Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep when Determining Associations with Markers of Health
Abstract
1. Introduction
2. Materials and Methods
- Inclusion criteria
- The participant is willing and able to give informed consent for participation in the study.
- Established T2DM (>6 months since diagnosis).
- Male or female.
- Aged 18 to 75 years inclusive.
- Body mass index (BMI) of less than or equal to 45 kg/m2 inclusive.
- No known sleep disorders except obstructive sleep apnea (OSA).
- Glycated hemoglobin (HbA1c) of up to and below 10% (86 mmol/mol).
- Type 1 diabetes.
- Good command of the English language.
- Exclusion criteria
- The participant is unwilling or unable to give informed consent.
- Anyone without a good command of the English language.
- Anyone of <18 years of age and >75 years of age.
- HbA1c above 10% (86 mmol/mol).
- BMI greater than 45 kg/m2.
- A regular cannabis user, that is, weekly use.
- A terminal illness.
- A known sleep disorder that is not OSA.
- Regular use (≥weekly) of medications including those promoting wakefulness, sedatives, melatonin, and medications for nocturnal movement disorders.
- -
- Feet together.
- -
- Semi-tandem.
- -
- Tandem.
2.1. Accelerometer Processing
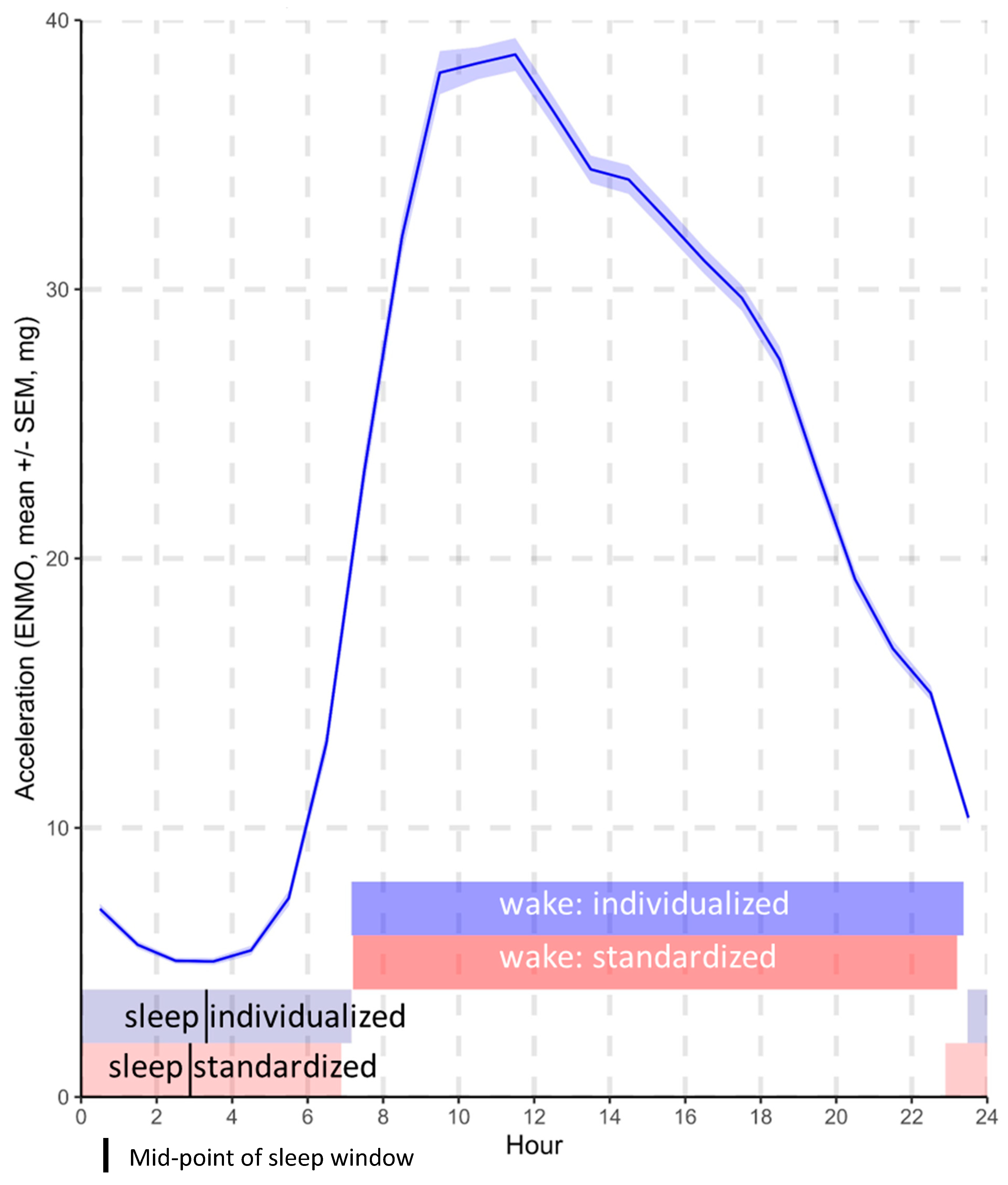
- The average acceleration is a proxy for the average intensity of physical activity over a given duration and was calculated over 3 separate time windows for the purposes of this analysis: (1) across the full 24 h day, (2) across the standardized wake and sleep windows, and (3) across the individualized wake and sleep windows. The resulting values were indicative of the overall level of physical activity undertaken within each of the specified time windows [8].
- The RA is a composite index of physical activity (average acceleration during the most active continuous 10 h, M10h) and movement during sleep (average acceleration during the least active continuous 5 h, L5h) and is calculated as (M10h − L5h)/(M10h + L5h) [20]. A high RA results from greater waking physical activity and reduced movement during sleep. Scores range from 0 to 1, with higher values indicating a higher amplitude or ‘healthier balance’.
- The intensity gradient describes the intensity distribution of physical activity and was calculated over the 24 h day, standardized wake window, and individualized wake window. Specifically, it describes the negative curvilinear relationship between the physical activity intensity and the time accumulated at that intensity [21]. Higher values indicate proportionally more time accumulated in higher-intensity activities or more time spread across the intensity distribution. The intensity gradient is always negative, reflecting the decrease in time accumulated as intensity increases.
2.2. Analyses
- The average acceleration over the 24 h day, over standardized wake and sleep windows (mutually adjusted for one another), and over individualized wake and sleep windows (mutually adjusted for one another).
- The RA.
- The intensity distribution of accelerations over 24 h, the standardized wake window, and the individualized wake window.
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Cui, X.; Meng, Y.; Zhou, M.; Xiao, L.; Ma, J.; Yi, G.; Chen, W. Sleep duration and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Int. J. Cardiol. 2016, 219, 231–239. [Google Scholar] [CrossRef]
- Shan, Z.; Majewski, C.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep Duration and Risk of Type 2 Diabetes: A Meta-analysis of Prospective Studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef]
- Doherty, A.; Jackson, D.; Hammerla, N.; Plötz, T.; Olivier, P.; Granat, M.H.; White, T.; van Hees, V.T.; Trenell, M.I.; Owen, C.G.; et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS ONE 2017, 12, e0169649. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Davies, M.J.; Dempsey, P.C.; Edwardson, C.L.; Razieh, C.; Yates, T. Wrist-worn accelerometers: Recommending ~1.0 mg as the minimum clinically important difference (MCID) in daily average acceleration for inactive adults. Br. J. Sports Med. 2020, 55, 814–815. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Kloecker, D.E.; Chudasama, Y.; Davies, M.J.; Dawkins, N.P.; Edwardson, C.L.; Gillies, C.; Khunti, K.; Razieh, C.; Islam, N.; et al. Association of Timing and Balance of Physical Activity and Rest/Sleep With Risk of COVID-19: A UK Biobank Study. Mayo Clin. Proc. 2021, 96, 156–164. [Google Scholar] [CrossRef]
- van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef]
- van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, N.P.; Yates, T.; Edwardson, C.L.; Maylor, B.; Davies, M.J.; Dunstan, D.; Highton, P.J.; Herring, L.Y.; Khunti, K.; Rowlands, A.V. Comparing 24 h physical activity profiles: Office workers, women with a history of gestational diabetes and people with chronic disease condition(s). J. Sports Sci. 2020, 39, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mickute, M.; Henson, J.; Rowlands, A.V.; Sargeant, J.A.; Webb, D.; Hall, A.P.; Edwardson, C.L.; Baldry, E.L.; Brady, E.M.; Khunti, K.; et al. Device-measured physical activity and its association with physical function in adults with type 2 diabetes mellitus. Diabet. Med. 2020, 38, e14393. [Google Scholar] [CrossRef]
- Brady, E.M.; Hall, A.P.; Baldry, E.; Chatterjee, S.; Daniels, L.J.; Edwardson, C.; Khunti, K.; Patel, M.I.; Henson, J.J.; Rowlands, A.; et al. Rationale and design of a cross-sectional study to investigate and describe the chronotype of patients with type 2 diabetes and the effect on glycaemic control: The CODEC study. BMJ Open 2019, 9, e027773. [Google Scholar] [CrossRef]
- Puthoff, M.L. Outcome Measures in Cardiopulmonary Physical Therapy: Short Physical Performance Battery. Cardiopulm. Phys. Ther. J. 2008, 19, 16–22. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Fang, Z.; Langford, J.; Assah, F.; Mohammad, A.; Da Silva, I.C.M.; Trenell, M.I.; White, T.; Wareham, N.J.; Brage, S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: An evaluation on four continents. J. Appl. Physiol. 2014, 117, 738–744. [Google Scholar] [CrossRef]
- Rock, P.; Goodwin, G.; Harmer, C.; Wulff, K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiol. Int. 2014, 31, 290–296. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Harrington, D.M.; Yates, T. Beyond Cut Points: Accelerometer Metrics that Capture the Physical Activity Profile. Med. Sci. Sports Exerc. 2018, 50, 1323–1332. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Etain, B.; Bellivier, F. More comprehensive models are needed to understand how relative amplitude might affect wellbeing and risk of mood disorders. Lancet Psychiatry 2018, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, N.P.; Yates, T.; Razieh, C.; Edwardson, C.L.; Maylor, B.; Zaccardi, F.; Khunti, K.; Rowlands, A.V. Differences in Accelerometer-Measured Patterns of Physical Activity and Sleep/Rest Between Ethnic Groups and Age: An Analysis of UK Biobank. J. Phys. Act. Health 2022, 19, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Clarke, A.E.; Carson, V.; Chaput, J.-P.; Giangregorio, L.M.; Kho, M.E.; Poitras, V.J.; Ross, R.; Saunders, T.J.; Ross-White, A.; et al. A systematic review of compositional data analysis studies examining associations between sleep, sedentary behaviour, and physical activity with health outcomes in adults. Appl. Physiol. Nutr. Metab. 2020, 45, S248–S257. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD, Median (IQR) *, or Count (%) | |
|---|---|
| Age | 66.0 (59.2, 71.0) * |
| Sex (female) | 230 (37.8) |
| Body mass index (BMI, kg·m−2) | 30.8 (5.0) |
| Diagnosis of sleep apnea | 46 (7.6) |
| Years since diabetes diagnosis | 8 (4, 14.3) * |
| Number of diabetes medications | 2 (1, 3) * |
| Medications | |
| Number on lipid-lowering medication | 436 (71.6) |
| Number on blood pressure medication | 398 (65.4) |
| Number of additional co-morbidities | |
| 0 | 79 (13.0) |
| 1 | 171 (28.1) |
| 2 | 208 (34.2) |
| 3 | 94 (15.4) |
| 4+ | 57 (9.4) |
| Ethnicity Caucasian South Asian Other | 506 (83.1) 73 (12.0) 30 (4.9) |
| Index of multiple deprivation † | 19,658 ± 9350.1 |
| Adiposity | |
| Fat percentage (%) | 34.2 ± 8.9 |
| Waist circumference (cm) | 106.2 ± 14.5 |
| Fitness | |
| SPPB score | 10.4 ± 1.8 |
| Sit-to-stand reps | 22.3 ±7.7 |
| Resting heart rate (bpm) | 72.0 ± 11.4 |
| Accelerometer variables | |
| Number of valid days | 6.9 ± 0.3 |
| Sleep duration (h:mm) | 7:42 ± 1:08 |
| Timings | |
| Standardized windows (M16h/L8h) | |
| Wake (hh:mm) | 07:11 ± 01:15 |
| Sleep onset (hh:mm) | 22:54 ± 01:13 |
| Sleep mid-point (hh:mm) | 02:54 ± 01:13 |
| Individualized (accelerometer determined) | |
| Wake (hh:mm) | 07:11 ± 01:20 |
| Sleep onset (hh:mm) | 23:28 ± 01:26 |
| Sleep mid-point (hh:mm) | 03:19 ± 01:16 |
| Average acceleration (mg) | |
| 24 h | 22.4 ± 7.0 |
| Standardized windows (M16h/L8h) | |
| Wake | 31.0 ± 10.2 |
| Sleep | 5.2 ± 1.5 |
| Individualized (accelerometer-determined) | |
| Wake | 30.8 ± 10.2 |
| Sleep | 4.5 ± 1.2 |
| Intensity gradient | |
| 24 h | −2.726 ± 0.197 |
| Standardized wake | −2.631 ± 0.188 |
| Individualized wake | −2.638 ± 0.205 |
| Relative amplitude | 0.94 ± 0.02 |
| Variable | Window | Regression Coefficient ** | 95% Confidence Interval | p-Value † |
|---|---|---|---|---|
| Waist circumference (cm) | ||||
| Average acceleration (mg) | 24 h | −3.18 | −4.21, −2.15 | <0.001 |
| Standardized wake | −4.24 | −5.31, −3.17 | <0.001 | |
| Standardized sleep | 3.01 | 1.82, 4.21 | <0.001 | |
| Individualized wake | −3.70 | −4.80, −2.60 | <0.001 | |
| Individualized sleep | 1.95 | 0.71, 3.19 | 0.002 | |
| Relative amplitude | −4.63 | −5.86, −3.41 | <0.001 | |
| Intensity gradient | 24 h | −2.41 | −3.43, −1.39 | <0.001 |
| Standardized wake | −2.42 | −3.46, −1.38 | <0.001 | |
| Individualized wake | −1.93 | −3.02, −0.84 | 0.001 | |
| Body fat percentage (%) | ||||
| Average acceleration (mg) | 24 h | −1.32 | −1.87, −0.78 | <0.001 |
| Standardized wake | −1.63 | −2.18, −1.07 | <0.001 | |
| Standardized sleep | 0.86 | 0.25, 1.48 | 0.006 | |
| Individualized wake | −1.54 | −2.06, −1.02 | <0.001 | |
| Individualized sleep | 0.91 | 0.38, 1.44 | 0.001 | |
| Relative amplitude | −1.88 | −2.56, −1.19 | <0.001 | |
| Intensity gradient | 24 h | −1.24 | −1.81, −0.68 | <0.001 |
| Standardized wake | −1.26 | −1.83, −0.69 | <0.001 | |
| Individualized wake | −0.99 | −1.57, −0.42 | 0.001 | |
| SPPB * score | ||||
| Average acceleration (mg) | 24 h | 0.17 | 0.02, 0.33 | 0.027 |
| Standardized wake | 0.27 | 0.11, 0.43 | 0.001 | |
| Standardized sleep | −0.24 | −0.40, −0.08 | 0.004 | |
| Individualized wake | 0.25 | 0.10, 0.40 | 0.001 | |
| Individualized sleep | −0.21 | −0.38, −0.02 | 0.028 | |
| Relative amplitude | 0.43 | 0.25, 0.61 | <0.001 | |
| Intensity gradient | 24 h | 0.37 | 0.23, 0.51 | <0.001 |
| Standardized wake | 0.36 | 0.22, 0.50 | <0.001 | |
| Individualized wake | 0.36 | 0.22, 0.50 | <0.001 | |
| Sit-to-stand repetitions (per 60 s) | ||||
| Average acceleration (mg) | 24 h | 1.15 | 0.44, 1.85 | <0.001 |
| Standardized wake | 1.72 | 1.00, 2.44 | <0.001 | |
| Standardized sleep | −1.44 | −2.20, −0.69 | <0.001 | |
| Individualized wake | 1.32 | 0.64, 1.99 | <0.001 | |
| Individualized sleep | −0.94 | −1.59, −0.29 | 0.005 | |
| Relative amplitude | 2.51 | 1.65, 3.37 | <0.001 | |
| Intensity gradient | 24 h | 1.70 | 1.09, 2.31 | <0.001 |
| Standardized wake | 1.65 | 1.04, 2.26 | <0.001 | |
| Individualized wake | 1.45 | 0.83, 2.06 | <0.001 | |
| Resting heart rate (bpm) | ||||
| Average acceleration | 24 h | −2.04 | −2.99, −1.09 | <0.001 |
| Standardized wake | −2.62 | −3.66, −1.59 | <0.001 | |
| Standardized sleep | 1.48 | 0.39, 2.58 | 0.008 | |
| Individualized wake | −2.11 | −3.07, −1.15 | <0.001 | |
| Individualized sleep | 1.34 | 0.43, 2.25 | 0.004 | |
| Relative amplitude | −2.93 | −4.11, −1.76 | <0.001 | |
| Intensity gradient | 24 h | −1.65 | −2.56, −0.73 | <0.001 |
| Standardized wake | −1.73 | −2.65, −0.80 | <0.001 | |
| Individualized wake | −1.19 | −2.10, −0.28 | 0.010 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowlands, A.V.; van Hees, V.T.; Dawkins, N.P.; Maylor, B.D.; Plekhanova, T.; Henson, J.; Edwardson, C.L.; Brady, E.M.; Hall, A.P.; Davies, M.J.; et al. Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep when Determining Associations with Markers of Health. Sensors 2023, 23, 5382. https://doi.org/10.3390/s23125382
Rowlands AV, van Hees VT, Dawkins NP, Maylor BD, Plekhanova T, Henson J, Edwardson CL, Brady EM, Hall AP, Davies MJ, et al. Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep when Determining Associations with Markers of Health. Sensors. 2023; 23(12):5382. https://doi.org/10.3390/s23125382
Chicago/Turabian StyleRowlands, Alex V., Vincent T. van Hees, Nathan P. Dawkins, Benjamin D. Maylor, Tatiana Plekhanova, Joseph Henson, Charlotte L. Edwardson, Emer M. Brady, Andrew P. Hall, Melanie J. Davies, and et al. 2023. "Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep when Determining Associations with Markers of Health" Sensors 23, no. 12: 5382. https://doi.org/10.3390/s23125382
APA StyleRowlands, A. V., van Hees, V. T., Dawkins, N. P., Maylor, B. D., Plekhanova, T., Henson, J., Edwardson, C. L., Brady, E. M., Hall, A. P., Davies, M. J., & Yates, T. (2023). Accelerometer-Assessed Physical Activity in People with Type 2 Diabetes: Accounting for Sleep when Determining Associations with Markers of Health. Sensors, 23(12), 5382. https://doi.org/10.3390/s23125382






