A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis
Abstract
:1. Introduction
2. Synthesis of MOFs
2.1. Hydrothermal/Solvothermal Methods
2.2. Microwave Synthesis
2.3. Electrochemical Synthesis
2.4. Mechanochemical Synthesis
2.5. Sonochemical Synthesis
3. Characterization of MOFs
3.1. N2 Adsorption/Desorption Isotherms
3.2. PXRD
3.3. SEM and TEM
3.4. Thermogravimetric Analyses
3.5. Fourier Transform Infrared Spectroscopy
3.6. NMR Spectroscopy
4. MOF-Based Electrochemical Biosensors for Biomedical Uses
4.1. Detection of the Pharmaceutical Drugs
4.2. Detection of Disease Biomarker
4.3. Detection of Small Biomolecules
4.4. Detection of Proteins
4.5. Detection of Pathogens
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz-Fernández, A.; Lorenzo-Gómez, R.; Miranda-Castro, R.; De-Los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Electrochemical aptasensors for cancer diagnosis in biological fluids—A review. Anal. Chim. Acta 2020, 1124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Darvishi, S.; Preet, A.; Huang, T.Y.; Lin, S.H.; Girault, H.H.; Lin, T.E. A review: Electrochemical biosensors for oral cancer. Chemosensors 2020, 8, 54. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Ozcelikay, G.; Ozkan, S.A. Recent progress on the sensitive detection of cardiovascular disease markers by electrochemical-based biosensors. J. Pharma. Biomed. Anal. 2018, 159, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.; Amin, H.M.; Compton, R.G. Electrochemical detection of pathogenic bacteria—Recent strategies, advances and challenges. Chem. Asian J. 2018, 13, 2758–2769. [Google Scholar] [CrossRef]
- Santhanam, K.S. Electrochemical approaches towards sensing viruses: A mini review. Med. Dev. Sens. 2021, 4, e10148. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiș, M.; Cernat, A.; Săndulescu, R.; Cristea, C. Electrochemical methods based on molecularly imprinted polymers for drug detection. A review. Int. J. Electrochem. Sci. 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Yarman, A.; Kurbanoglu, S.; Jetzschmann, K.J.; Ozkan, S.A.; Wollenberger, U.; Scheller, F.W. Electrochemical MIP-sensors for drugs. Curr. Med. Chem. 2018, 25, 4007–4019. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Frias, C.; Marques, T.; Frazão, O. Smart sensors/actuators for biomedical applications. Measurement 2012, 45, 1675–1688. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.L.; Reaz, M.B.I. Evolution of a capacitive electromyography contactless biosensor: Design and modelling techniques. Measurement 2019, 145, 460–471. [Google Scholar] [CrossRef]
- French, D. Advances in bioanalytical techniques to measure steroid hormones in serum. Bioanalysis 2016, 8, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wright, G.; Yang, Y. Materials and techniques for electrochemical biosensor design and construction. Biosens. Bioelectron. 2000, 15, 273–282. [Google Scholar] [CrossRef]
- Sahin, B.; Kaya, T. Electrochemical amperometric biosensor applications of nanostructured metal oxides: A review. Mater. Res. Express 2019, 6, 042003. [Google Scholar] [CrossRef]
- Hamdan, S.K. In vivo electrochemical biosensor for brain glutamate detection: A mini review. Malays. J. Med. Sci. 2014, 21, 12. [Google Scholar]
- Cheng, A.K.; Sen, D.; Yu, H.Z. Design and testing of aptamer-based electrochemical biosensors for proteins and small molecules. Bioelectrochemistry 2009, 77, 1–12. [Google Scholar] [CrossRef]
- Ulhakim, M.T.; Rezki, M.; Dewi, K.K.; Abrori, S.A.; Harimurti, S.; Septiani, N.L.W.; Yuliarto, B. Review–Recent Trend on Two-Dimensional Metal-Organic Frameworks for Electrochemical Biosensor Application. J. Electrochem. Soc. 2020, 165, 136509. [Google Scholar] [CrossRef]
- Liao, X.; Fu, H.; Yan, T.; Lei, J. Electroactive metal–organic framework composites: Design and biosensing application. Biosens. Bioelectron. 2019, 146, 111743. [Google Scholar] [CrossRef]
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic acid-functionalized metal–organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef]
- Liu, T.Z.; Hu, R.; Zhang, X.; Zhang, K.L.; Liu, Y.; Zhang, X.B.; Yang, Y.H. Metal–organic framework nanomaterials as novel signal probes for electron transfer mediated ultrasensitive electrochemical immunoassay. Anal. Chem. 2016, 88, 12516–12523. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-organic frameworks for the development of biosensors: A current overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Applications of Functional Metal-Organic Frameworks in Biosensors. Biotechnol. J. 2021, 16, 1900424. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lü, W.; Zuo, X.; Zhu, Q.; Pan, C.; Niu, X.; Chen, X. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells. Biosens. Bioelectron. 2017, 95, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B Chem. 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Jin, H.; Wei, M.; Ren, W.; Zhang, Y.; He, B. Electrochemical biosensor for sensitive detection of Hg2+ baesd on clustered peonylike copper-based metal-organic frameworks and DNAzyme-driven DNA Walker dual amplification signal strategy. Sens. Actuators B Chem. 2021, 329, 129215. [Google Scholar] [CrossRef]
- Burnett, B.J.; Barron, P.M.; Hu, C.; Choe, W. Stepwise synthesis of metal–organic frameworks: Replacement of structural organic linkers. J. Am. Chem. Soc. 2011, 133, 9984–9987. [Google Scholar] [CrossRef]
- Bosch, M.; Yuan, S.; Rutledge, W.; Zhou, H.C. Stepwise synthesis of metal–organic frameworks. Acc. Chem. Res. 2017, 50, 857–865. [Google Scholar] [CrossRef]
- Walton, R.I. Perovskite Oxides Prepared by Hydrothermal and Solvothermal Synthesis: A Review of Crystallisation, Chemistry, and Compositions. Chem. Eur. J. 2020, 26, 9041–9069. [Google Scholar] [CrossRef]
- Chen, W.; Du, L.; Wu, C. Hydrothermal synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 141–157. [Google Scholar]
- Pachfule, P.; Das, R.; Poddar, P.; Banerjee, R. Solvothermal synthesis, structure, and properties of metal organic framework isomers derived from a partially fluorinated link. Crys. Growth Des. 2011, 11, 1215–1222. [Google Scholar] [CrossRef]
- Tzitzios, V.; Kostoglou, N.; Giannouri, M.; Basina, G.; Tampaxis, C.; Charalambopoulou, G.; Rebholz, C. Solvothermal synthesis, nanostructural characterization and gas cryo-adsorption studies in a metal–organic framework (IRMOF-1) material. Int. J. Hydrog. Energy 2017, 42, 23899–23907. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal–organic framework (MOF) nanoparticles for curing with epoxy. J. Alloy. Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Haque, E.; Khan, N.A.; Park, J.H.; Jhung, S.H. Synthesis of a metal–organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: A kinetic study. Chem. Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Dau, P.V.; Cohen, S.M.; Ranocchiari, M.; Van Bokhoven, J.A.; Costantino, F.; Vivani, R. Efficient microwave assisted synthesis of metal–organic framework UiO-66: Optimization and scale up. Dalton Trans. 2015, 44, 14019–14026. [Google Scholar] [CrossRef]
- George, P.; Dhabarde, N.R.; Chowdhury, P. Rapid synthesis of titanium based metal organic framework (MIL-125) via microwave route and its performance evaluation in photocatalysis. Mater. Lett. 2017, 186, 151–154. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.V.; Nageswaran, G. Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar]
- Kumar, R.S.; Kumar, S.S.; Kulandainathan, M.A. Efficient electrosynthesis of highly active Cu3 (BTC) 2-MOF and its catalytic application to chemical reduction. Microporous Mesoporous Mater. 2013, 168, 57–64. [Google Scholar] [CrossRef]
- Wei, J.Z.; Gong, F.X.; Sun, X.J.; Li, Y.; Zhang, T.; Zhao, X.J.; Zhang, F.M. Rapid and low-cost electrochemical synthesis of UiO-66-NH2 with enhanced fluorescence detection performance. Inorg. Chem. 2019, 58, 6742–6747. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical synthesis of metal–organic frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Prochowicz, D.; Sokołowski, K.; Justyniak, I.; Kornowicz, A.; Fairen-Jimenez, D.; Friščić, T.; Lewiński, J. A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors. Chem. Commun. 2015, 51, 4032–4035. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, J.; Lv, D.; Huang, T.; Xu, F.; Sun, X.; Li, Z. Highly efficient mechanochemical synthesis of an indium based metal-organic framework with excellent water stability. Chem. Eng. Sci. 2017, 158, 539–544. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Liu, Z.; Sun, X.; Xia, Q.; Li, Z. Liquid-assisted mechanochemical synthesis of copper based MOF-505 for the separation of CO2 over CH4 or N2. Ind. Eng. Chem. Res. 2018, 57, 703–709. [Google Scholar] [CrossRef]
- Son, W.J.; Kim, J.; Kim, J.; Ahn, W.S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 47, 6336–6338. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Fang, M.; Hyeon, T. Sonochemical synthesis of iron colloids. J. Am. Chem. Soc. 1996, 118, 11960–11961. [Google Scholar] [CrossRef]
- Li, Z.Q.; Qiu, L.G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kim, J.; Kim, S.N.; Ahn, W.S. High yield 1-L scale synthesis of ZIF-8 via a sonochemical route. Microporous Mesoporous Mater. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Alp, E.E.; Mini, S.M.; Ramanathan, M. X-ray Absorption Spectroscopy: EXAFS and XANES-A Versatile Tool to Study the Atomic and Electronic Structure of Materials (No. ANL/APS/TM-7) United States; INIS: Vienna, Austria, 1990; Volume 22, pp. 25–36. [Google Scholar]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Pierotti, R.; Rouquerol, J.J.P.A.C. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr 6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 2014, 50, 8944–8946. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Wang, T.C.; Bury, W.; Gómez-Gualdrón, D.A.; Vermeulen, N.A.; Mondloch, J.E.; Deria, P.; Farha, O.K. Ultrahigh surface area zirconium MOFs and insights into the applicability of the BET theory. J. Am. Chem. Soc. 2015, 137, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, G.; Afzali, D.; Daldosso, N.; Kazemian, H.; Chauhan, N.P.S.; Sadeghian, Z.; Mozafari, M. A systematic study on the use of ultrasound energy for the synthesis of nickel–metal organic framework compounds. Ultrason. Sonochem. 2015, 27, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale screening of hypothetical metal–organic frameworks. Nature Chem. 2012, 4, 83. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data; the use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Fujii, K.; Garay, A.L.; Hill, J.; Sbircea, E.; Pan, Z.; Xu, M.; Harris, K.D. Direct structure elucidation by powder X-ray diffraction of a metal–organic framework material prepared by solvent-free grinding. Chem. Commun. 2010, 46, 7572–7574. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Budnyk, A.P.; Charykov, K.M.; Vetlitsyna-Novikova, K.S.; Bugaev, A.L.; Guda, A.A.; Lamberti, C. Partial and complete substitution of the 1, 4-benzenedicarboxylate linker in UiO-66 with 1, 4-naphthalenedicarboxylate: Synthesis, characterization, and H2-adsorption properties. Inorg. Chem. 2019, 58, 1607–1620. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 77–85. [Google Scholar]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. M@ MIL-100 (Fe)(M = Au, Pd, Pt) nanocomposites fabricated by a facile photodeposition process: Efficient visible-light photocatalysts for redox reactions in water. Nano Res. 2015, 8, 3237–3249. [Google Scholar] [CrossRef]
- Cik, R.C.H.; Foo, C.T.; Nor, A.F.O. Field Emission Scanning Electron Microscope (FESEM) Facility in BTI; Oral Presentation; Malaysian Nuclear Agency Document Delivery Center: Kajang, Malaysia, 2015; Volume 47, pp. 3–5. [Google Scholar]
- Zhu, Y.; Ciston, J.; Zheng, B.; Miao, X.; Czarnik, C.; Pan, Y.; Han, Y. Unravelling surface and interfacial structures of a metal–organic framework by transmission electron microscopy. Nature Mater. 2017, 16, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Yang, Z. Transmission electron microscopy (TEM). Membr. Character. 2017, 145–159. [Google Scholar]
- Yang, T.; Xiang, Q.; Feng, L.; Dong, C.; Zhang, X.; Ning, Z.; Gao, D. Yttrium-based metal-organic frameworks: Controllable synthesis, growth mechanism and the phase transformation to Y2O3: Eu3+ phosphors. J. Lumin. 2019, 214, 116567. [Google Scholar] [CrossRef]
- Butova, V.V.E.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280. [Google Scholar] [CrossRef]
- Pei, X.; Wu, Y.; Wang, J.; Chen, Z.; Liu, W.; Su, W.; Liu, F. Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal–organic framework for efficient biocatalysis. Nanoscale 2020, 12, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Duygu, D.; Baykal, T.; Açikgöz, İ.; Yildiz, K. Fourier transform infrared (FT-IR) spectroscopy for biological studies. Gazi Univ. J. Sci. 2009, 22, 117–121. [Google Scholar]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuang, Y.; Liu, M.; Wang, J.; Pei, R. Synthesis of metal–organic framework nanosheets with high relaxation rate and singlet oxygen yield. Chem. Mater. 2018, 30, 7511–7520. [Google Scholar] [CrossRef]
- Wang, T.C.; Vermeulen, N.A.; Kim, I.S.; Martinson, A.B.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. Scalable synthesis and post-modification of a mesoporous metal-organic framework called NU-1000. Nat. Protoc. 2016, 11, 149–162. [Google Scholar] [CrossRef]
- Klein, N.; Herzog, C.; Sabo, M.; Senkovska, I.; Getzschmann, J.; Paasch, S.; Kaskel, S. Monitoring adsorption-induced switching by 129Xe NMR spectroscopy in a new metal–organic framework Ni2(2,6-ndc)2 (dabco). Phys. Chem. Chem. Phys. 2010, 12, 11778–11784. [Google Scholar] [CrossRef]
- Özkan, S.A.; Uslu, B.; Aboul-Enein, H.Y. Analysis of pharmaceuticals and biological fluids using modern electroanalytical techniques. Crit. Rev. Anal. Chem. 2003, 33, 155–181. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dubey, R.S.; Malik, J.K. Application of modern electroanalytical techniques: Recent trend in pharmaceutical and drug analysis. Int. J. Pharma. Sci. Res. 2013, 4, 2450. [Google Scholar]
- Pradhan, S.; Jityen, A.; Juagwon, T.; Sinsarp, A.; Osotchan, T. Development of Electrochemical Electrodes Using Carbon Nanotube and Metal Phthalocyanine to Classify Pharmaceutical Drugs. Mater. Today Proc. 2020, 23, 732–737. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; Wang, J. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Hu, X.; Zhang, Y.; Teng, M.; Deng, R.; Xing, G.; Zhang, G. Label-free electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@ graphene composites for sensitive detection of monensin in milk. Sens. Actuators B Chem. 2019, 288, 571–578. [Google Scholar] [CrossRef]
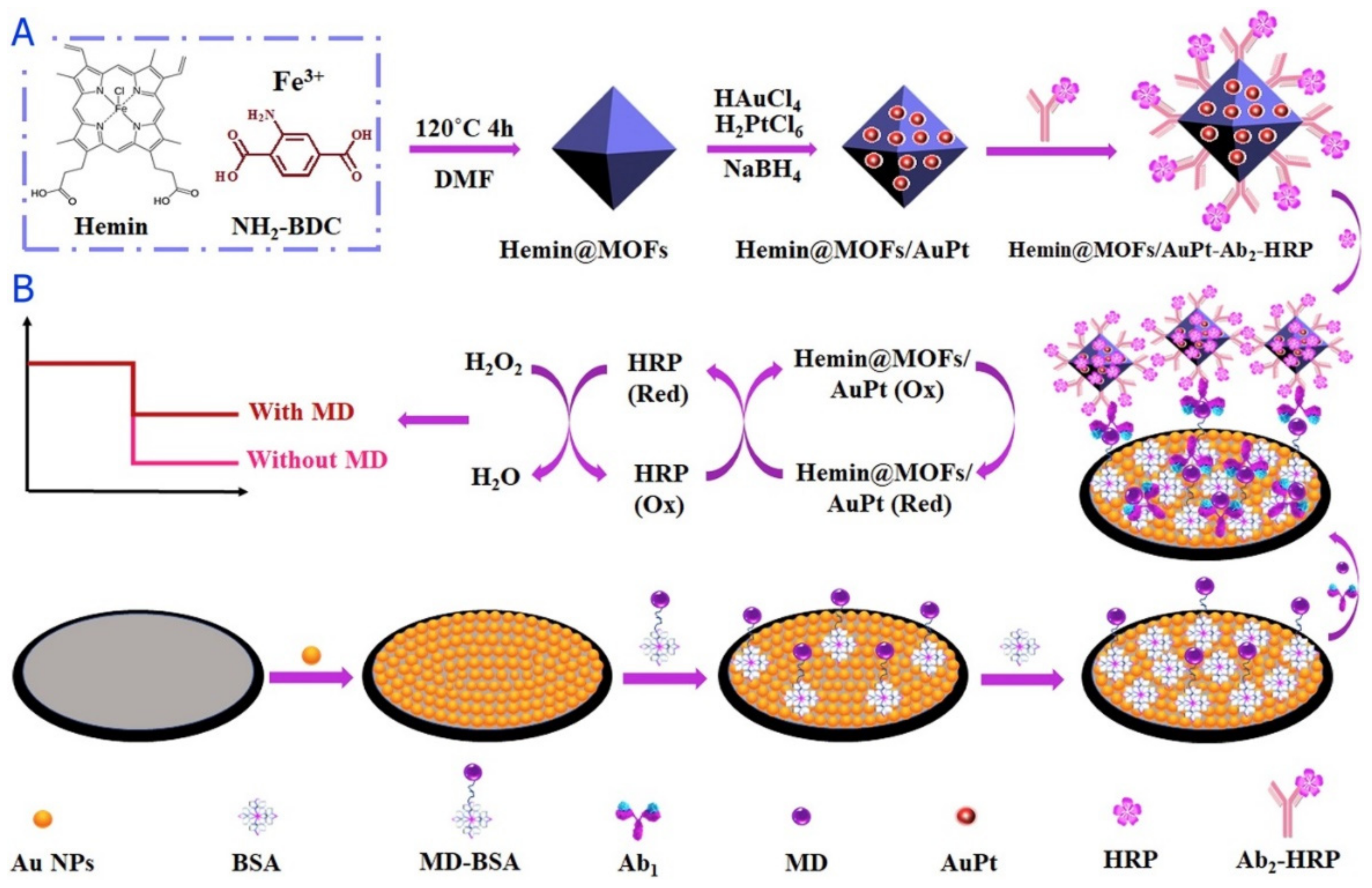
- Hu, M.; Wang, Y.; Yang, J.; Sun, Y.; Xing, G.; Deng, R.; Zhang, G. Competitive electrochemical immunosensor for maduramicin detection by multiple signal amplification strategy via hemin@ Fe-MIL-88NH2/AuPt. Biosens. Bioelectron. 2019, 142, 111554. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.S.; Gadhari, N.S.; Srivastava, A.K. Biomimetic sensor for ethambutol employing β-cyclodextrin mediated chiral copper metal organic framework and carbon nanofibers modified glassy carbon electrode. Biosens. Bioelectron. 2020, 165, 112397. [Google Scholar] [CrossRef]
- Wu, X.Q.; Feng, P.Q.; Guo, Z.; Wei, X. Water-Stable 1D Double-Chain Cu Metal–Organic Framework-based Electrochemical Biosensor for Detecting l-Tyrosine. Langmuir 2020, 36, 14123–14129. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Liu, X.; Li, Z.; Wang, C.; Jia, Q.; Du, M. A label-free enrofloxacin electrochemical aptasensor constructed by a semiconducting CoNi-based metal–organic framework (MOF). Electrochim. Acta 2021, 368, 137609. [Google Scholar] [CrossRef]
- Zhang, H.W.; Li, H.K.; Han, Z.Y.; Yuan, R.; He, H. Incorporating Fullerenes in Nanoscale Metal–Organic Matrixes: An Ultrasensitive Platform for Impedimetric Aptasensing of Tobramycin. ACS Appl. Mater. Interfaces 2022, 14, 7350–7357. [Google Scholar] [CrossRef]
- Rajeev, G.; Prieto Simon, B.; Marsal, L.F.; Voelcker, N.H. Advances in Nanoporous Anodic Alumina-Based Biosensors to Detect Biomarkers of Clinical Significance: A Review. Adv. Healthc. Mater. 2018, 7, 1700904. [Google Scholar] [CrossRef]
- Solhi, E.; Hasanzadeh, M. Critical role of biosensing on the efficient monitoring of cancer proteins/biomarkers using label-free aptamer based bioassay. Biomed. Pharmacother. 2020, 132, 110849. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Z.; Morrin, A.; Luo, X. Low fouling strategies for electrochemical biosensors targeting disease biomarkers. Anal. Methods 2019, 11, 702–711. [Google Scholar] [CrossRef]
- Reddy, K.K.; Bandal, H.; Satyanarayana, M.; Goud, K.Y.; Gobi, K.V.; Jayaramudu, T.; Kim, H. Recent trends in electrochemical sensors for vital biomedical markers using hybrid nanostructured materials. Adv. Sci. 2020, 7, 1902980. [Google Scholar] [CrossRef] [PubMed]
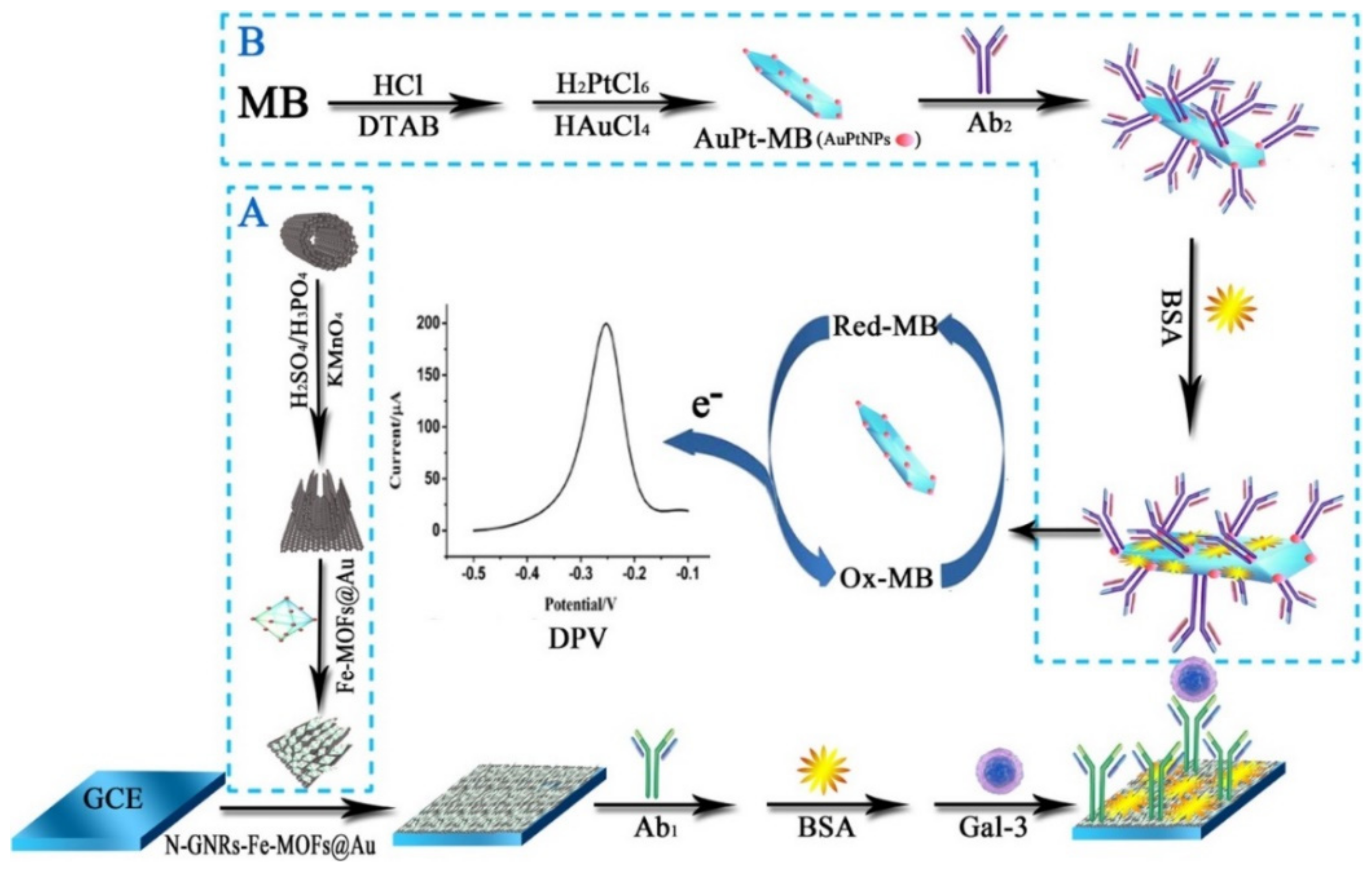
- Tang, Z.; He, J.; Chen, J.; Niu, Y.; Zhao, Y.; Zhang, Y.; Yu, C. A sensitive sandwich-type immunosensor for the detection of galectin-3 based on N-GNRs-Fe-MOFs@ AuNPs nanocomposites and a novel AuPt-methylene blue nanorod. Biosens. Bioelectron. 2018, 101, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, C.; Yang, B.; Liu, Z.; Xia, P.; Wang, Q. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelectron. 2018, 102, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qin, Z.; Hao, Y.; He, Q.; Chen, S.; Zhang, Z.; Li, C. A signal-decreased electrochemical immunosensor for the sensitive detection of LAG-3 protein based on a hollow nanobox-MOFs/AuPt alloy. Biosens. Bioelectron. 2018, 113, 148–156. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.; Ma, Z.; Han, H. Cascade catalysis-initiated radical polymerization amplified impedimetric immunosensor for ultrasensitive detection of carbohydrate antigen 15-3. Biosens. Bioelectron. 2019, 137, 1–7. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Z.; Lu, J.; Zhang, S.; Che, T.; Chen, Z.; Zhang, L. Electrochemical dual-aptamer-based biosensor for nonenzymatic detection of cardiac troponin I by nanohybrid electrocatalysts labeling combined with DNA nanotetrahedron structure. Biosens. Bioelectron. 2019, 134, 49–56. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, X.Z.; Gu, H.W.; Yi, H.C.; Sun, W.Y. A dual-response biosensor for electrochemical and glucometer detection of DNA methyltransferase activity based on functionalized metal-organic framework amplification. Biosens. Bioelectron. 2019, 134, 117–122. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, D.; Tong, Y.; Zhong, Y.; Chen, Z. DNA nanotetrahedron linked dual-aptamer based voltammetric aptasensor for cardiac troponin I using a magnetic metal-organic framework as a label. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Rezaei, H.; Motovali-Bashi, M.; Radfar, S. An enzyme-free electrochemical biosensor for simultaneous detection of two hemophilia A biomarkers: Combining target recycling with quantum dots-encapsulated metal-organic frameworks for signal amplification. Anal. Chim. Acta 2019, 1092, 66–74. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, D.; Zhao, M.; Wu, H.; Shen, B.; Ding, S. Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@ Pt@ MOF nanozyme. Biosens. Bioelectron. 2020, 168, 112554. [Google Scholar] [CrossRef]
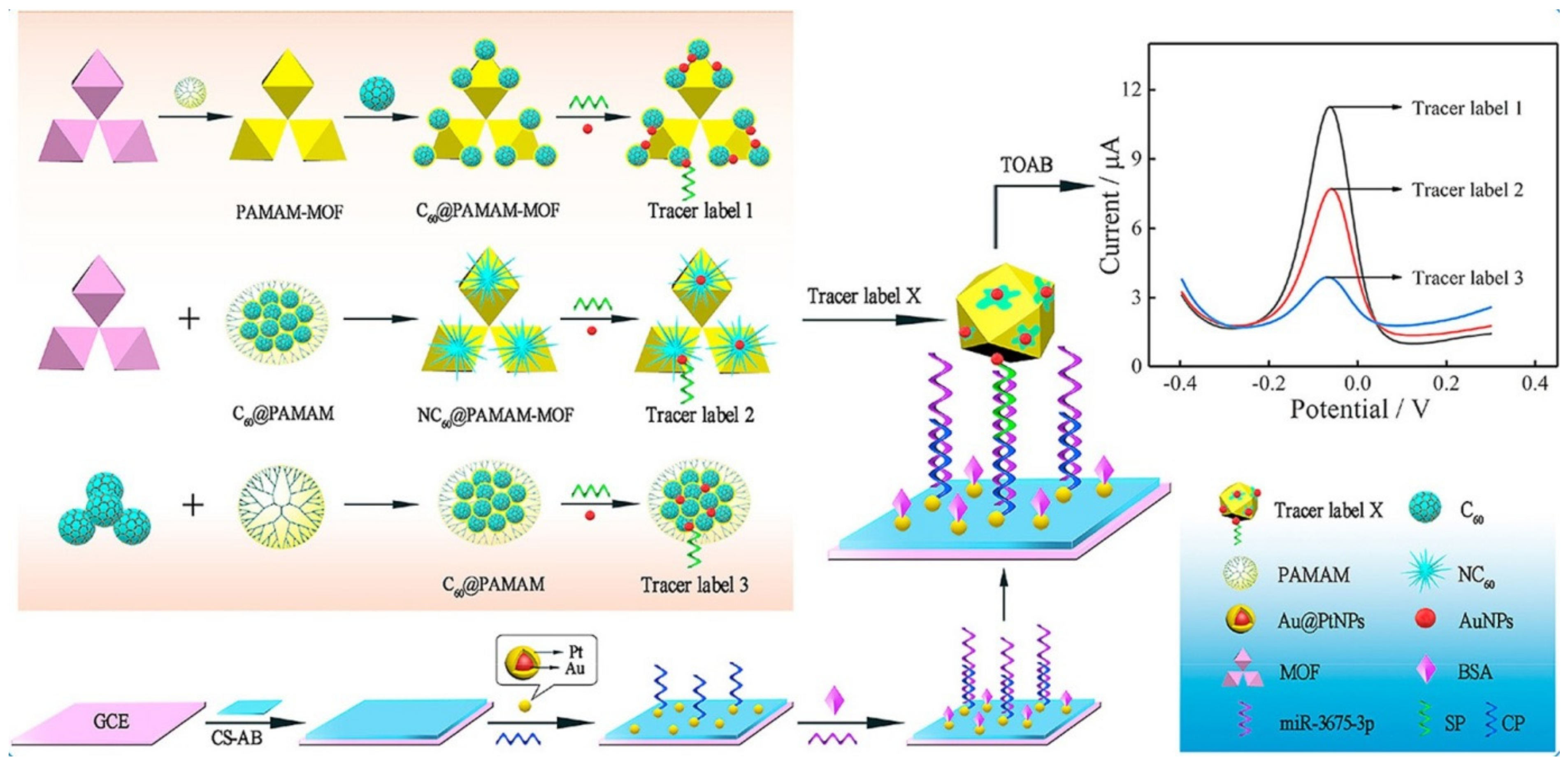
- Zuo, J.; Yuan, Y.; Zhao, M.; Wang, J.; Chen, Y.; Zhu, Q.; Bai, L. An efficient electrochemical assay for miR-3675-3p in human serum based on the nanohybrid of functionalized fullerene and metal-organic framework. Anal. Chim. Acta 2020, 1140, 78–88. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.K.; Pachauri, V.; Ingebrandt, S.; Singh, S.; Sharma, A.L.; Deep, A. Sensitive impedimetric detection of troponin I with metal–organic framework composite electrode. RSC Adv. 2021, 11, 2167–2174. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ming, D. Molybdenum Disulfide Supported on Metal–Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Appl. Bio Mater. 2021, 4, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Lu, X.; Wu, L.; Tan, D.; Li, Y.; Chen, J.; Jain, R. Voltammetric sensing of biomolecules at carbon based electrode interfaces: A review. TrAC Trends Anal. Chem. 2018, 98, 174–189. [Google Scholar]
- Lu, L. Recent advances in synthesis of three-dimensional porous graphene and its applications in construction of electrochemical (bio) sensors for small biomolecules detection. Biosens. Bioelectron. 2018, 110, 180–192. [Google Scholar] [CrossRef]
- Qiu, R.; Xu, Q.; Jiang, H.; Wang, X. A Novel Enzyme-Free Biosensor Based on Porous Core–Shell Metal Organic Frame Nanocomposites Modified Electrode for Highly Sensitive Detection of Uric Acid and Dopamine. J. Biomed. Nanotechnol. 2019, 15, 1443–1453. [Google Scholar] [CrossRef]
- Choi, H.S.; Yang, X.; Liu, G.; Kim, D.S.; Yang, J.H.; Lee, J.H.; Kim, S.W. Development of Co-hemin MOF/chitosan composite based biosensor for rapid detection of lactose. J. Taiwan Inst. Chem. Eng. 2020, 113, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ye, C.; Bao, S.J.; Zhang, Y.; Yu, Y.N.; Xu, M.W. Carbon nanotubes implanted manganese-based MOFs for simultaneous detection of biomolecules in body fluids. Analyst 2016, 141, 1279–1285. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Sun, Z.; Liu, H.; Wang, X. Innovative Integration of Phase-Change Microcapsules with Metal–Organic Frameworks into an Intelligent Biosensing System for Enhancing Dopamine Detection. ACS Appl. Mater. Interfaces 2021, 13, 41753–41772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, X.; Gu, Q.; Zhang, J.; Ding, Y.; Xue, L.; Wu, Q. Cascade amplification based on PEI-functionalized metal–organic framework supported gold nanoparticles/nitrogen–doped graphene quantum dots for amperometric biosensing applications. Electrochim. Acta 2022, 405, 139803. [Google Scholar] [CrossRef]
- Leca-Bouvier, B.; Blum, L.J. Biosensors for protein detection: A review. Anal. Lett. 2005, 38, 1491–1517. [Google Scholar] [CrossRef]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. Ultra-sensitive and selective electrochemical biosensor with aptamer recognition surface based on polymer quantum dots and C60/MWCNTs-polyethylenimine nanocomposites for analysis of thrombin protein. Bioelectrochemistry 2020, 138, 107701. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muñiz, A.; Merkoçi, A. Electrochemical detection of proteins using nanoparticles: Applications to diagnostics. Expert Opin. Med. Diagn. 2010, 4, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, S.; Gao, J.; Zhao, J.; Xue, S.; Xu, W. Glucose oxidase-initiated cascade catalysis for sensitive impedimetric aptasensor based on metal-organic frameworks functionalized with Pt nanoparticles and hemin/G-quadruplex as mimicking peroxidases. Biosens. Bioelectron. 2017, 98, 83–90. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.; Li, C.C.; Wang, C.M.; Zhang, C.Y. An electrochemical biosensor based on the enhanced quasi-reversible redox signal of prussian blue generated by self-sacrificial label of iron metal-organic framework. Biosens. Bioelectron. 2018, 122, 168–174. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Wang, Z.; Yu, H.; Han, J.; Xie, G.; Chen, S. Highly stable Ni-MOF comprising triphenylamine moieties as a high-performance redox indicator for sensitive aptasensor construction. Anal. Chim. Acta 2019, 1049, 74–81. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Ding, S.; Fan, J.; Luo, Z.; Liu, K.; Zang, G. A highly sensitive label-free electrochemical immunosensor based on AuNPs-PtNPs-MOFs for nuclear matrix protein 22 analysis in urine sample. J. Electroanal. Chem. 2019, 834, 33–42. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, D.; Cui, H.; Huang, T. ZnO/porous carbon composite from a mixed-ligand MOF for ultrasensitive electrochemical immunosensing of C-reactive protein. Sens. Actuators B Chem. 2019, 284, 354–361. [Google Scholar] [CrossRef]
- Hatami, Z.; Jalali, F.; Tabrizi, M.A.; Shamsipur, M. Application of metal-organic framework as redox probe in an electrochemical aptasensor for sensitive detection of MUC1. Biosens. Bioelectron. 2019, 141, 111433. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Teng, H.; Ding, C.; Xu, G.; Luo, X. Designed zwitterionic peptide combined with sacrificial Fe-MOF for low fouling and highly sensitive electrochemical detection of T4 polynucleotide kinase. Sens. Actuators B Chem. 2020, 305, 127329. [Google Scholar] [CrossRef]
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@ Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Rocha-Gaso, M.I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Stevenson, K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst 2019, 144, 6461–6478. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, L.; Lin, L.; Hu, Y.; Zheng, F.; Hu, Y.; Wang, Q. Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: A meta-analysis. J. Infect. 2013, 67, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wan, K. MPT64 polymorphisms of Mycobacterium tuberculosis strains suggest ongoing immune evasion. Tuberculosis 2014, 94, 712–714. [Google Scholar] [CrossRef]
- Li, N.; Huang, X.; Sun, D.; Yu, W.; Tan, W.; Luo, Z.; Chen, Z. Dual-aptamer-based voltammetric biosensor for the Mycobacterium tuberculosis antigen MPT64 by using a gold electrode modified with a peroxidase loaded composite consisting of gold nanoparticles and a Zr (IV)/terephthalate metal-organic framework. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, Y.; Chen, Y.; Zhang, P.; Bai, Y.; Bai, L. Aptamer based voltammetric biosensor for Mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, G.; Gou, D.; Luo, P.; Yao, Y.; Chen, H. A novel enzyme-free electrochemical biosensor for rapid detection of Pseudomonas aeruginosa based on high catalytic Cu-ZrMOF and conductive Super P. Biosens. Bioelectron. 2019, 142, 111486. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Kim, K.H.; Deep, A. Development of an advanced electrochemical biosensing platform for E. coli using hybrid metal-organic framework/polyaniline composite. Environ. Res. 2019, 171, 395–402. [Google Scholar] [CrossRef]
- Wang, W.; Tan, L.; Wu, J.; Li, T.; Xie, H.; Wu, D.; Gan, N. A universal signal-on electrochemical assay for rapid on-site quantitation of vibrio parahaemolyticus using aptamer modified magnetic metal–organic framework and phenylboronic acid-ferrocene co-immobilized nanolabel. Anal. Chim. Acta 2020, 1133, 128–136. [Google Scholar] [CrossRef]
- Panhwar, S.; Ilhan, H.; Hassan, S.S.; Zengin, A.; Boyacı, I.H.; Tamer, U. Dual Responsive Disposable Electrode for the Enumeration of Escherichia coli in Whole Blood. Electroanalysis 2020, 32, 2244–2252. [Google Scholar] [CrossRef]
- Dai, G.; Li, Z.; Luo, F.; Lu, Y.; Chu, Z.; Zhang, J.; He, P. Simultaneous electrochemical determination of nuc and mecA genes for identification of methicillin-resistant Staphylococcus aureus using N-doped porous carbon and DNA-modified MOF. Microchim. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, J.; Ning, S.; Hou, J.; Waterhouse, G.I.; Cheng, Z.; Ai, S. An electrochemical immunosensor based on an etched zeolitic imidazolate framework for detection of avian leukosis virus subgroup J. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sheta, S.M.; El-Sheikh, S.M.; Osman, D.I.; Salem, A.M.; Ali, O.I.; Harraz, F.A.; Dionysiou, D.D. A novel HCV electrochemical biosensor based on a polyaniline@ Ni-MOF nanocomposite. Dalton Trans. 2020, 49, 8918–8926. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Lou, Y.; Rong, F.; Zhang, S.; Wang, M.; He, L.; Du, M. Silver nanoparticle embedded polymer–zirconium-based metal–organic framework (polyUiO-66) for electrochemical biosensors of respiratory viruses. J. Mater. Chem. C 2021, 9, 14190–14200. [Google Scholar] [CrossRef]
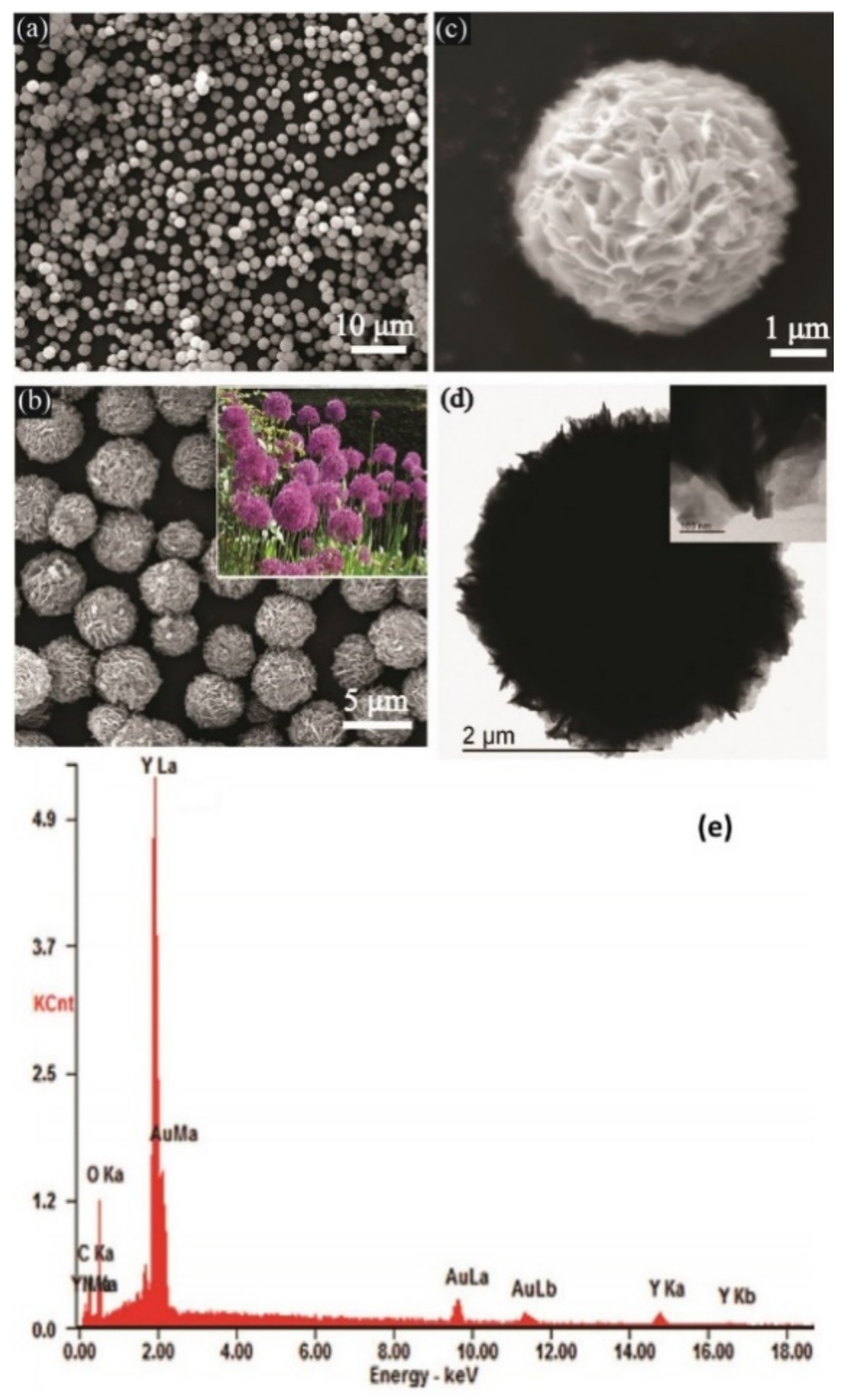





| Biosensor Materials | Analyte | Technique | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| anti-monensin antibodies and Zn/Ni-ZIF-8-800@grahene composites | Monensin | DPV | 0.25–100.0 ng mL−1 | 0.11 ng mL−1 | [77] |
| hemin@MOFs/AuPt-Ab2-HRP/HRP | Maduramicin | i-t | 0.1–50.0 ng mL−1 | 0.045 ng mL−1 | [78] |
| CD-CuMOF-CNF and artificial enzyme model | RR-ethambutol | SWV | 1.0 × 10−7–1.0 × 10−4 | 3.10 × 10−8 M | [79] |
| SS-ethambutol | 5.0 × 10−7–2.5 × 10−4 | 8.52 × 10−8 M | |||
| Cu-1 | L-tyrosine | DPV | 0.01–0.09 mM | 5.822 μM | [80] |
| CoxNi3-x(HITP)2/Apt | Enrofloxacin | EIS | 0.001–1.0 pg·mL−1 | 0.2 fg·mL−1 | [81] |
| C60@UiO-66-NH2 and aptamer | Tobramycin | EIS | 2.1 × 10−3–106.9 nM | 0.377 pM | [82] |
| Biosensor Materials | Biomarker | Technique | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| N-GNRs-Fe-MOFs@AuNPs-Ab1 and AuPt-MB-Ab2 | Galectin-3 | DPV | 100.0 fg mL−1–50.0 ng mL−1 | 33.33 fg mL−1 | [87] |
| PdNPs@Fe-MOFs/SA/SPs and hairpin assembly | miR-122 | Amperometric | 0.01 fM–10.0 pM | 0.003 fM | [88] |
| rGO-SnO2/HNMs/AuPt and SiO2-Ab2 | Lymphocyte activation gene-3 | Amperometric | 0.01 ng·mL−1–1.0 μg·mL−1 | 1.1 pg·mL−1 | [89] |
| Ab2-functionalized GOx-Cu-MOF | carbohydrate antigen 15–3 | Impedimetric | 10.0 μU/mL–100.0 U/mL | 5.06 μU/mL | [90] |
| Fe3O4@UiO-66/Cu@Au nanocomposites and NTH-Tro4 + Tro6 | Cardiac troponin I | DPV | 0.05–100.0 ng/mL | 16.0 pg/mL | [91] |
| Invertase/CP/Au/CuMOF and hairpin probe 1 & 2 | DNA methyltransferase | DPV | 0.002-12.0 U mL−1 | 0.001 U mL−1 | [92] |
| Fe3O4@UiO-66/Au@PtNP nanocomposites and GQH DNAzyme | Cardiac troponin I | DPV | 0.01–100.0 ng·mL−1 | 5.7 pg·mL−1 | [93] |
| Au nanoparticles, PbS and CdS QDs encapsulated ZIF-8 particles and catalytic hairpin assembly | miR-1246 | DPV | 1.0 fM–1.0 mM | 0.19 fM | [94] |
| miR-4521 | 1.0 fM–1.0 mM | 0.28 fM | |||
| MOF@Pt@MOF nanozyme | Exosomal miRNA | DPV | 1.0 fM–1.0 nM | 0.29 fM | [95] |
| C60@PAMAM-MOF coupled with CS-AB and Au@PtNPs | miR-3675-3p | DPV | 10.0 fM–10.0 nM | 2.99 fM | [96] |
| b/Cu3(BTC)2/PANI | Troponin I | Impedimetric | 1.0–400.0 ng mL−1 | 0.8 ng mL−1 | [97] |
| MOF-808/CNT/Ab | carbohydrate antigen 125 | DPV | 0.001–30.0 ng·mL−1 | 0.5 pg·mL−1 | [98] |
| CuBTC@MoS2-AuNPs/CA125 Ab | ovarian cancer biomarker CA125 | DPV | 0.5 mU/Ml–500.0 U/mL | 0.0005 U/mL | [99] |
| Biosensor Materials | Analytes | Technique | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Au@NC@GC | Uric acid | DPV | 10.0–600.0 μM | 10.0–150.0 μM | [102] |
| Dopamine | 0.773 nM | 0.746 nM | |||
| Co-hemin MOF/chitosan/PcCDH | Lactose | Amperometric | 10.0–100.0 mM | 4.0 mM | [103] |
| GOD-GA-Ni/Cu-MOFs-FET | Glucose | Amperometric | 1.0 μM–20.0 mM | 0.51 μM | [104] |
| Mn-BDC@MWCNTs | Ascorbic acid | DPV | 0.1–1150.0 μM | 0.01 μM | [105] |
| Dopamine | 0.01–500.0 μM | 0.002 μM | |||
| Uric acid | 0.02–1100.0 μM | 0.005 μM | |||
| ZIF-8/PPy@SiO2-MEPCM and laccase | Dopamine | DPV | 40.0–90.0 μM | 0.0069 μM | [106] |
| GOx-AuNPs/N-GQDs-P-MOF | Glucose | Amperometric | 0.02–10.0 μM | 0.7 μM | [107] |
| Biosensor Materials | Proteins | Technique | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Pt@CuMOFs-hGq-GOx | Carcinoembryonic antigen | Impedimetric | 0.05 pg mL−1–20.0 ng mL−1 | 0.023 pg mL−1 | [111] |
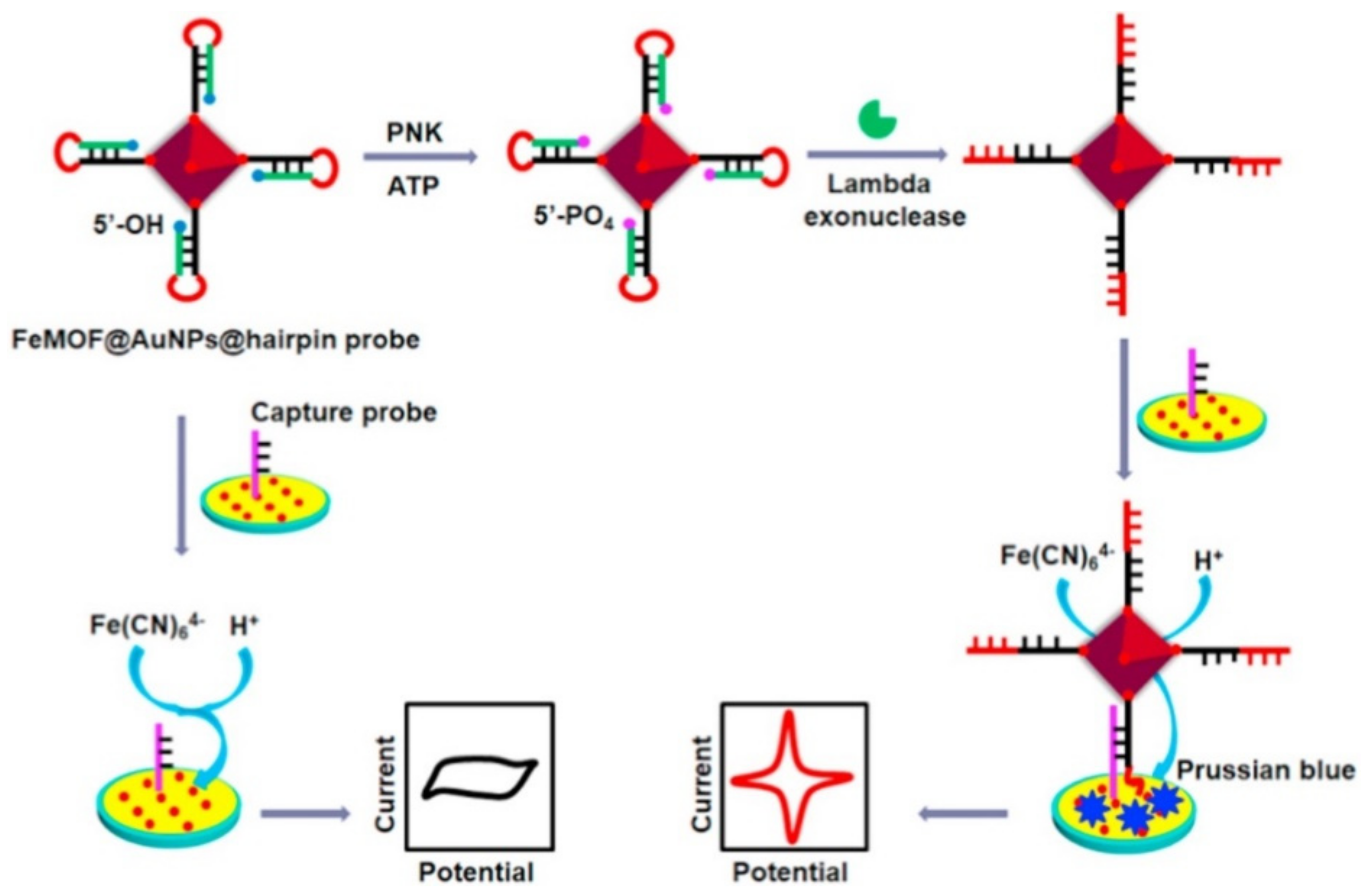
| FeMOF@AuNPs-hairpin | T4 polynucleotide kinase | CV | 0.0005–10.0 U mL−1 | 2.344 × 10−4 | [112] |
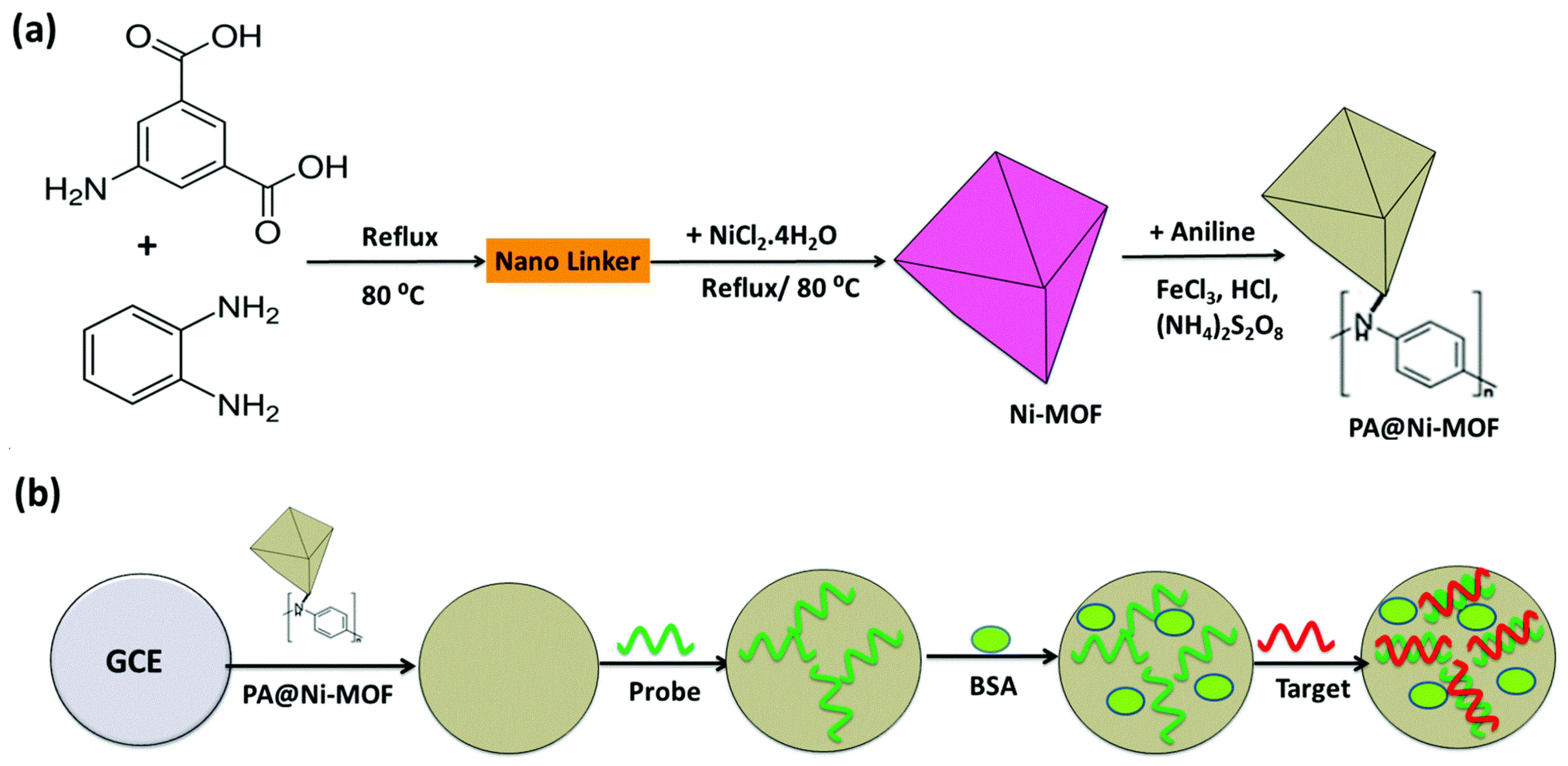
| AP II/AuNPs/Ni-MOF | Thrombin | DPV | 0.05 pM–50.0 nM | 0.016 pM | [113] |
| rGO-TEPA/AuNPs-PtNPs-MOFs | Nuclear matrix protein 22 | DPV | 0.005–20.0 ng·mL−1 | 1.7 pg·mL−1 | [114] |
| anti-CRP/ZnO/MPC/IL | C-reactive protein | DPV | 0.01–1000.0 ng·mL−1 | 5.0 pg·mL−1 | [115] |
| Cu-MOF-GO and MUC1–aptamer | Mucin 1 | DPV | 0.1 pM–10.0 nM | 0.033 pM | [116] |
| Fe-MOF/AuNPs/ DNA | T4 polynucleotide kinase | DPV | 1.0 × 10−3–10.0 U·mL−1 | 3.5 × 10−4 U·mL−1 | [117] |
| GQH DNAzyme/Dual-aptamer/HRP/Au@Pt/MIL-53 | COVID-19 nucleocapsid protein | DPV | 0.025–50.0 ng mL−1 | 8.33 pg mL−1 | [118] |
| Biosensor Materials | Pathogens | Technique | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| aptamer/HRP/AuNP/UiO-66-NH2 | Mycobacterium tuberculosis antigen MPT64 | DPV | 0.02–1000.0 pg·mL−1 | 10.0 fg·mL−1 | [124] |
| ESAT-6/BSA/EBA/Pt@Au/TB/P-MOF-rGO | Mycobacterium tuberculosis antigen ESAT-6 | CV | 1.0 × 10−4–2.0 × 102 ng⋅mL−1 | 3.3 × 10−5 ng⋅mL−1 | [125] |
| Cu-ZrMOF@Aptamer@DNA | Pseudomonas aeruginosa | DPV | 10–106 CFU mL−1 | 2.0 CFU mL−1 | [126] |
| Ab/Cu3(BTC)2-PANI | Escherichia coli | Impedimetric | 2.0–2.0 × 108 cfu/mL | 2.0 cfu/mL | [127] |
| Fe3O4@NMOF-Apt | Vibrio parahaemolyticus | SWV | 10–109 cfu/mL | 3.0 cfu/mL | [128] |
| MOFs-Ab-E. coli-AuNP | Escherichia coli K12 | CV | 101–107 cfu/mL | 1.0 cfu/mL | [129] |
| UiO-66/BMZIF-derived NPCs | MecA gene in methicillin-resistant Staphylococcus aureus | DPV | 5.0 × 10−15–1.0 × 10−10 M | 3.7 fM | [130] |
| Nuc gene in methicillin-resistant Staphylococcus aureus | 5.0 × 10−15–1.0 × 10−10 M | 1.6 fM | |||
| rGO-TA-Fe3O4/ BSA/Ab1/ALV-J/eZIF-Ab2-HRP | Avian leukosis virus | DPV | 152.0–10,000 TCID50 mL−1 | 140.0 TCID50 mL−1 | [131] |
| polyaniline@Ni-MOF/DNA/BSA | Hepatitis-C virus | Impedimetric | 1.0 fM–100.0 nM | 0.75 fM | [132] |
| Ab/polyUiO-66@AgNPs | H1N1 virus | EIS | 100.0–1.0 × 109 fg mL−1 | 54.7 fg mL−1 | [133] |
| DPV | 100.0–1.0 × 109 fg mL−1 | 49.4 fg mL−1 | |||
| Apt/polyUiO-66@AgNPs | SARS-CoV2 virus | EIS | 100.0–1.0 × 106 fg mL−1 | 23.4 fg mL−1 | |
| DPV | 100.0–1.0 × 106 fg mL−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Nejad, F.G.; Sheikhshoaie, I.; Di Bartolomeo, A. A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors 2022, 22, 2238. https://doi.org/10.3390/s22062238
Dourandish Z, Tajik S, Beitollahi H, Jahani PM, Nejad FG, Sheikhshoaie I, Di Bartolomeo A. A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors. 2022; 22(6):2238. https://doi.org/10.3390/s22062238
Chicago/Turabian StyleDourandish, Zahra, Somayeh Tajik, Hadi Beitollahi, Peyman Mohammadzadeh Jahani, Fariba Garkani Nejad, Iran Sheikhshoaie, and Antonio Di Bartolomeo. 2022. "A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis" Sensors 22, no. 6: 2238. https://doi.org/10.3390/s22062238
APA StyleDourandish, Z., Tajik, S., Beitollahi, H., Jahani, P. M., Nejad, F. G., Sheikhshoaie, I., & Di Bartolomeo, A. (2022). A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors, 22(6), 2238. https://doi.org/10.3390/s22062238









