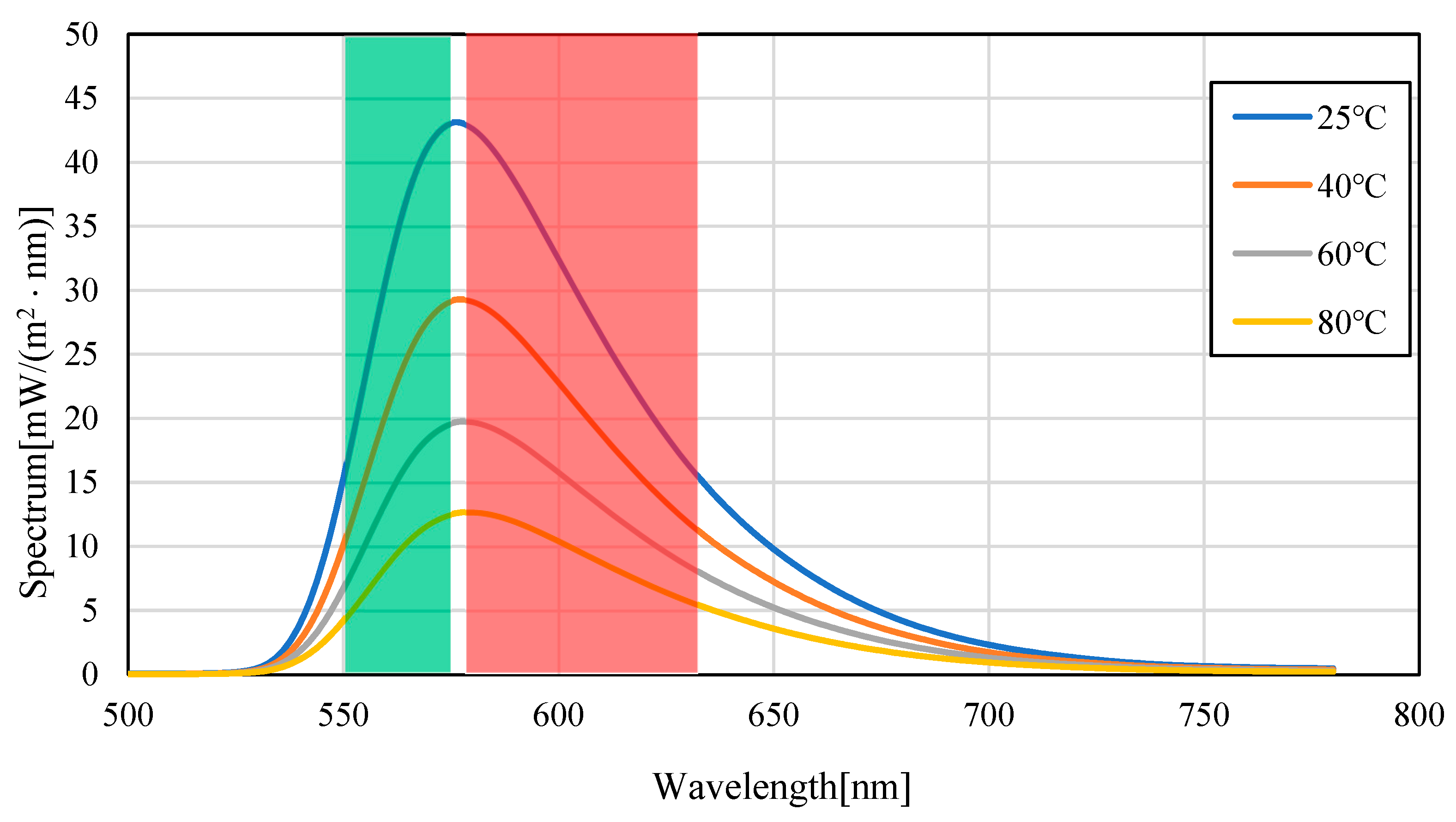
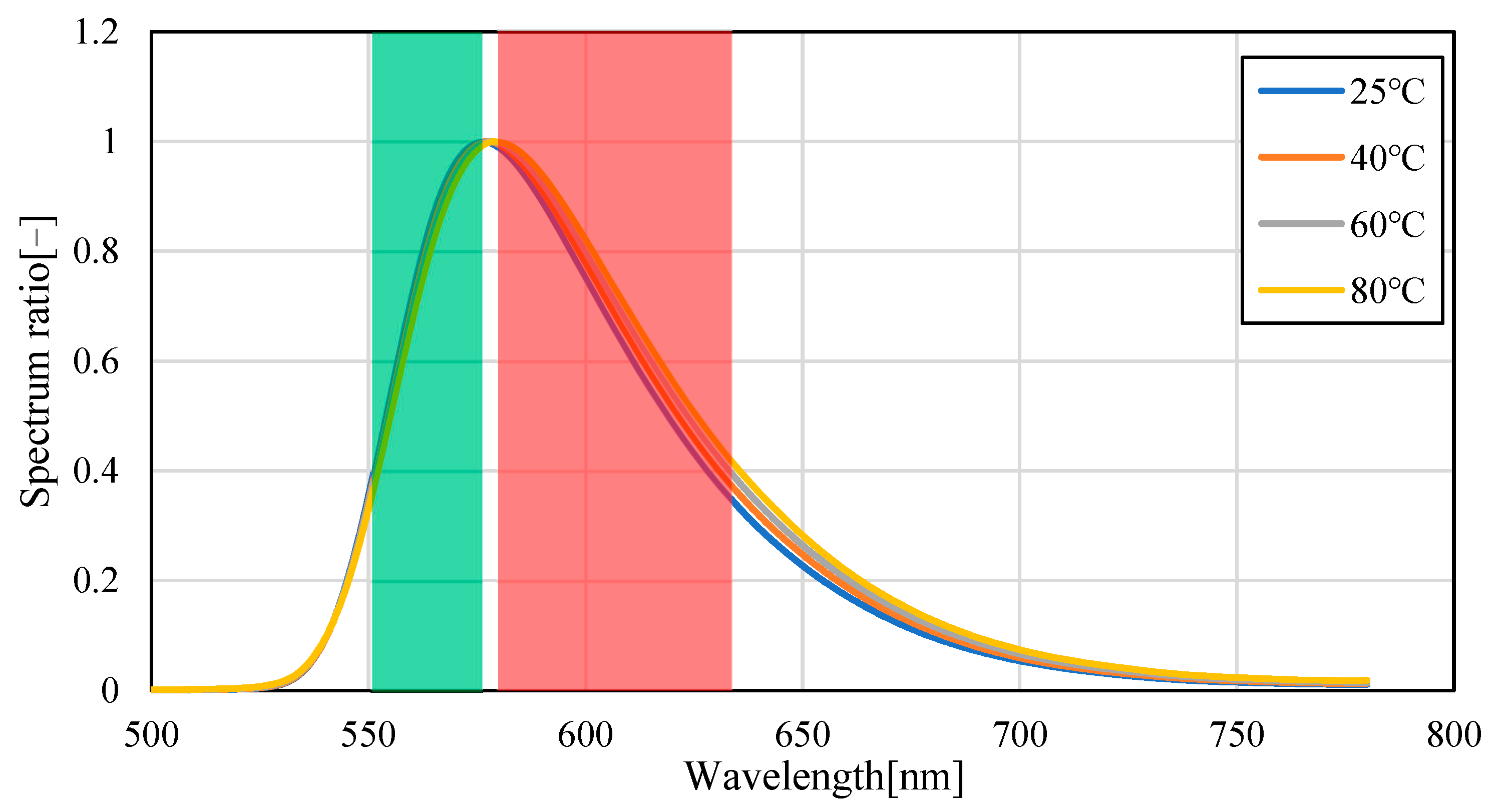
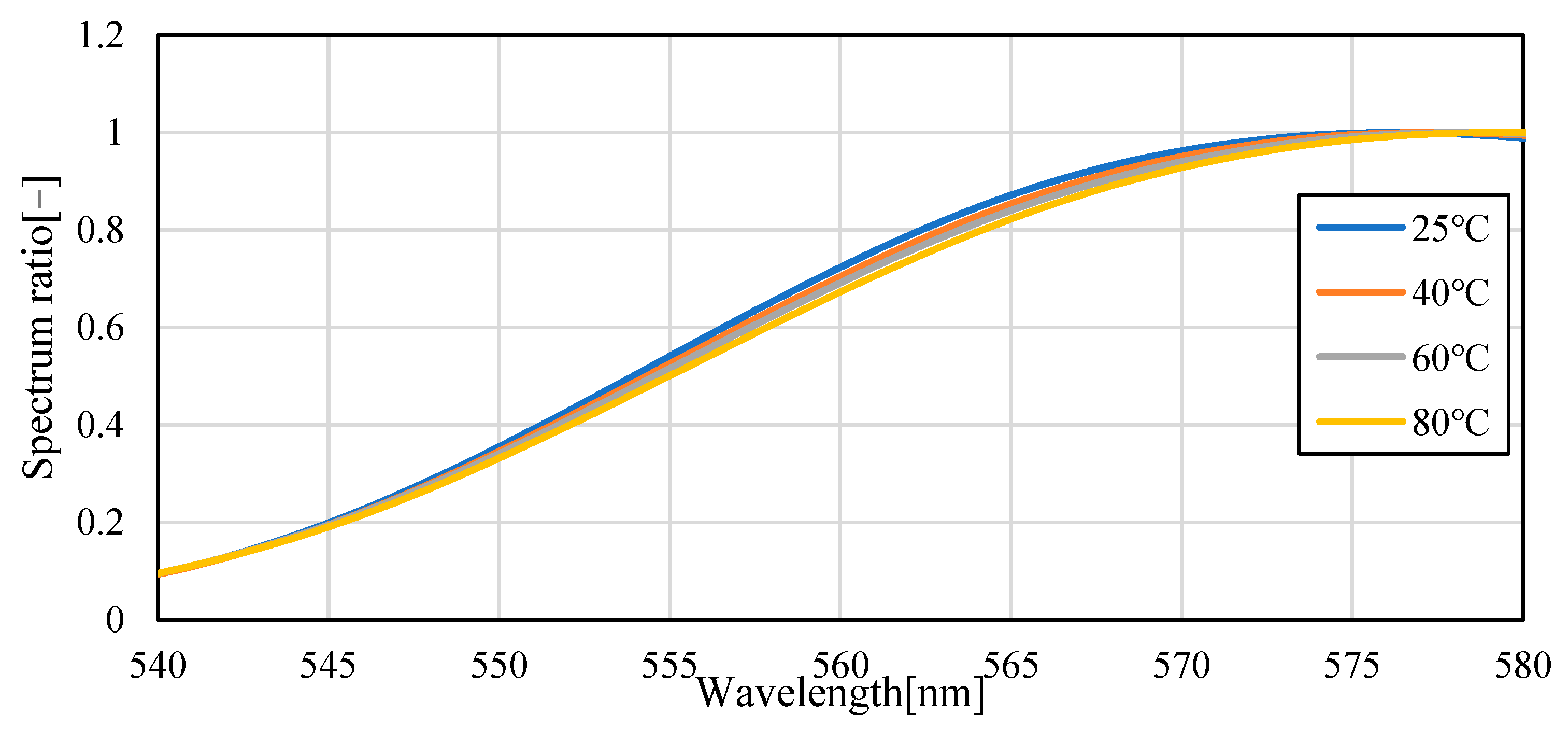
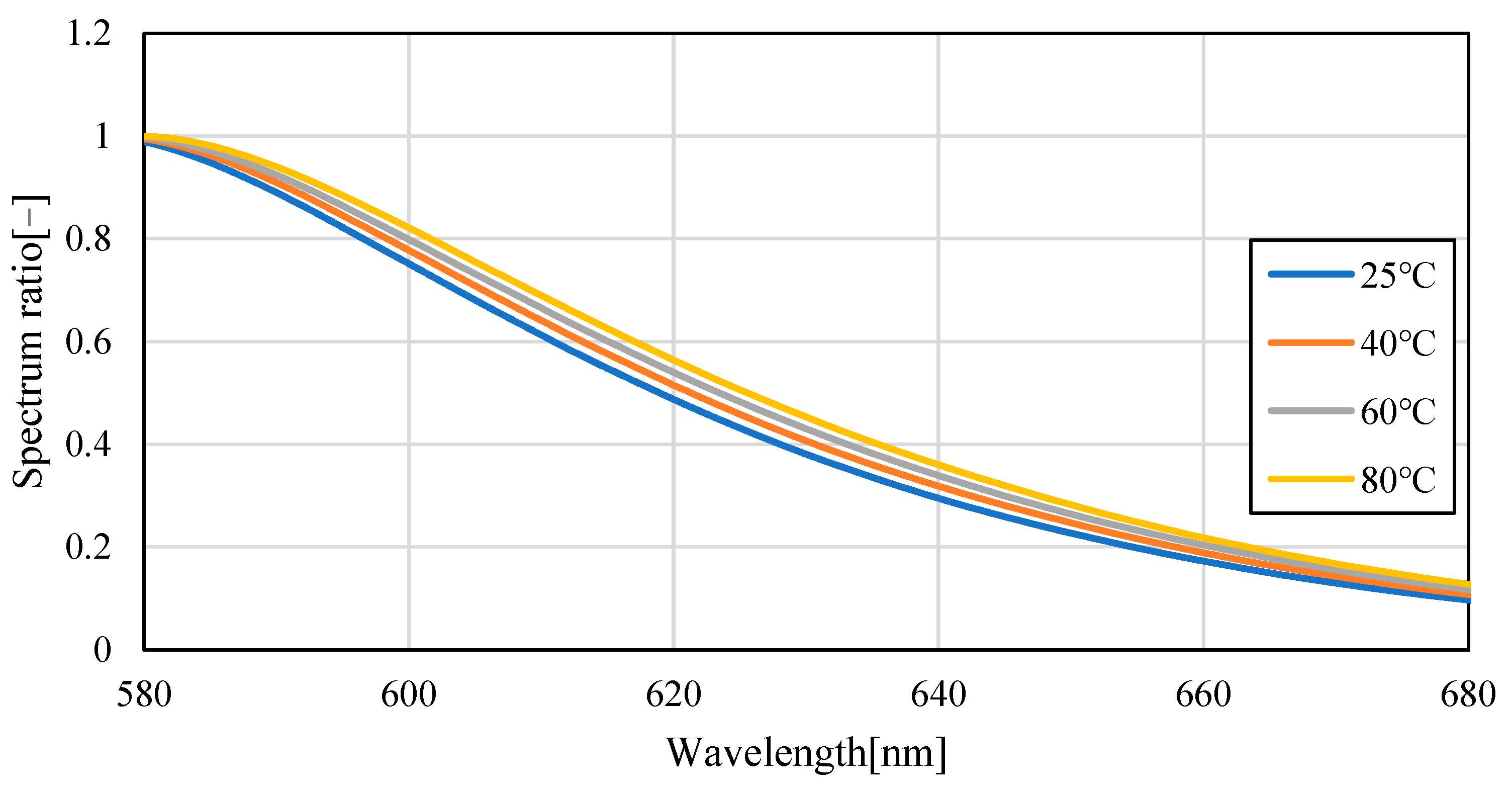
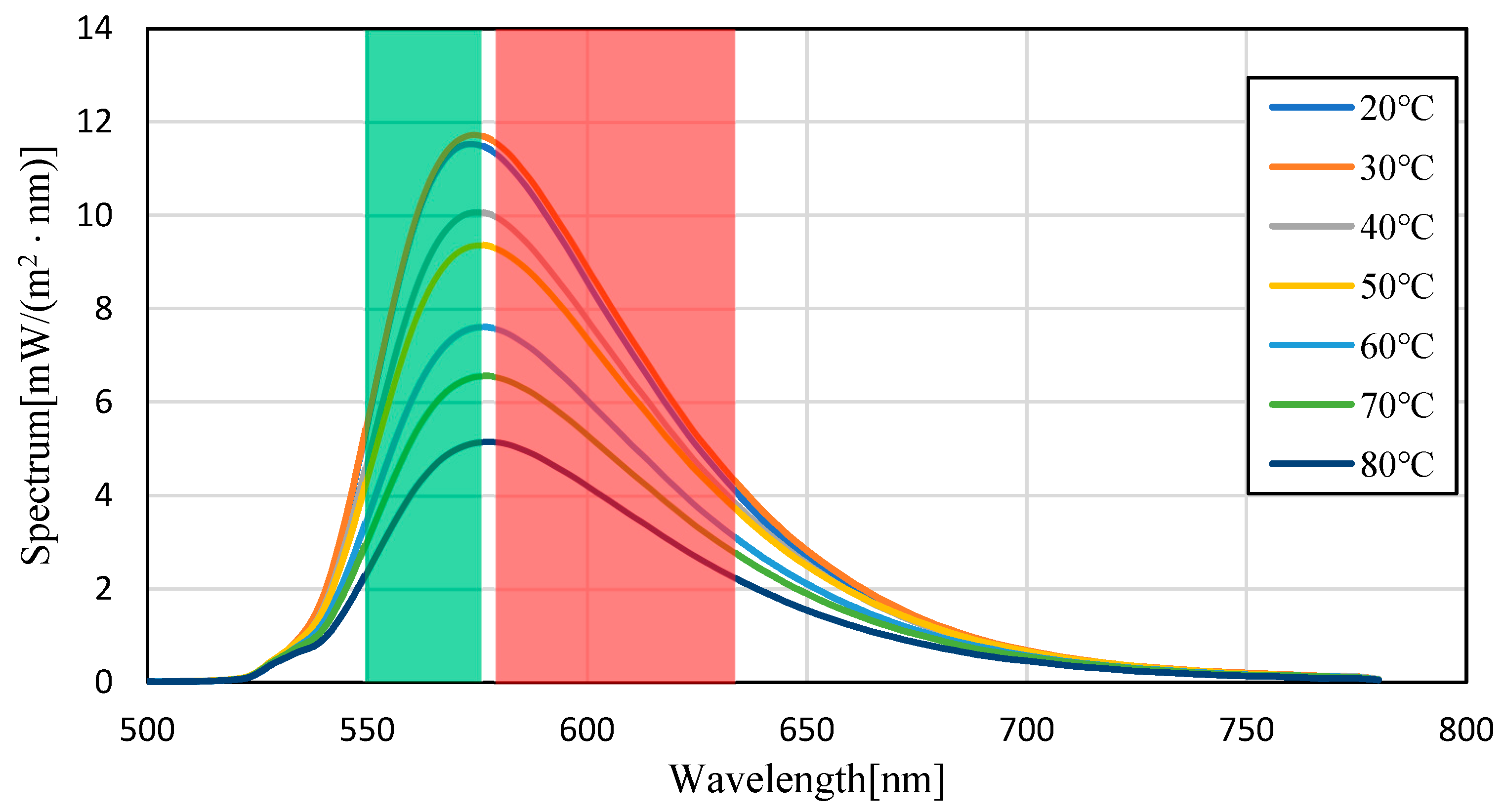
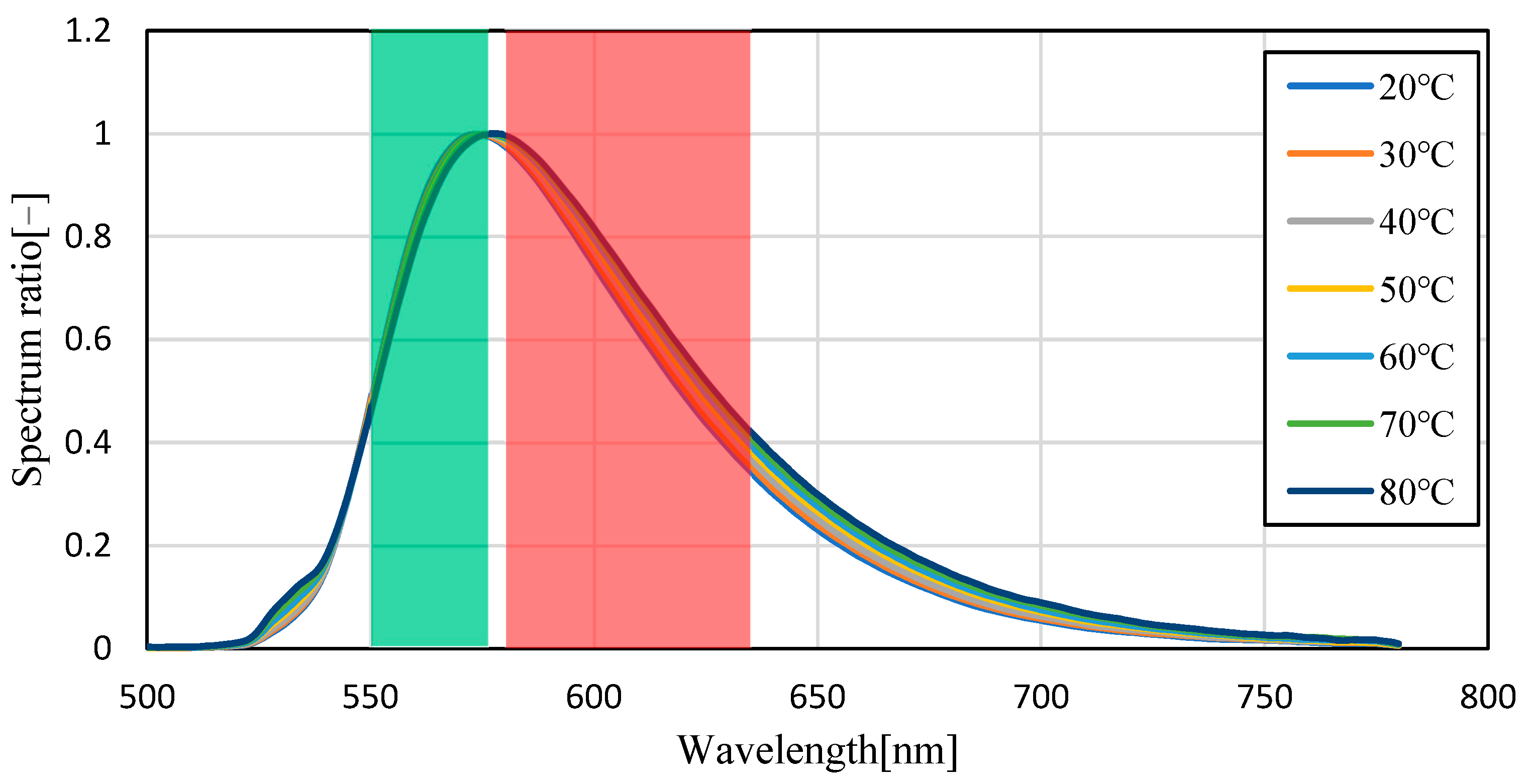
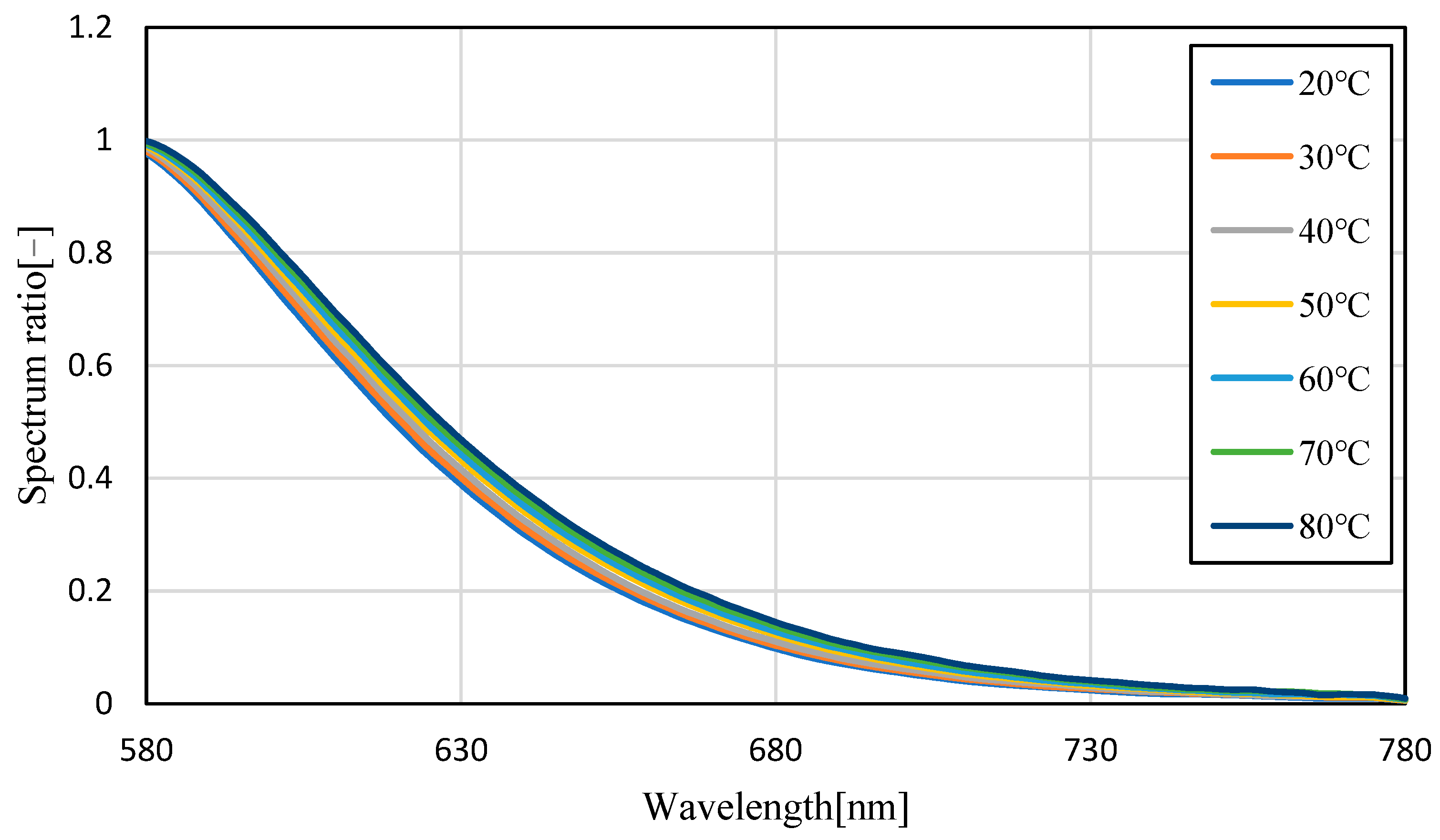
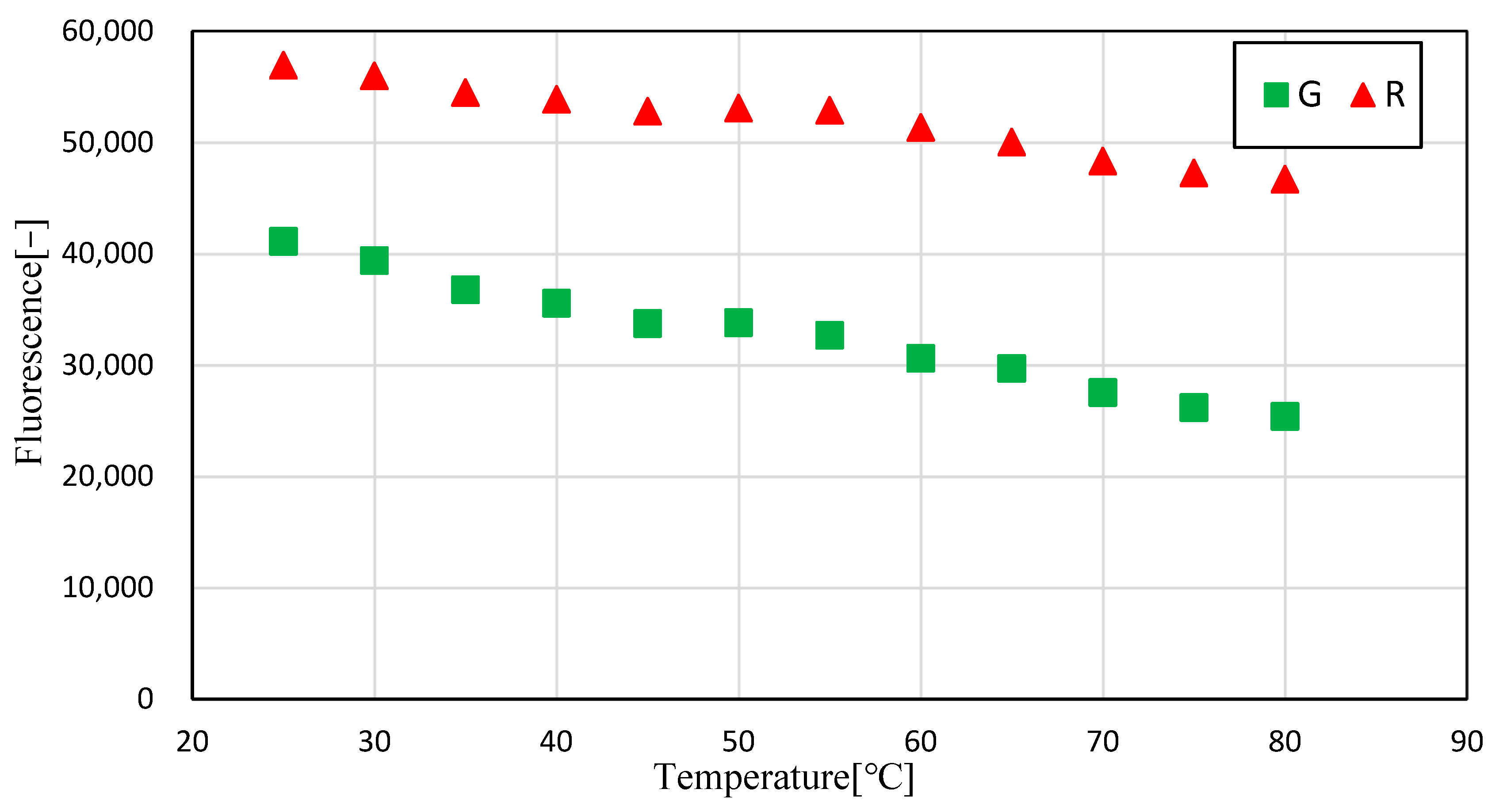
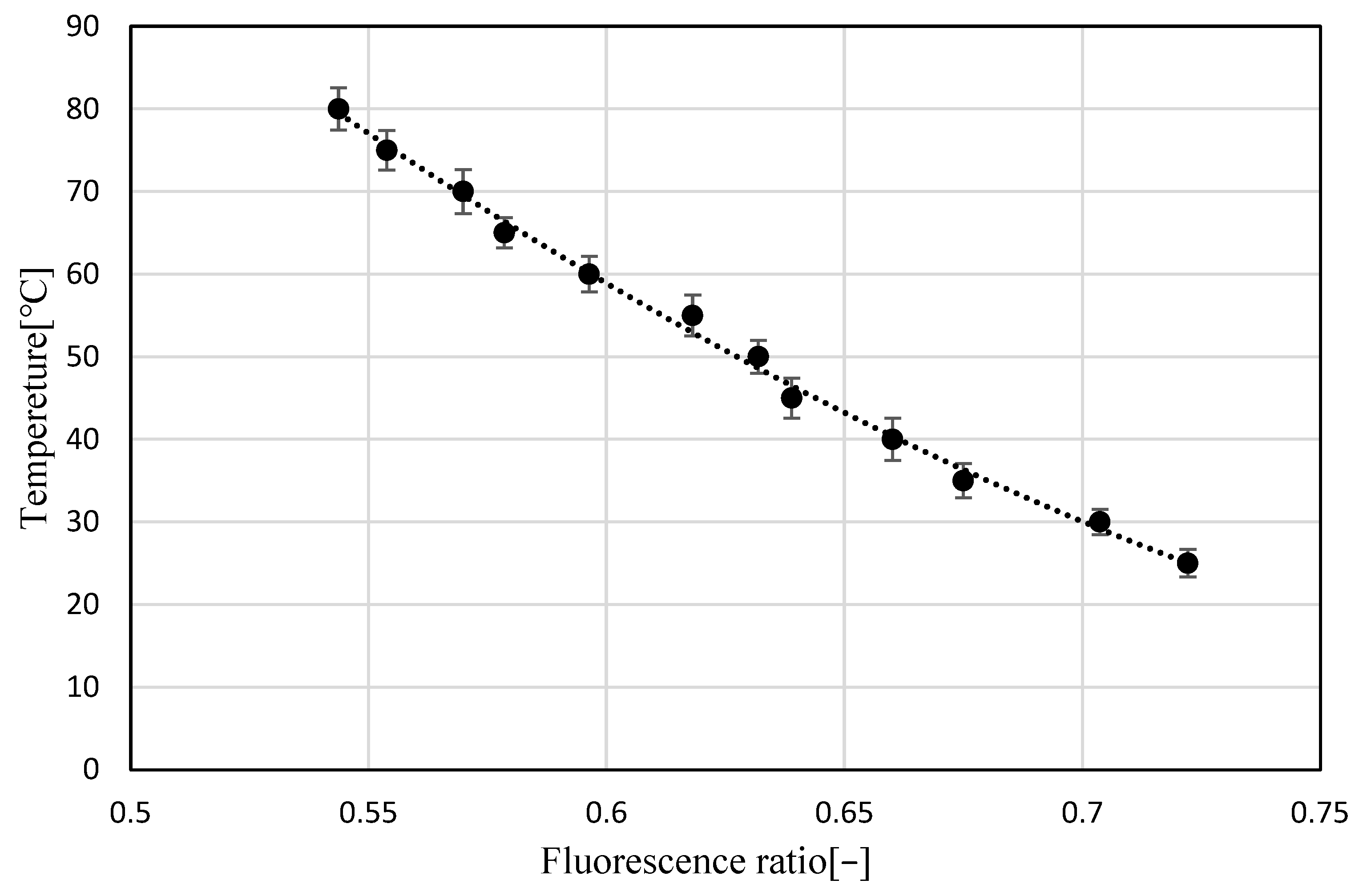
3.1. Temperature Dependence of Pyrromethene 597
3.2. Uncertainty Analysis
4. Application to Flow Fields
- (1)
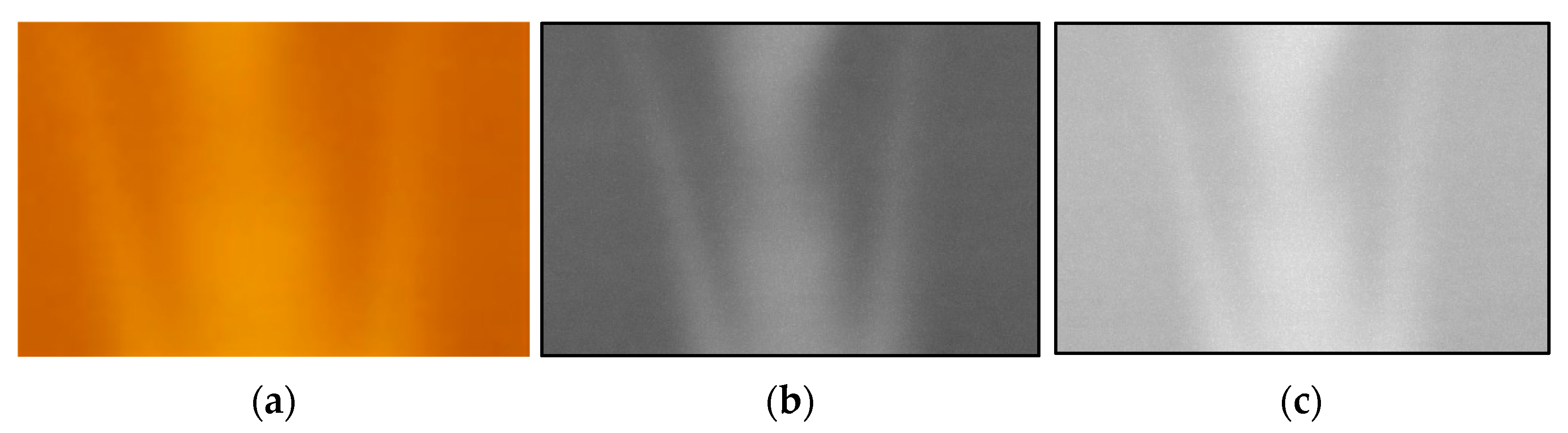
- The analysis area of the acquired R and G images was cropped and converted into a grayscale image.
- (2)
- Noise was removed from the analyzed images.
- (3)
- The luminance value of each pixel in the denoised image was expressed as a numerical data distribution:
- (4)
- The distribution of luminance data for each of the R and G images was calculated (Figure 14b,c) using the numerical values of the corresponding coordinates to obtain G/R.
- (5)
- The approximate curve obtained in Figure 13 was applied to the luminance ratio distribution to obtain the temperature distribution data.
- (6)
- The temperature distribution data were converted into a two-dimensional color map (Figure 15).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Yang, G.; Wu, G.; Li, X. Oxidative Deterioration Effect of Cavitation Heat Generation on Hydraulic Oil. IEEE Access 2020, 8, 119720–119727. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Y.; Okhai, T.A.; Snyman, L.W. Micro optical sensors based on avalanching silicon light-emitting devices monolithically integrated on chips. Opt. Mater. Express 2019, 9, 3985–3997. [Google Scholar] [CrossRef]
- Sakakibara, J.; Adrian, R.J. Whole field measurement of temperature in water using two-color laser induced fluorescence. Exp. Fluids 1999, 26, 7–15. [Google Scholar] [CrossRef]
- Hishida, K.; Sakakibara, J. Combined planar laser-induced fluorescence-particle image velocimetry technique for velocity and temperature fields. Exp. Fluids 2000, 29, 129–140. [Google Scholar] [CrossRef]
- Sakakibara, J.; Adrian, R.J. Measurement of temperature field of a Rayleigh-Bénard convection using two-color laser-induced fluorescence. Exp. Fluids 2004, 37, 331–340. [Google Scholar] [CrossRef]
- Kashanj, S.; Nobes, D.S. Application of 4D two-colour LIF to explore the temperature field of laterally confined turbulent Rayleigh–Bénard convection. Exp. Fluids 2023, 64, 46. [Google Scholar] [CrossRef]
- Abdelghany, A.; Kuribayashi, K.; Tange, M. Ratiometric laser-induced fluorescence for liquid-phase thermometry around boiling bubbles at extended temperatures above 70 °C. Exp. Fluids 2022, 63, 52. [Google Scholar] [CrossRef]
- Doering, C.R. Thermal forcing and ‘classical’ and ‘ultimate’ regimes of Rayleigh–Bénard convection. J. Fluid Mech. 2019, 868, 1–4. [Google Scholar] [CrossRef]
- Doering, C.R. Turning up the heat in turbulent thermal convection. Proc. Natl. Acad. Sci. USA 2020, 117, 9671–9673. [Google Scholar] [CrossRef] [PubMed]
- Ecke, R.E.; Shishkina, O. Turbulent rotating Rayleigh–Bénard convection. Annu. Rev. Fluid Mech. 2023, 55, 603–638. [Google Scholar] [CrossRef]
- Hartmann, R.; Chong, K.L.; Stevens, R.J.A.M.; Verzicco, R.; Lohse, D. Heat transport enhancement in confined Rayleigh–Bénard convection feels the shape of the container. Europhys. Lett. 2021, 135, 24004. [Google Scholar] [CrossRef]
- Hartmann, R.; Verzicco, R.; Kranenbarg, L.K.; Lohse, D.; Stevens, R.J. Multiple heat transport maxima in confined-rotating Rayleigh–Bénard convection. J. Fluid Mech. 2022, 939, A1. [Google Scholar] [CrossRef]
- Funatani, S.; Tsukamoto, Y.; Toriyama, K. Temperature Measurement of Hot Airflow Using Ultra-Fine Thermo-Sensitive Fluorescent Wires. Sensors 2022, 22, 3175. [Google Scholar] [CrossRef]
- Labergue, A.; Deprédurand, V.; Delconte, A.; Castanet, G.; Lemoine, F. New insight into two-color LIF thermometry applied to temperature measurements of droplets. Exp. Fluids 2010, 49, 547–556. [Google Scholar] [CrossRef]
- Labergue, A.; Delconte, A.; Castanet, G.; Lemoine, F. Study of the droplet size effect coupled with the laser light scattering in sprays for two-color LIF thermometry measurements. Exp. Fluids 2012, 52, 1121–1132. [Google Scholar] [CrossRef][Green Version]
- Tsukamoto, Y.; Funatani, S. Application of feature matching trajectory detection algorithm for particle streak velocimetry. J. Vis. 2020, 23, 971–979. [Google Scholar] [CrossRef]
- Prenting, M.M.; Shilikhin, M.; Dreier, T.; Schulz, C.; Endres, T. Characterization of tracers for two-color laser-induced fluorescence thermometry of liquid-phase temperature in ethanol,2–ethylhexanoic-acid/ethanol mixtures, 1-butanol, and oxylene. Appl. Opt. 2021, 60, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.B.; Arbeloa, F.L.; Martínez, V.M.; López, T.A.; Arbeloa, I.L. Photophysical Properties of the Pyrromethene 597 Dye: Solvent Effect. J. Phys. Chem. A 2004, 108, 5503–5508. [Google Scholar] [CrossRef]
- Jain, P.; Motosuke, M. Temperature sensitivity of BODIPY dye (pyrromethene 597) over different linear organic solvents. Jpn. J. Appl. Phys. 2022, 61, 056504. [Google Scholar] [CrossRef]
- Xu, K. Silicon electro-optic micro-modulator fabricated in standard CMOS technology as components for all silicon monolithic integrated optoelectronic systems. J. Micromech. Microeng. 2021, 31, 054001. [Google Scholar] [CrossRef]
- Funatani, S.; Fujisawa, N.; Ikeda, H. Simultaneous measurement of temperature and velocity using two-colour LIF combined with PIV with a colour CCD camera and its application to the turbulent buoyant plume. Meas. Sci. Technol. 2004, 15, 983. [Google Scholar] [CrossRef]




| Temperature, °C | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | 80 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard deviation, K | 1.68 | 1.52 | 2.07 | 2.56 | 2.41 | 1.98 | 2.47 | 2.16 | 1.82 | 2.66 | 2.37 | 2.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funatani, S.; Takei, R.; Tsukamoto, Y. Development of a Temperature Distribution Measurement System for Transmission Oil for Transportation Equipment. Sensors 2023, 23, 5499. https://doi.org/10.3390/s23125499
Funatani S, Takei R, Tsukamoto Y. Development of a Temperature Distribution Measurement System for Transmission Oil for Transportation Equipment. Sensors. 2023; 23(12):5499. https://doi.org/10.3390/s23125499
Chicago/Turabian StyleFunatani, Shumpei, Ryoga Takei, and Yusaku Tsukamoto. 2023. "Development of a Temperature Distribution Measurement System for Transmission Oil for Transportation Equipment" Sensors 23, no. 12: 5499. https://doi.org/10.3390/s23125499
APA StyleFunatani, S., Takei, R., & Tsukamoto, Y. (2023). Development of a Temperature Distribution Measurement System for Transmission Oil for Transportation Equipment. Sensors, 23(12), 5499. https://doi.org/10.3390/s23125499







