Air–Oxygen Blenders for Mechanical Ventilators: A Literature Review
Abstract
1. Introduction
- a systematic review of the literature on air–oxygen blender technologies;
- a comparative analysis of the most relevant proposals available in the literature;
- the use of air–oxygen blenders in newborns;
- identification of relevant open research topics on air–oxygen blender technologies.
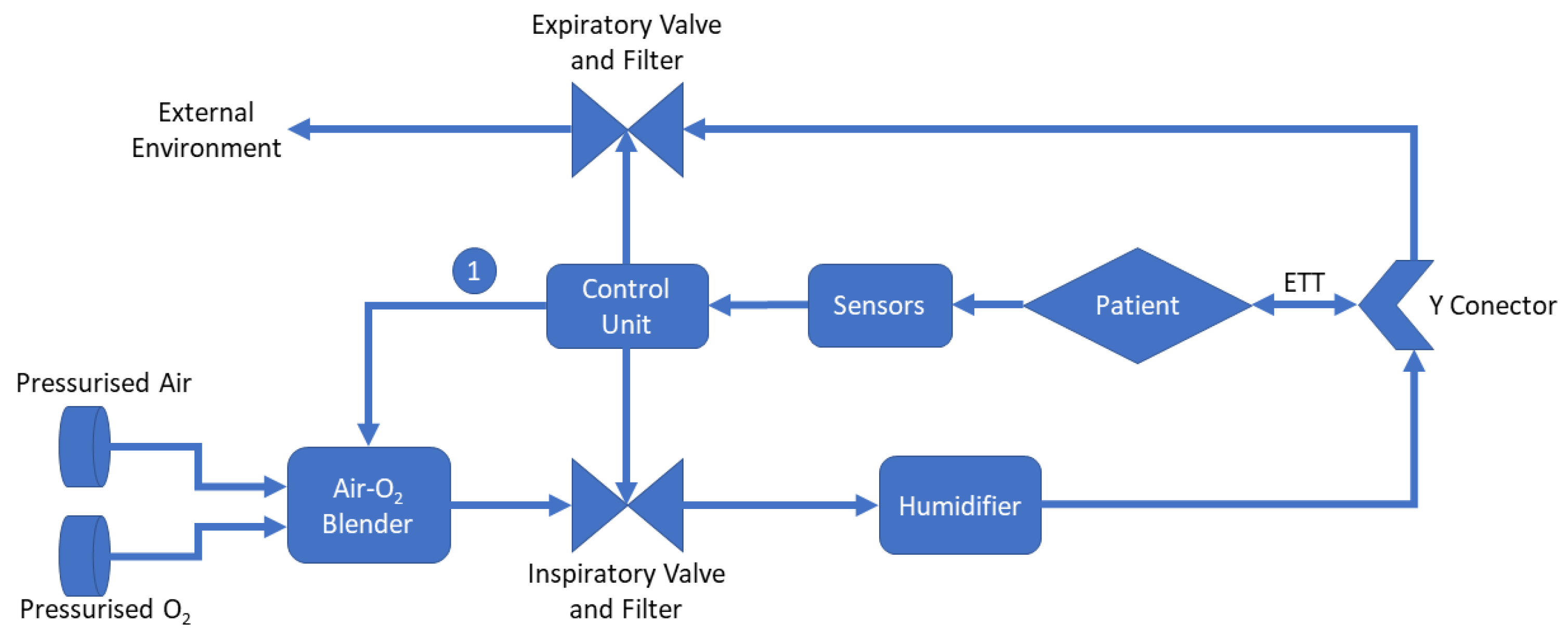
2. Mechanical Ventilation
- Air-O2 Blender: Equipment responsible for mixing air and oxygen according to the healthcare professional’s settings. It is important to highlight that depending on the mechanical fan model, such adjustment can be performed automatically through the control unit, as exemplified by indicator “1” in the figure [36,37];
- Control Unit: Control unit is responsible for processing all the data in the system. From the information obtained by the sensors, it adjusts the system parameters, displays information, and, depending on the existence of automatic controls in the blender, it changes the air–oxygen ratio;
- Humidifier: Humidifier is essential to avoid cases of hypothermia, rupture of the airway epithelium, bronchospasm, atelectasis, and airway obstruction [38];
- Y Conector: The Y-connector is used to join the inspiratory tube with the expiratory tube;
- Endotracheal Tube (ETT): It is a tube placed between the vocal cords through the trachea enabling oxygen delivering, inhaled gases to the lungs, and protecting the lungs from contamination [39].
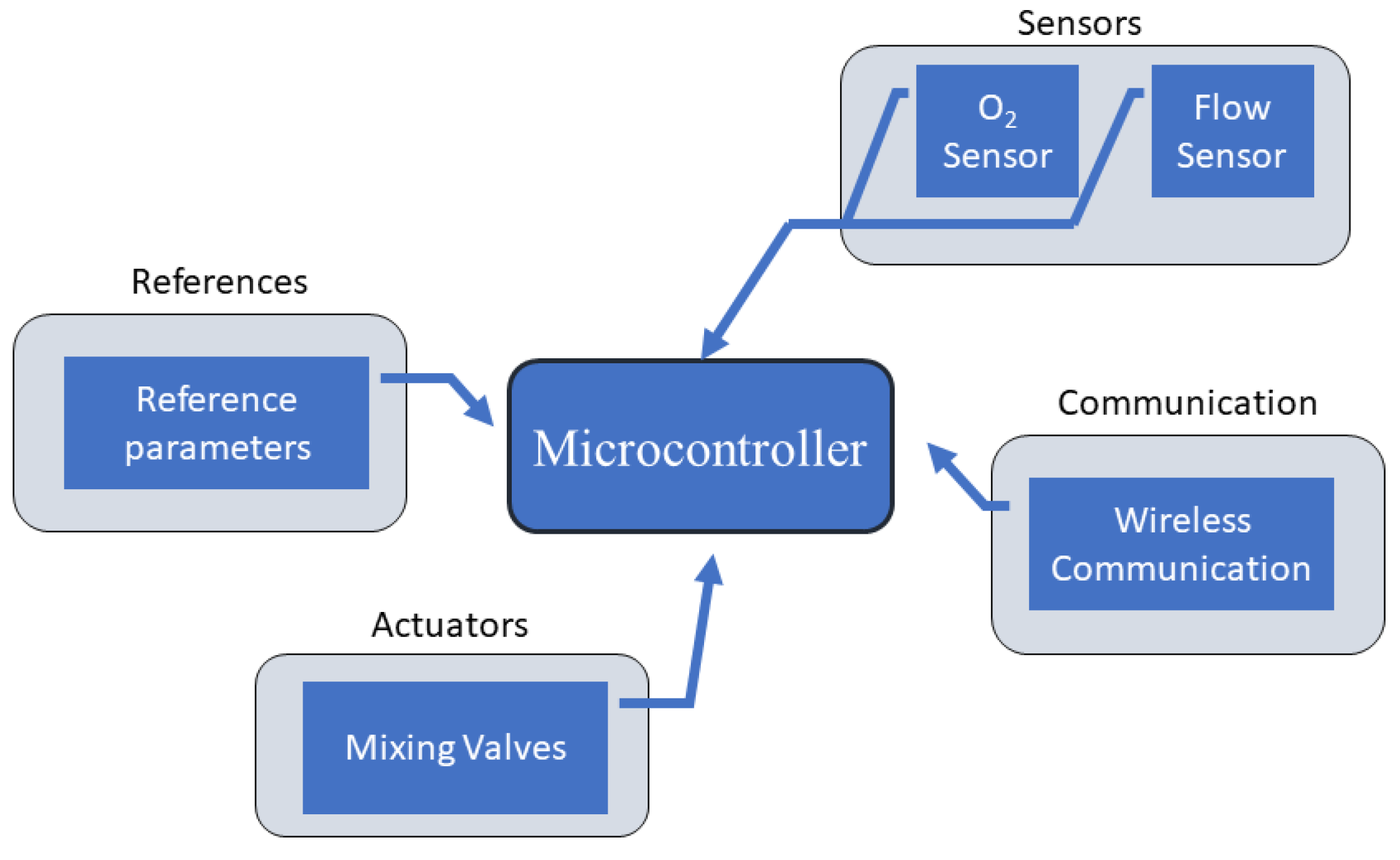
3. Main Technologies in Air–Oxygen Blenders
- Air Input and Oxygen Input: At the entry stage of each gas, there are filters to prevent contamination and one-way valves to ensure the gas flow does not return;
- Bypass and Alarm: The bypass system checks the differential pressure between both gases. If this value is higher than the established limit, a command is sent to the alarm in order to provide an audible indication that something is wrong. In addition, the gas flow is diverted all the way to the system outlet, thus ignoring the balancing stage;
- Balancing Stage: The balancing stage considers two chambers in sequence. At this point, the pressure of the two gases is equalized in order to ensure that, at the mixing stage, the pressures are equal, always limited by the lowest pressure value between the two gases;
- Mixer: The mixing of gases is usually carried out through a proportional valve. This mechanism is based on an adjustment made by the equipment operator where the percentage of oxygen is defined. The higher percentage of oxygen desired, the smaller the airflow will be and the greater the oxygen flow at the mixing point. The equipment manufactured commonly has this control occurring manually; however, it is possible to find works with application of automatic controllers. It is important to mention that in the structure of an air–oxygen blender there is also a presence of flow and pressure sensors to inform operators how the device is working.
3.1. Types of Blenders
3.2. Control Algorithms
3.3. Adjustment Modes
3.4. Use of Blenders in Newborns Treatment
4. Comparative Analysis and Discussion
5. Open Issues
- Use of open source embedded systems: Open source platforms can significantly reduce the costs for creating and developing automated blenders due to the popularity of such platforms and the simplified implementation of the algorithms, while ensuring information security using protocols already established in the literature;
- The application of IoT in blenders and cloud data storage: a remote monitoring system for several blenders can be developed. Then, health professionals, even at distance, can monitoring patients evolution and send commands to blenders for making needed adjustments. All data generated is stored in the cloud, making all the clinic patients registries saved and easily available when needed;
- Artificial intelligence and optimization in blenders: in conjunction with IoT, it would be possible to obtain a device with extremely high precision and the ability to adapt to unforeseen events that may occur, since the machine itself would detect any anomalies and immediately communicate to the health staff when making adjustments for return the patient to normal condition. It was also observed the possibility of reducing the production costs of a blender from the use of 3D printing, which can facilitate the access to this important equipment;
- Development of modular blenders: The development of modular equipment is an area that can add positively to hospital electronics. This would allow for easy integration of an external blender into a mechanical fan through standardized protocols. In addition, it would only allow the removal and replacement of the blender for periodic maintenance without compromising the use of the mechanical fan. Modular devices tend to be more versatile and more cost-effective.
6. Lessons Learned and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Lausanne, Switzerland, 2017. [Google Scholar]
- Gibson, G.; Loddenkemper, R.; Sibille, Y.; Lundbäck, B. The European Lung White Book: Respiratory Health and Disease in Europe; European Respiratory Society: Lausanne, Switzerland, 2013. [Google Scholar]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zheng, B.; He, Y.; Liu, X.; Zhuang, Z.; Cheung, C.; Luo, S.; Li, P.; Zhang, L.; Guan, Y.; et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 2003, 302, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hu, Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008, 133, 74–87. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Chan-Yeung, M.; Xu, R.H. SARS: Epidemiology. Respirology 2003, 8, S9–S14. [Google Scholar] [CrossRef]
- Bermingham, A.; Chand, M.; Brown, C.; Aarons, E.; Tong, C.; Langrish, C.; Hoschler, K.; Brown, K.; Galiano, M.; Myers, R.; et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Eurosurveillance 2012, 17, 20290. [Google Scholar] [CrossRef]
- Azhar, E.I.; El-Kafrawy, S.A.; Farraj, S.A.; Hassan, A.M.; Al-Saeed, M.S.; Hashem, A.M.; Madani, T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014, 370, 2499–2505. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E. MERS coronavirus: Diagnostics, epidemiology and transmission. Virol. J. 2015, 12, 222. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278. [Google Scholar] [CrossRef]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019—COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Halaji, M.; Farahani, A.; Ranjbar, R.; Heiat, M.; Dehkordi, F.S. Emerging coronaviruses: First SARS, second MERS and third SARS-CoV-2: Epidemiological updates of COVID-19. Infez Med. 2020, 28, 6–17. [Google Scholar] [PubMed]
- Sun, J.; He, W.T.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J.; et al. COVID-19: Epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020, 26, 483–495. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Holanda, M.A.; Pinheiro, B.V. COVID-19 pandemic and mechanical ventilation: Facing the present, designing the future. J. Bras. Pneumol. 2020, 46, e20200282. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 variants—Clinical, public health, and vaccine implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: What new variants are emerging and how are they being investigated? BMJ 2021, 372, n158. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Update on Omicron; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ahmed, I.; Ahmad, M.; Rodrigues, J.J.; Jeon, G.; Din, S. A deep learning-based social distance monitoring framework for COVID-19. Sustain. Cities Soc. 2020, 65, 102571. [Google Scholar] [CrossRef] [PubMed]
- Le, D.N.; Parvathy, V.S.; Gupta, D.; Khanna, A.; Rodrigues, J.J.; Shankar, K. IoT enabled depthwise separable convolution neural network with deep support vector machine for COVID-19 diagnosis and classification. Int. J. Mach. Learn. Cybern. 2021, 12, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Y.; Mehta, M.; Gupta, D.; Khann, A.; Sharma, R.; Rodrigues, J.J.P.C. Efficient-CovidNet: Deep Learning Based COVID-19 Detection From Chest X-Ray Images. In Proceedings of the 22nd International Conference on E-Health Networking, Applications and Services (IEEE HEALTHCOM), Shenzhen, China, 1–2 March 2021. [Google Scholar]
- Preethi, S.R.; Revathi, A.R.; Murugan, M. Exploration of Cough Recognition Technologies Grounded on Sensors and Artificial Intelligence. In Internet of Medical Things for Smart Healthcare: Covid-19 Pandemic; Chakraborty, C., Banerjee, A., Garg, L., Rodrigues, J.J.P.C., Eds.; Springer: Singapore, 2020; pp. 193–214. [Google Scholar] [CrossRef]
- Ali, A.M.; Ghafoor, K.Z.; Maghdid, H.S.; Mulahuwaish, A. Diagnosing COVID-19 Lung Inflammation Using Machine Learning Algorithms: A Comparative Study. In Internet of Medical Things for Smart Healthcare: Covid-19 Pandemic; Chakraborty, C., Banerjee, A., Garg, L., Rodrigues, J.J.P.C., Eds.; Springer: Singapore, 2020; pp. 91–105. [Google Scholar] [CrossRef]
- Santos, P.R.S.; Souza, L.B.M.; Lélis, S.P.B.D.; Ribeiro, H.B.; Borges, F.A.S.; Silva, R.R.V.; Filho, A.O.C.; Araujo, F.H.D.; Rabêlo, R.d.A.L.; Rodrigues, J.J.P.C. Prediction of COVID-19 using Time-Sliding Window: The case of Piauí State-Brazil. In Proceedings of the 22nd International Conference on E-Health Networking, Applications and Services (IEEE HEALTHCOM), Shenzhen, China, 1–2 March 2021; pp. 1–6. [Google Scholar]
- Rahal, L.; Garrido, A.G.; Cruz, R.J., Jr. Ventilaç ao n ao-invasiva: Quando utilizar? Rev. Assoc. Med. Bras. 2005, 51, 245–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Technical Specifications for Invasive and Non-Invasive Ventilators for COVID-19: Interim Guidance, 15 April 2020; Technical Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Popat, B.; Jones, A.T. Invasive and non-invasive mechanical ventilation. Medicine 2012, 40, 298–304. [Google Scholar] [CrossRef]
- Walter, J.M.; Corbridge, T.C.; Singer, B.D. Invasive mechanical ventilation. South. Med. J. 2018, 111, 746. [Google Scholar] [CrossRef]
- Dellaca, R.L.; Veneroni, C. Trends in mechanical ventilation: Are we ventilating our patients in the best possible way? Breathe 2017, 13, 84–98. [Google Scholar] [CrossRef]
- Hess, D.R.; Kacmarek, R.M. Essentials of Mechanical Ventilation; McGraw Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Bevis, R. Mechanical Ventilation: Humidification (Respiratory Therapy); Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ahmed, R.A.; Boyer, T.J. Endotracheal Tube. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539747/ (accessed on 12 February 2022).
- Pham, T.; Brochard, L.J.; Slutsky, A.S. Mechanical ventilation: State of the art. Mayo Clin. Proc. 2017, 92, 1382–1400. [Google Scholar] [CrossRef]
- Cruz, D.A.; de Lima Sousa, I.; Santana, P.V.D.; de Abreu Oliveira, L.K.; dos Santos Sousa, F.W.; de Araújo, Á.M.X.; da Silva, K.M.P.; de Araújo, G.S.S.; Costa, J.d.N.S.; do Nascimento, I.R. Impactos da ventilaç ao mecânica invasiva em pacientes de COVID-19: Revis ao integrativa. Res. Soc. Dev. 2021, 10, e380101119656. [Google Scholar] [CrossRef]
- Lim, Z.J.; Subramaniam, A.; Ponnapa Reddy, M.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef]
- Dave, C.; Cameron, P.; Basmaji, J.; Campbell, G.; Buga, E.; Slessarev, M. Frugal Innovation: Enabling Mechanical Ventilation During Coronavirus Disease 2019 Pandemic in Resource-Limited Settings. Crit. Care Explor. 2021, 3, e0410. [Google Scholar] [CrossRef]
- Rao, C.P.; Lister, D.C. Mechanical Ventilator System. US Patent 20090133695A1, 28 May 2009. [Google Scholar]
- Gattinoni, L.; Quintel, M.; Marini, J.J. “Less is More” in mechanical ventilation. Intensive Care Med. 2020, 46, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.; Pisani, L. Nasal high flow oxygen in acute respiratory failure. Pulmonology 2021, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.d. Modelagem e controle de um dispositivo de ventilação mecânica pulmonar. Master’s Thesis, Escola Politécnica da Universidade de São Paulo, São Paulo, Brazil, 2011. [Google Scholar]
- Genstar Technologies Co., Inc. Medical Air/Oxygen Blender Service Manual: Model No. GMX30U-AIR/O2 and GMX120U-AIR/O2; Genstar Technologies Co., Inc.: Chino, CA, USA, 2017. [Google Scholar]
- Precision Medical. Air–Oxygen Blender—Service Manual: Model No. PM5200 Series and PM5300 Series; Precision Medical: Northampton, PA, USA, 2021. [Google Scholar]
- Maxtec. Air–Oxygen Blender—Service Manual: Model No. R203P13 Series and R203P14 Series; Maxtec: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Crapo, J.D. Morphologic changes in pulmonary oxygen toxicity. Annu. Rev. Physiol. 1986, 48, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.M. Pulmonary oxygen toxicity. Chest 1985, 88, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Karmann, U.; Roth, F. Prevention of accidents associated with air–oxygen mixers. Anaesthesia 1982, 37, 680–682. [Google Scholar] [CrossRef]
- Caso, R.B.; Quam, I. Auto-Controlled air–oxygen Blender. US Patent 20140254305A1, 11 September 2014. [Google Scholar]
- DeVries, D.F. Ambient Pressure Air/Oxygen Blender. US Patent 5,014,694, 14 May 1991. [Google Scholar]
- Guillaume, D.; DeVries, D. Ambient pressure air/oxygen blender. J. Biomed. Eng. 1992, 14, 153–156. [Google Scholar] [CrossRef]
- Burke, T.F.; Bellare, A.; Moghaddam, K.M. Adjustable Ambient Air–Oxygen Blender. US Patent 20180333555A1, 22 November 2018. [Google Scholar]
- Moghaddam, K.M.; Burke, T.F.; Dundek, M.; Yeung, S.H.; Sharma, R.; Ravi, R.; Bellare, A. A Low-Cost Venturi Ambient air–oxygen Blender for Neonatal Oxygen Therapy. Acad. J. Pediatr. Neonatol. 2020, 8, 69–77. [Google Scholar]
- Taube, J. Solenoid Air/Oxygen System for Use with an Adaptive Oxygen Controller and Therapeutic Methods of Use. US Patent 20080156328A1, 3 July 2008. [Google Scholar]
- Dixon, P.; Westfall, T. Automated Oxygen Delivery Method. US Patent 20100224192A1, 9 September 2010. [Google Scholar]
- Taube, J.C. Oxygen Mixing and Delivery. US Patent 10514662B1, 24 December 2019. [Google Scholar]
- Chanyagorn, P.; Kiratiwudhikul, P. Fuzzy control of oxygen gas content for premature labor infants oxygen therapy. In Proceedings of the 2016 International Conference on Electronics, Information, and Communications (ICEIC), Danang, Vietnam, 27–30 January 2016; pp. 1–5. [Google Scholar]
- Tehrani, F. A control system for oxygen therapy of premature infants. In Proceedings of the 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; Volume 2, pp. 2059–2062. [Google Scholar]
- Urschitz, M.S.; Horn, W.; Seyfang, A.; Hallenberger, A.; Herberts, T.; Miksch, S.; Popow, C.; Müller-Hansen, I.; Poets, C.F. Automatic control of the inspired oxygen fraction in preterm infants: A randomized crossover trial. Am. J. Respir. Crit. Care Med. 2004, 170, 1095–1100. [Google Scholar] [CrossRef]
- Morozoff, E.P.; Smyth, J.A. Evaluation of three automatic oxygen therapy control algorithms on ventilated low birth weight neonates. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 3079–3082. [Google Scholar]
- Mantena, S.; Burke, T.F. Oxygen Blending is Urgently Needed in Resource-Limited Settings. J. Pediatr. 2021, 237, 288–291. [Google Scholar] [CrossRef]
- Ali, S.K.; Mohammed, N.; Qureshi, N.; Gupta, S. Oxygen therapy in preterm infants: Recommendations for practice. Paediatr. Child Health 2021, 31, 1–6. [Google Scholar] [CrossRef]
- Gottimukkala, S.B.; Sotiropoulos, J.X.; Lorente-Pozo, S.; Sharma, A.M.; Vento, M.; Saugstad, O.D.; Oei, J.L. Oxygen saturation (SpO2) targeting for newborn infants at delivery: Are we reaching for an impossible unknown? In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2021; Volume 26, p. 101220. [Google Scholar]
- Mathias, M.; Chang, J.; Perez, M.; Saugstad, O. Supplemental Oxygen in the Newborn: Historical Perspective and Current Trends. Antioxidants 2021, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Herrod, S.K.; Stevenson, A.; Vaucher, Y.E.; Lambert, S.R.; Isenberg, S.J.; Yap, V.L.; Ezeaka, V.C.; Carlo, W.A. Oxygen management among infants in neonatal units in sub-Saharan Africa: A cross-sectional survey. J. Perinatol. 2021, 41, 2631–2638. [Google Scholar] [CrossRef] [PubMed]

| — Fuzzy Control | —Rule-Based Control | |
|---|---|---|
| Maximum | 60.80% | 100% |
| Average | 23.49% | 49.99% |
| Minimum | 21% | 21% |
| Parameter | Routine Manual Adjustment | Optimized Manual Adjustment | Closed-Loop Automatic Control |
|---|---|---|---|
| Adjustments per hour | 3 | 7.7 | 0.3 |
| Episodes of hyperoxia per hour | 9.3 | 4 | 4.7 |
| Average duration of hyperoxia episodes (seconds) | 19.2 | 16.4 | 10.1 |
| Cases of hypoxia per hour | 12.7 | 8.7 | 9.3 |
| Average duration of hypoxia cases (seconds) | 19 | 16.4 | 12.4 |
| Percentage of time that the value remained as desired | 81.7% | 91% | 90.5% |
| Type of Control | Total Hours | Total Manual Adjustments | Adjustments per Hour |
|---|---|---|---|
| Adaptive control | 21.42 | 5 | 0.2 |
| PID | 42.2 | 19 | 0.45 |
| State machine | 14.72 | 7 | 0.48 |
| Manual adjustment | 18.43 | 69 | 3.74 |
| Venturi Tube | Poppet-Seat Valves | |
|---|---|---|
| Range of mixing volume obtained | 1 L/min to 15 L/min | 5 L/min to 160 L/min |
| Main Advantage | It is a technology that can be successfully applied in regions with less economic resources or cases that do not require significant changes in the rates of delivered to the patient | Capable of providing greater mixing flow and greater precision compared to the Venturi tube model. |
| Main Disadvantage | This type of blender is more inaccurate, in addition to not being able to be applied in the hospital environment, only residential. | Not suitable for hospital use. |
| Adjustments per Hour | Percentage of Time That the Value Remained as Desired | |
|---|---|---|
| Routine manual adjustment | 3 | 81.7% |
| Optimized manual adjustment | 7.7 | 91% |
| Closed-loop automatic control | 0.3 | 90.5% |
| Adaptive control | 0.2 | 90% |
| PID | 0.45 | ≈86% |
| State machine | 0.48 | ≈88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, G.F.; Almeida, O.M.; Menezes, J.W.M.; Kozlov, S.S.A.; Rodrigues, J.J.P.C. Air–Oxygen Blenders for Mechanical Ventilators: A Literature Review. Sensors 2022, 22, 2182. https://doi.org/10.3390/s22062182
Soares GF, Almeida OM, Menezes JWM, Kozlov SSA, Rodrigues JJPC. Air–Oxygen Blenders for Mechanical Ventilators: A Literature Review. Sensors. 2022; 22(6):2182. https://doi.org/10.3390/s22062182
Chicago/Turabian StyleSoares, Gabryel F., Otacílio M. Almeida, José W. M. Menezes, Sergei S. A. Kozlov, and Joel J. P. C. Rodrigues. 2022. "Air–Oxygen Blenders for Mechanical Ventilators: A Literature Review" Sensors 22, no. 6: 2182. https://doi.org/10.3390/s22062182
APA StyleSoares, G. F., Almeida, O. M., Menezes, J. W. M., Kozlov, S. S. A., & Rodrigues, J. J. P. C. (2022). Air–Oxygen Blenders for Mechanical Ventilators: A Literature Review. Sensors, 22(6), 2182. https://doi.org/10.3390/s22062182