Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sonochemical Preparation of Silver Citrate Coated Au@Ag NPs
2.3. General Procedure for Colorimetric Assay of Morphine
2.4. Morphine Assay in Real Sample
2.5. Characterization and Apparatus
3. Results
3.1. Optimization of Au@Ag NPs Synthesis Conditions
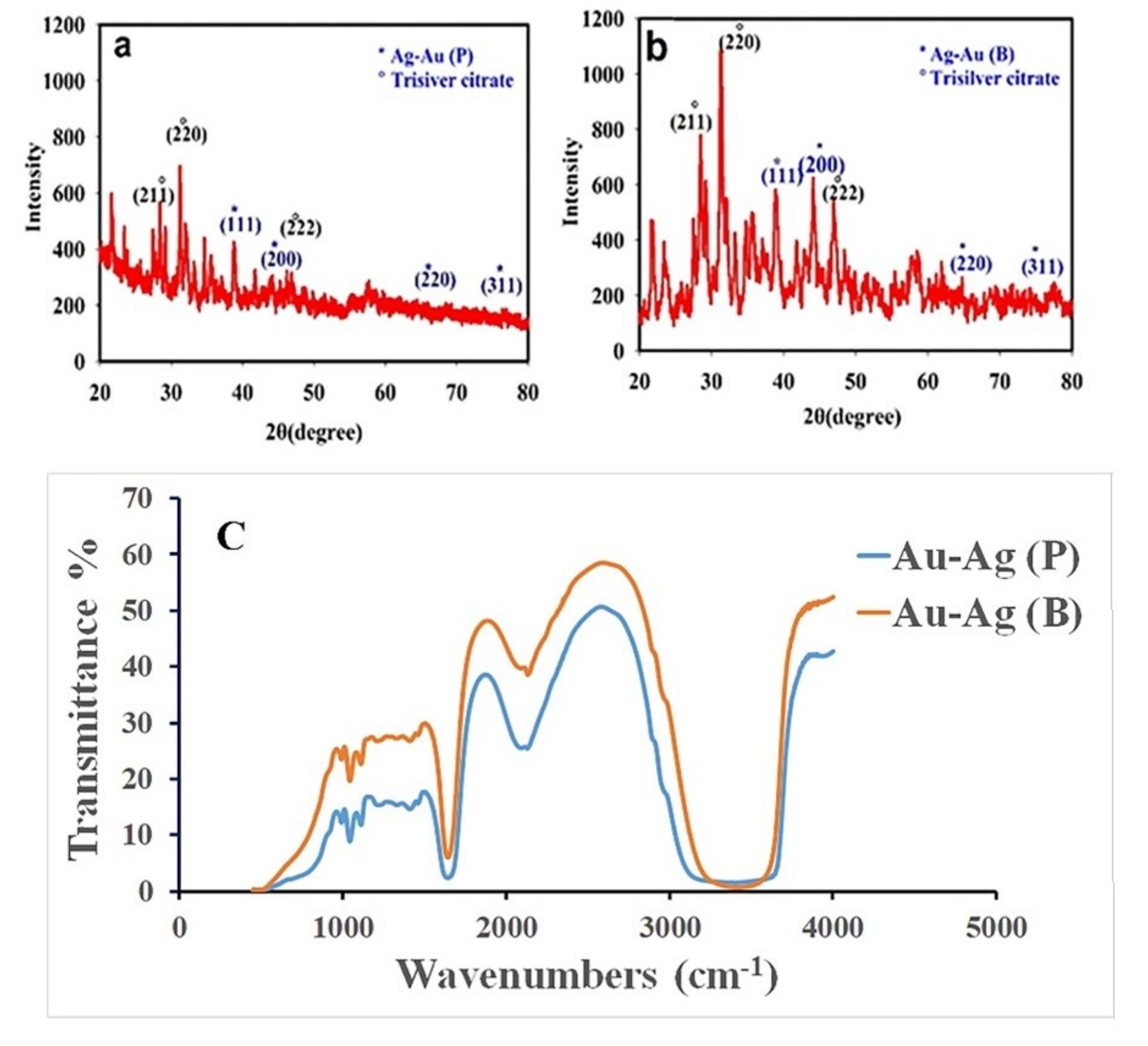
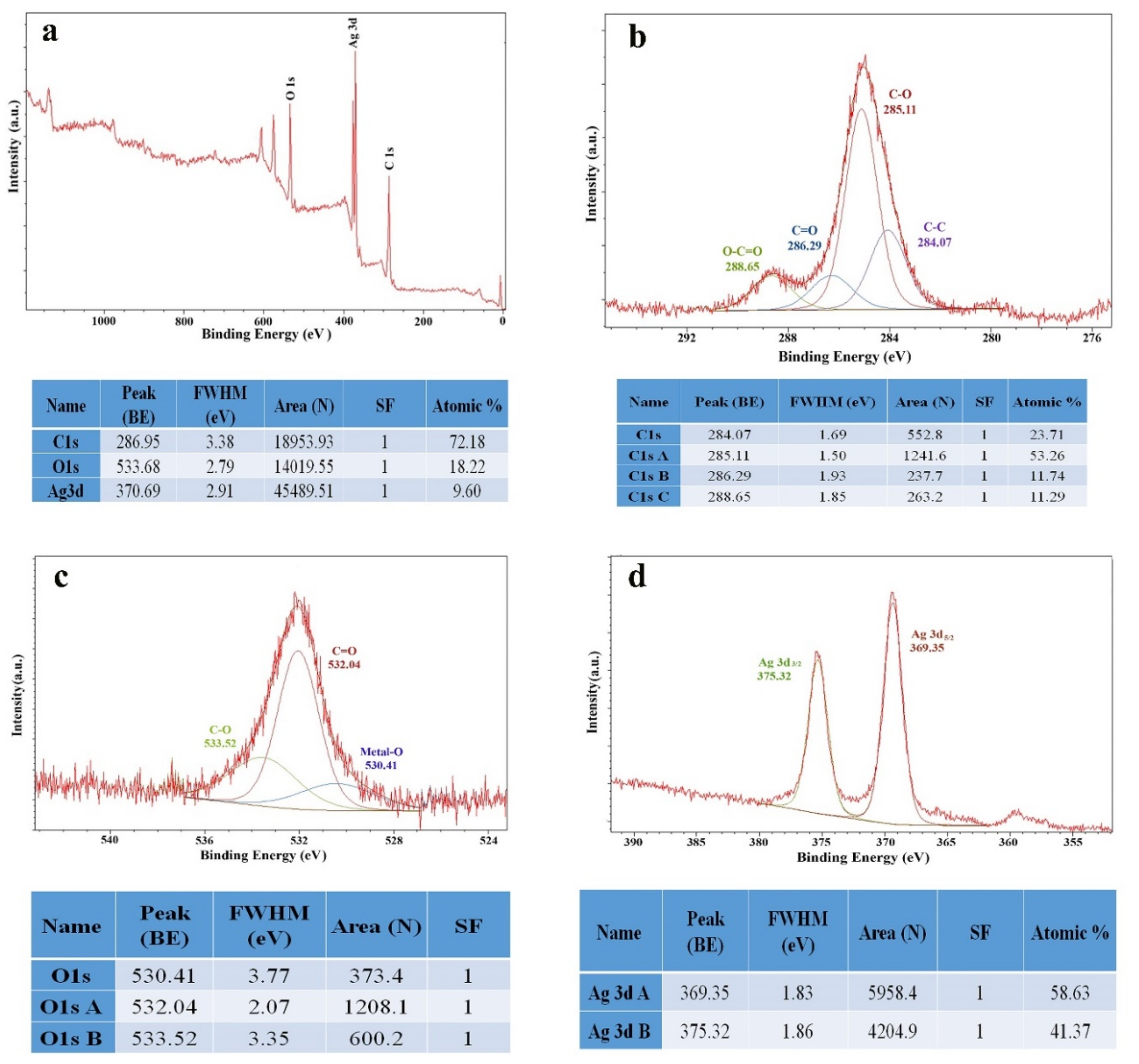
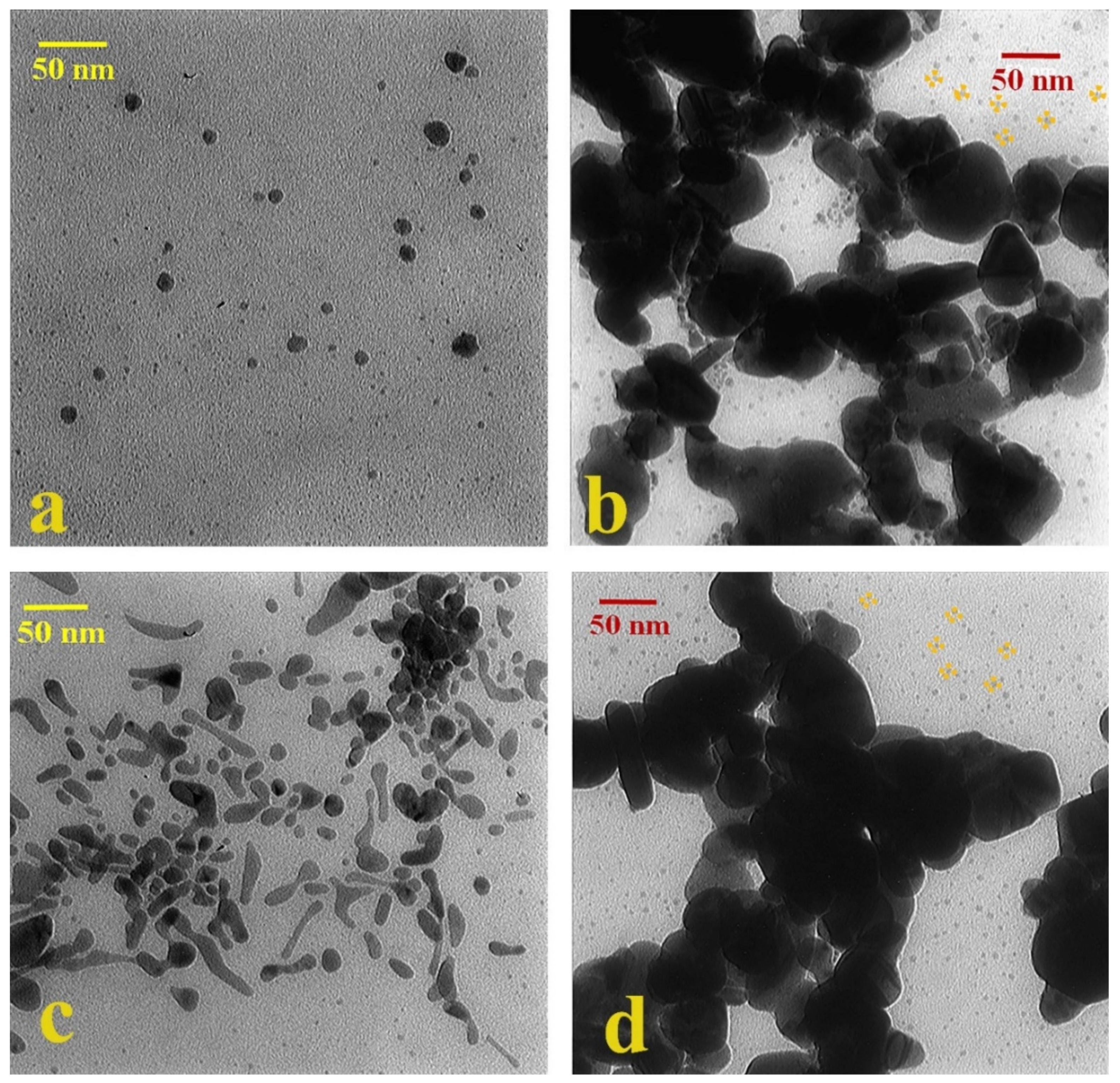
3.2. Characterization of the Optimized Au@Ag NPs
3.3. Optimization for Colorimetric Assay of Morphine
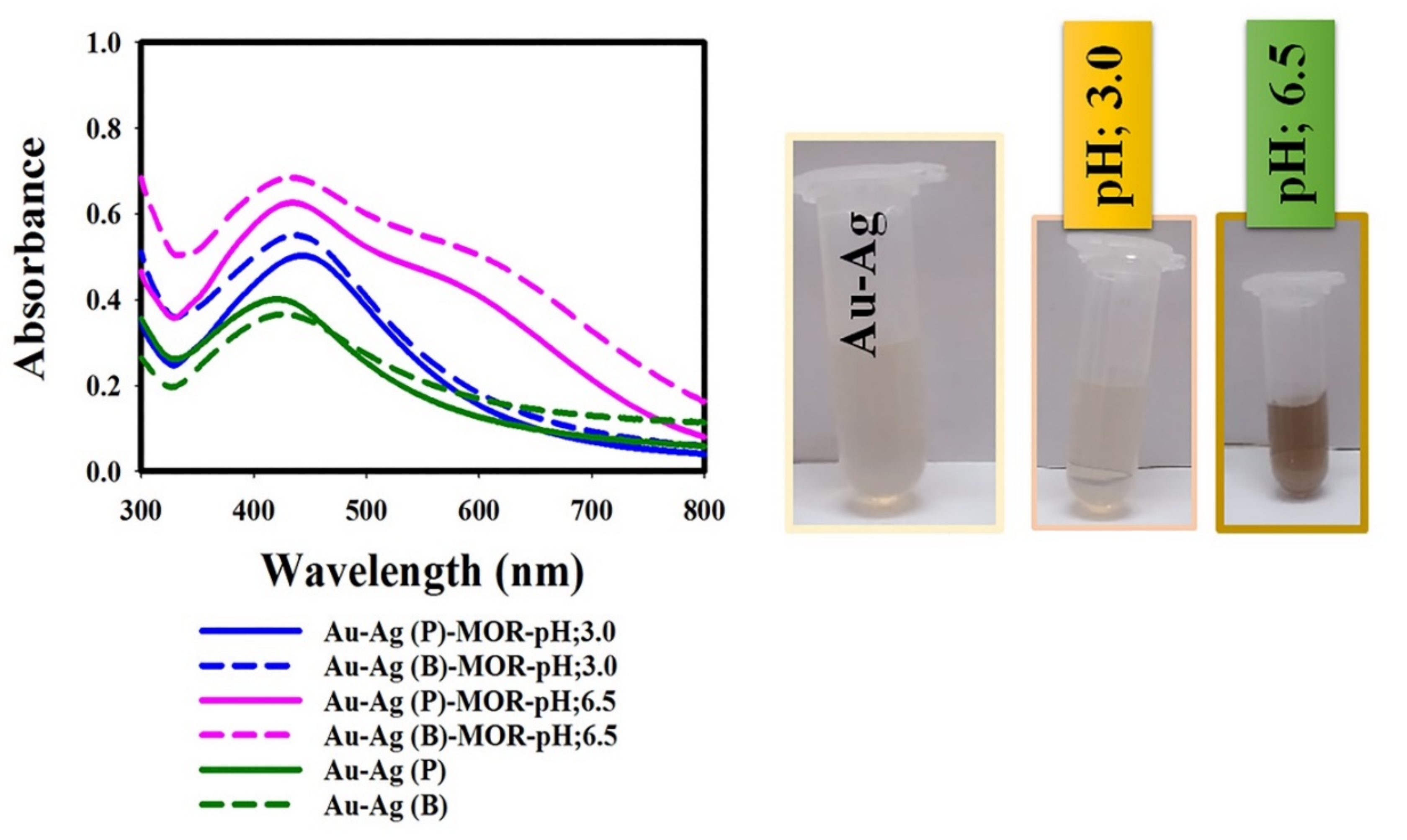
3.3.1. Effect of Sample pH Value
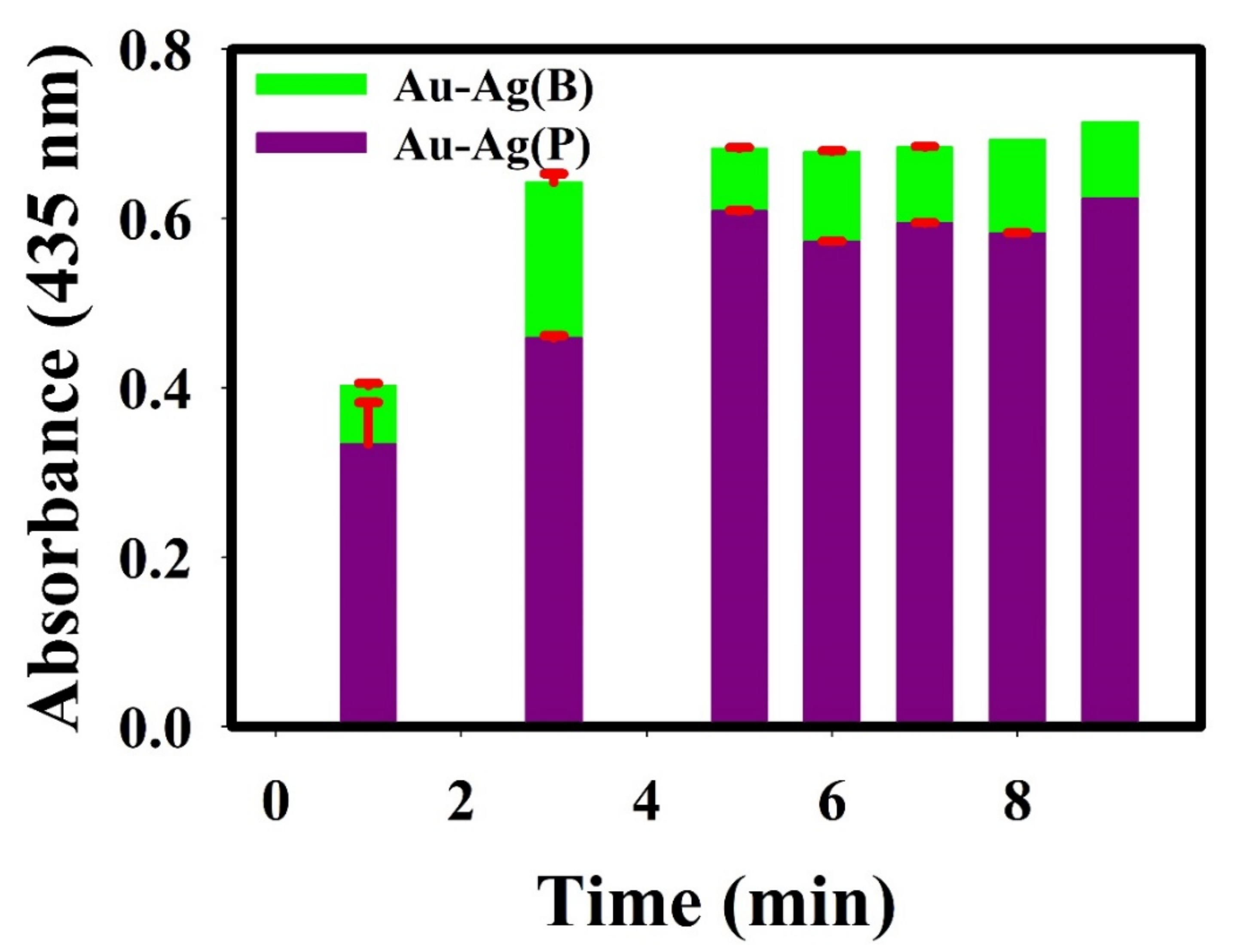
3.3.2. Effect of Incubation Time
3.4. Linear Range of Calibration Curves
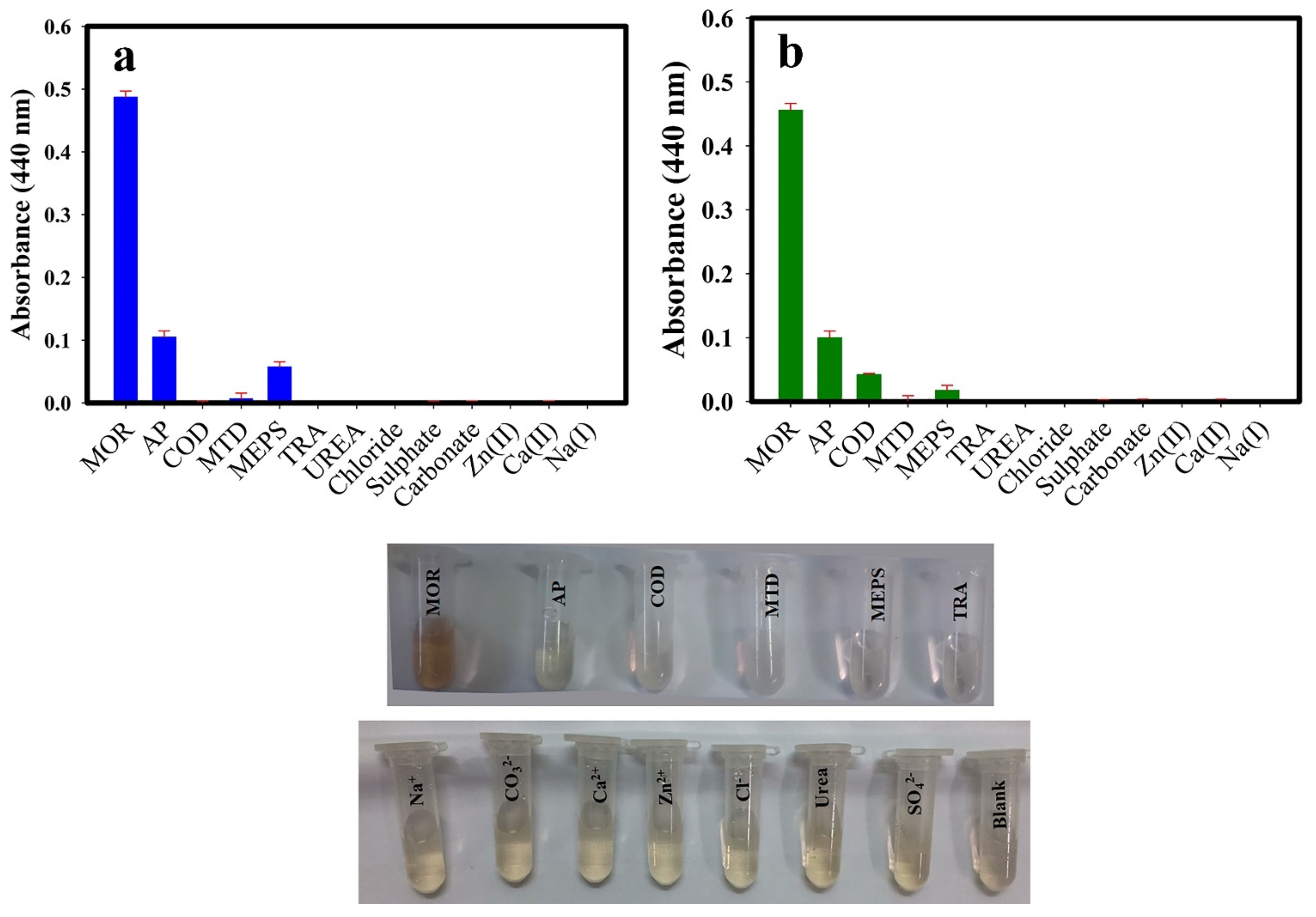
3.5. Interference Effect
3.6. Real Sample
3.7. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gholivand, M.-B.; Jalalvand, A.R.; Goicoechea, H.C.; Gargallo, R.; Skov, T.; Paimard, G. Combination of electrochemistry with chemometrics to introduce an efficient analytical method for simultaneous quantification of five opium alkaloids in complex matrices. Talanta 2015, 131, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Mell, L.D., Jr.; Mitchell, J.M.; Irving, J.; Novak, A.J. Simultaneous Identification and Quantitation of Codeine and Morphine in Urine by Capillary Gas Chromatography and Mass Spectroscopy. J. Anal. Toxicol. 1985, 9, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.E.; Harris, S.C.; Peek, M.W. Determination of morphine by liquid chromatography with electrochemical detection. Anal. Chem. 1980, 52, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Merchaoui, S.; Ben Said, A.; Louati, K.; Hajri, A.; Safta, F.; Kallel, M. Optimization of morphine extraction method for the assay of its urinary 3-glucuronideconjuguate by gas chromatography-mass spectrometry. Ann. Pharm. Franc. 2019, 77, 468–487. [Google Scholar] [CrossRef]
- Maccaferri, G.; Terzi, F.; Xia, Z.; Vulcano, F.; Liscio, A.; Palermo, V.; Zanardi, C. Highly sensitive amperometric sensor for morphine detection based on electrochemically exfoliated graphene oxide. Application in screening tests of urine samples. Sens. Actuators B Chem. 2019, 281, 739–745. [Google Scholar] [CrossRef]
- Sheibani, A.; Shishehbore, M.R.; Mirparizi, E. Kinetic spectrophotometric method for the determination of morphine in biological samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 535–538. [Google Scholar] [CrossRef]
- Espinosa Bosch, M.; Ruiz Sanchez, A.; Anchez Rojas, F.S.; Bosch Ojeda, C. Morphine and its metabolites: Analytical methodologies for its determination. J. Pharm. Biomed. Anal. 2007, 43, 799–815. [Google Scholar] [CrossRef]
- Sverrisdóttir, E.; Lund, T.M.; Olesen, A.E.; Drewes, A.M.; Christrup, L.L.; Kreilgaard, M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic–pharmacodynamic relationships in experimental and clinical pain. Eur. J. Pharm. Sci. 2015, 74, 45–62. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent Biosensing Advances in the Rapid Detection of Illicit Drugs. Trends Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mosleh, N. Functionalization of graphene quantum dots with antimorphine: Design of selective nanosensor for detection of morphine. Mater. Lett. 2019, 241, 206–209. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Khademabbasi, K.; Mollatayefeh, N. A selective morphine nanosensor derived from functionalized CdS quantum dots. Mater. Lett. 2018, 228, 68–71. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, N.; Bahram, M. Mean centering of ratio spectra for colorimetric determination of morphine and codeine in pharmaceuticals and biological samples using melamine modified gold nanoparticles. Anal. Methods 2016, 8, 6739–6747. [Google Scholar] [CrossRef]
- Darroudi, M.; Khorsand Zak, A.; Muhamad, M.R.; Huang, N.M.; Hakimi, M. Green synthesis of colloidal silver nanoparticles by sonochemical method. Mater. Lett. 2012, 66, 117–120. [Google Scholar] [CrossRef]
- Ary, K.; Róna, K. LC determination of morphine and morphine glucuronides in human plasma by coulometric and UV detection. J. Pharm. Biomed. Anal. 2001, 26, 179–187. [Google Scholar] [CrossRef]
- Stojadinovic, U.; Matenco, L.; Andriessen, P.; Toljić, M.; Rundić, L.; Ducea, M.N. Structure and provenance of Late Cretaceous–Miocene sediments located near the NE Dinarides margin: Inferences from kinematics of orogenic building and subsequent extensional collapse. Tectonophysics 2017, 710, 184–204. [Google Scholar] [CrossRef]
- Yu, L.; Song, Z.; Peng, J.; Yang, M.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Ghaedi, A.; Mitchell, S.G.; Javadian-Saraf, A.; Karimi, M. Sonochemical synthesis of polyoxometalate-stabilized gold nanoparticles for point-of-care determination of acetaminophen levels: Preclinical study in an animal model. RSC Adv. 2020, 10, 16805–16816. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Dabirifar, Z. AuNPs@ PMo 12 nanozyme: Highly oxidase mimetic activity for sensitive and specific colorimetric detection of acetaminophen. RSC Adv. 2020, 10, 35949–35956. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Jamaluddin, N.D.; Tan, L.L.; Mohd Yusof, N.Y. A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus. Sensors 2021, 21, 5114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, J.; Li, J.-J.; Zhao, J.-W. A promising direct visualization of an Au@Ag nanorod-based colorimetric sensor for trace detection of alpha-fetoprotein. J. Mater. Chem. C 2015, 3, 6035–6045. [Google Scholar] [CrossRef]
- Huang, H.; Li, Q.; Wang, J.; Li, Z.; Yu, X.-F.; Chu, P.K. Sensitive and Robust Colorimetric Sensing of Sulfide Anion by Plasmonic Nanosensors Based on Quick Crystal Growth. Plasmonics 2014, 9, 11–16. [Google Scholar] [CrossRef]
- Park, G.; Lee, C.; Seo, D.; Song, H. Full-Color Tuning of Surface Plasmon Resonance by Compositional Variation of Au@Ag Core–Shell Nanocubes with Sulfides. Langmuir 2012, 28, 9003–9009. [Google Scholar] [CrossRef]
- Gao, Z.; Deng, K.; Wang, X.-D.; Miró, M.; Tang, D. High-Resolution Colorimetric Assay for Rapid Visual Readout of Phosphatase Activity Based on Gold/Silver Core/Shell Nanorod. ACS Appl. Mater. Interfaces 2014, 6, 18243–18250. [Google Scholar] [CrossRef]
- Al-Masoodi, A.H.H.; Nazarudin, N.F.F.B.; Nakajima, H.; Tunmee, S.; Goh, B.T.; Majid, W.H.B.A. Controlled growth of silver nanoparticles on indium tin oxide substrates by plasma-assisted hot-filament evaporation: Physical properties, composition, and electronic structure. Thin Solid Film. 2020, 693, 137686. [Google Scholar] [CrossRef]
- Alula, M.T.; Lemmens, P.; Bo, L.; Wulferding, D.; Yang, J.; Spende, H. Preparation of silver nanoparticles coated ZnO/Fe3O4 composites using chemical reduction method for sensitive detection of uric acid via surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2019, 1073, 62–71. [Google Scholar] [CrossRef]
- Rivera-Rangel, R.D.; González-Muñoz, M.P.; Avila-Rodriguez, M.; Razo-Lazcano, T.A.; Solans, C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 60–67. [Google Scholar] [CrossRef]
- Banu, R.; Ramakrishna, D.; Reddy, G.B.; Veerabhadram, G.; Mangatayaru, K.G. Facile one-pot microwave-assisted green synthesis of silver nanoparticles using Bael gum: Potential application as catalyst in the reduction of organic dyes. Mater. Today Proc. 2021, 43, 2265–2273. [Google Scholar] [CrossRef]
- Joy Prabu, H.; Johnson, I. Plant-mediated biosynthesis and characterization of silver nanoparticles by leaf extracts of Tragia involucrata, Cymbopogon citronella, Solanum verbascifolium and Tylophora ovata. Karbala Int. J. Mod. Sci. 2015, 1, 237–246. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Entezari, M.H. Sono-synthesis approach in uniform loading of ultrafine Ag nanoparticles on reduced graphene oxide nanosheets: An efficient catalyst for the reduction of 4-Nitrophenol. Ultrason. Sonochem. 2018, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rohani Bastami, T.; Entezari, M.H. A novel approach for the synthesis of superparamagnetic Mn3O4 nanocrystals by ultrasonic bath. Ultrason. Sonochem. 2012, 19, 560–569. [Google Scholar] [CrossRef]
- Okitsu, K.; Ashokkumar, M.; Grieser, F. Sonochemical Synthesis of Gold Nanoparticles: Effects of Ultrasound Frequency. J. Phys. Chem. B 2005, 109, 20673–20675. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Grieser, F.; Ashokkumar, M. Sonochemical Synthesis of Au−Ag Core−Shell Bimetallic Nanoparticles. J. Phys. Chem. C 2008, 112, 15102–15105. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Entezari, M.H. High stable suspension of magnetite nanoparticles in ethanol by using sono-synthesized nanomagnetite in polyol medium. Mater. Res. Bull. 2013, 48, 3149–3156. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Lim, H.N.; Zakaria, R.; Huang, N.M. Sonochemical synthesis of reduced graphene oxide uniformly decorated with hierarchical ZnS nanospheres and its enhanced photocatalytic activities. RSC Adv. 2015, 5, 12726–12735. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Neppolian, B.; Lightcap, I.V.; Grieser, F.; Ashokkumar, M.; Kamat, P.V. Sonolytic Design of Graphene−Au Nanocomposites. Simultaneous and Sequential Reduction of Graphene Oxide and Au(III). J. Phys. Chem. Lett. 2010, 1, 1987–1993. [Google Scholar] [CrossRef]
- Wang, X.-K.; Shao, L.; Guo, W.-L.; Wang, J.-G.; Zhu, Y.-P.; Wang, C. Synthesis of dendritic silver nanostructures by means of ultrasonic irradiation. Ultrason. Sonochem. 2009, 16, 747–751. [Google Scholar] [CrossRef]
- Yao, T.; Sun, Z.; Li, Y.; Pan, Z.; Wei, H.; Xie, Y.; Nomura, M.; Niwa, Y.; Yan, W.; Wu, Z.; et al. Insights into Initial Kinetic Nucleation of Gold Nanocrystals. J. Am. Chem. Soc. 2010, 132, 7696–7701. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Entezari, M.H. Synthesis of manganese oxide nanocrystal by ultrasonic bath: Effect of external magnetic field. Ultrason. Sonochem. 2012, 19, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Friedrich, A.; Taubert, A. Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride. J. Mater. Chem. 2008, 18, 1008–1014. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, T.; Liang, Z.; Zhu, L. Facile Preparation of AuPt Alloy Nanoparticles from Organometallic Complex Precursor. Chem. Mater. 2008, 20, 1688–1690. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Ren, H.; Xia, A.; Liu, Y. Direct double Z-scheme O-g-C3N4/Zn2SnO4N/ZnO ternary heterojunction photocatalyst with enhanced visible photocatalytic activity. Appl. Surf. Sci. 2019, 492, 690–702. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, Y.; Kim, K. Dodecanethiol-Derivatized Au/Ag Bimetallic Nanoparticles: TEM, UV/VIS, XPS, and FTIR Analysis. J. Colloid Interface Sci. 1998, 208, 272–278. [Google Scholar] [CrossRef]
- Wang, X.-j.; Li, X.; Yang, S. Influence of pH and SDBS on the Stability and Thermal Conductivity of Nanofluids. Energy Fuels 2009, 23, 2684–2689. [Google Scholar] [CrossRef]
- Alhaddad, M.; Sheta, M.S. Dual Naked-Eye and Optical Chemosensor for Morphine Detection in Biological Real Samples Based on Cr(III) Metal−Organic Framework Nanoparticles. ACS Omega 2020, 43, 28296–28304. [Google Scholar] [CrossRef]
- Chapman, D.V.; Way, E.L. Metal Ion Interactions with Opiates. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 553–579. [Google Scholar] [CrossRef]
- Wang, F.; Lu, Y.; Chen, Y.; Sun, J.; Liu, Y. Novel colorimetric nanosensor based on the aggregation of AuNP triggered by carbon quantum dots for detection of Ag+ ions. ACS Sustain. Chem. Eng. 2018, 6, 3706–3713. [Google Scholar] [CrossRef]
- Roy, S.D.; Flynn, G.L. Solubility Behavior of Narcotic Analgesics in Aqueous Media: Solubilities and Dissociation Constants of Morphine, Fentanyl, and Sufentanil. Pharm. Res. 1989, 6, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Traiwatcharanon, P.; Timsorn, K.; Wongchoosuk, C. Flexible room-temperature resistive humidity sensor based on silver nanoparticles. Mater. Res. Express 2017, 4, 085038. [Google Scholar] [CrossRef]
- He, Y.; Lu, J.; Liu, M.; Du, J.; Nie, F. Determination of morphine by molecular imprinting-chemiluminescence method. J. Anal. Toxicol. 2005, 29, 528–532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudo, K.; Ishida, T.; Nishida, N.; Yoshioka, N.; Inoue, H.; Tsuji, A.; Ikeda, N. Simple and sensitive determination of free and total morphine in human liver and kidney using gas chromatography–mass spectrometry. J. Chromatogr. B 2006, 830, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Javidnia, K.; Miri, R.; Miri, D. A Rapid and Sensitive Method for Determination of Morphine in Saliva of Opium Addicts. Iran. J. Med. Sci. 2006, 31, 213–215. [Google Scholar]
- Fryirs, B.; Dawson, M.; Mather, L.E. Highly sensitive gas chromatographic–mass spectrometric method for morphine determination in plasma that is suitable for pharmacokinetic studies. J. Chromatogr. B 1997, 693, 51–57. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, R.; Zhang, C.G. Column-switching high performance liquid chromatographic method for the determination of morphine and 6-monoacetylmorphine in urine. Shanghai Inst. Forensic Sci. 2002, 18, 89–91. [Google Scholar]
- Bahram, M.; Madrakian, T.; Alizadeh, S. Simultaneous colorimetric determination of morphine and ibuprofen based on the aggregation of gold nanoparticles using partial least square. J. Pharm. Anal. 2017, 7, 411–416. [Google Scholar] [CrossRef]

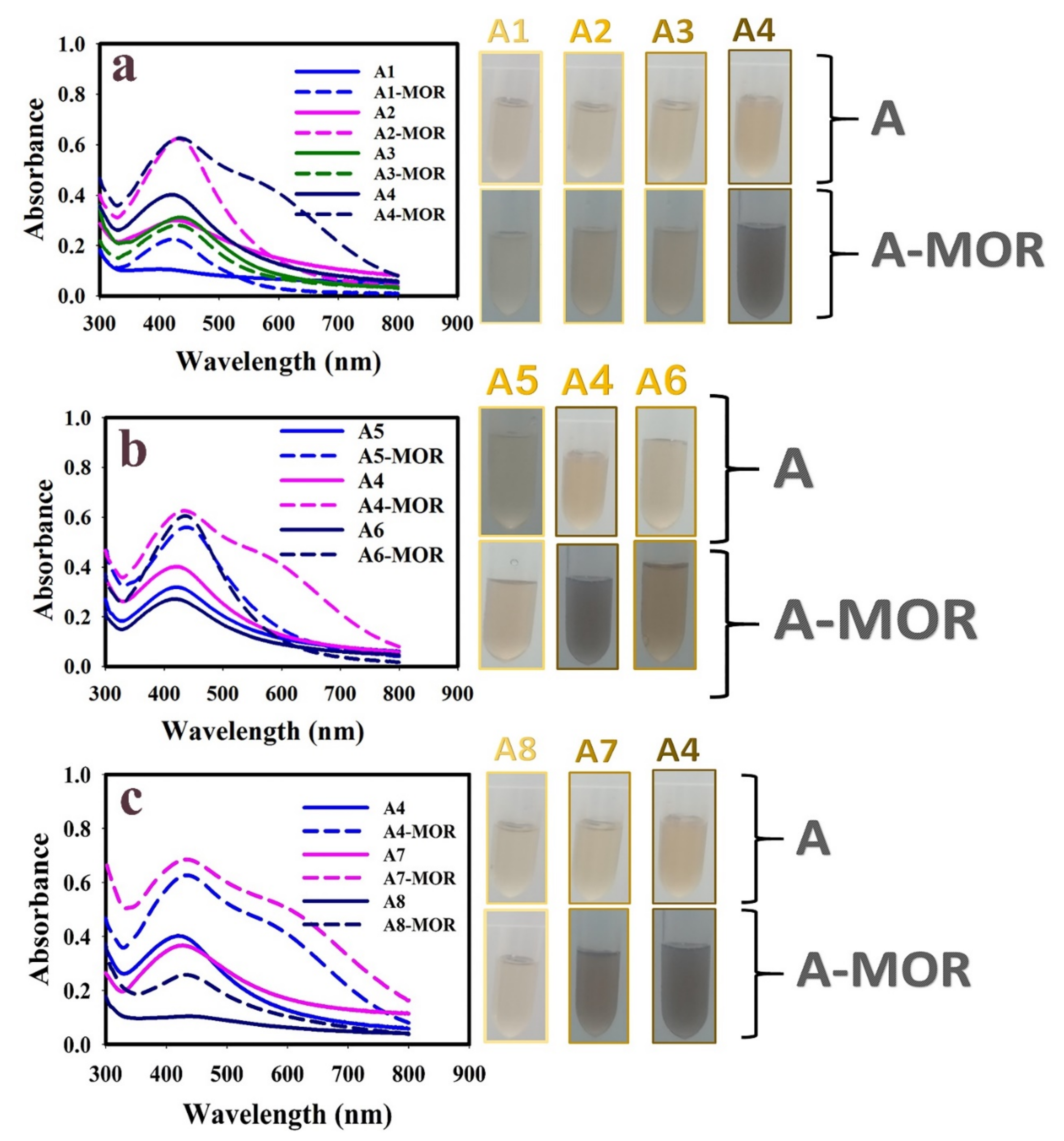



| Sample | Intensity (W/cm2) | Condition | Glycerol (M) | HAuCl4 (M) | AgNO3 (M) | Sodium Citrate (M) | λmax (nm) | λmax after Addition of Morphine (nm) |
|---|---|---|---|---|---|---|---|---|
| A1 | 26 | Probe type | - | - | 5.0 × 10−3 | 1.0 × 10−2 | No significant peak | 425 |
| A2 | 26 | Probe type | - | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | 431 | 435 |
| A3 | 26 | Probe type | 1 | - | 5.0 × 10−3 | 1.0 × 10−2 | 440 | 440 |
| A4 | 26 | Probe type | 1 | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | 420 | 440 |
| A5 | 21 | Probe type | 1 | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | 423 | 440 |
| A6 | 30.5 | Probe type | 1 | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | 420 | 440 |
| A7 | <0.3 | Bath type | 1 | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | 426 | 440 |
| A8 | - | Stirrer method | 1 | 1.0 × 10−5 | 5.0 × 10−3 | 1.0 × 10−2 | No significant peak | 435 |
| TEM | DLS | |||
|---|---|---|---|---|
| NPs (nm) | NPs +MOR (nm) | NPs (nm) | NPs + MOR (nm) | |
| Au@Ag (B) | 5 | 39 | 36 | 380 |
| Au@Ag (P) | 10 | 60 | 39 | 114 |
| Method | Linear Range | LOD (mg/L) | Reference |
|---|---|---|---|
| Molecular imprinting-chemiluminescence | 5 × 10−9–1 × 10−6 (g/mL) | 2 × 10−6 (g/mL) | [54] |
| Spectrophotometry | 0.07–7.98 (mg/L) | 0.03 (mg/L) | [7] |
| GC- MS | 0.05–5 (µg/g) | 0.005 (µg/g) | [55] |
| GC-MS | 5–500 (ng/mL) | 1 (ng/mL) | [56] |
| GC–MS | 0–750 (ng/mL) | 0.75 (ng/mL) | [57] |
| HPLC-MS | 50–1600 (ng/mL) | 40 (ng/mL) | [58] |
| Chemical sensor-Colorimetric method Citrate-capped gold nanoparticles | 1.33–33.29 (µg/mL) | 0.15 (µg/mL) | [59] |
| Chemical sensor-Colorimetric method Cr(III) Metal-Organic Framework | 0.1–350 (nM) | 0.167 (nM) | [49] |
| Chemical sensor-Colorimetric method Melamine modified gold nanoparticles (MA–AuNPs) | 0.07–3.0 (µM) | 17 (nM) | [14] |
| Au@Ag (P) | 0–50 (µM) | 0.1 (µg/mL) | Present work |
| Au@Ag (B) | 0–30 (µM) | 0.055 (µg/mL) | Present work |
| Sample | Added | Found | Recovery (%) | Std.Dev ** |
|---|---|---|---|---|
| (µg/mL) | (µg/mL) | |||
| Au@Ag (P) | 0 | ND * | - | - |
| Au@Ag (P) | 50 | 45.6 | 91.2 | 0.127 |
| Au@Ag (P) | 30 | 26.6 | 88.6 | 0.212 |
| Au@Ag (P) | 1 | 0.87 | 87 | 0.167 |
| Au@Ag (B) | 0 | ND | - | - |
| Au@Ag (B) | 30 | 31.4 | 104 | 0.141 |
| Au@Ag (B) | 10 | 9.7 | 97 | 0.112 |
| Au@Ag (B) | 1 | 1.01 | 101 | 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohani Bastami, T.; Bayat, M.; Paolesse, R. Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors. Sensors 2022, 22, 2072. https://doi.org/10.3390/s22052072
Rohani Bastami T, Bayat M, Paolesse R. Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors. Sensors. 2022; 22(5):2072. https://doi.org/10.3390/s22052072
Chicago/Turabian StyleRohani Bastami, Tahereh, Mansour Bayat, and Roberto Paolesse. 2022. "Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors" Sensors 22, no. 5: 2072. https://doi.org/10.3390/s22052072
APA StyleRohani Bastami, T., Bayat, M., & Paolesse, R. (2022). Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors. Sensors, 22(5), 2072. https://doi.org/10.3390/s22052072







