1. Introduction
Wireless Sensor Networks (WSNs) have attracted a significant amount of attention thanks to their pervasive nature and wide range of applications in emerging fields, such as the Internet of Things and Cyber–Physical Systems [
1,
2]. Since sensor nodes are located in hard-to-reach locations, it is usually expensive and inconvenient to replace the nodes’ batteries. The use of sensor networks is severely restricted by the energy constraints posed by sensor nodes [
3,
4]. All-solid-state polymer lithium batteries are employed as power sources for wireless sensors because of their excellent safety, stability, and high energy density [
5,
6]. However, the high interface resistance between the solid electrolyte and the cathode severely limits the broad application of all-solid-state polymer lithium batteries.
The contact at the interface between the polymer electrolyte and the cathode is poor because of their solid properties, and the existence of tiny gaps at the interface significantly reduces the contact area. Lithium ions cannot pass through the gaps, resulting in high interface resistance [
7]. In order to improve the contact at the interface and eliminate the gaps to reduce the interface resistance between the solid electrolyte and the cathode, researchers have conducted much research in recent years. Zipei Wan et al. [
8] used polyethylene oxide (PEO) and lithium bis(trifluoromethylsulfonyl)imide (LiTFSI) as the cathode binders and prepared a composite electrolyte matrix embedded with Li
7La
3Zr
2O
12 (LLZO) nanowires. The PEO in the cathode and the composite electrolyte were fused at a high temperature to form an integrated electrolyte/electrode structure, which effectively enhanced the interface compatibility and stability between the electrolyte and cathode and ensured efficient lithium-ion transport at the interface. Kyusung Park et al. [
9] intensely studied the cathode/electrolyte interface between LiCoO
2 and LLZO. LiBO
3, an inorganic material with ion conductivity and a low melting point, was used as a binder and inserted into the interface between the electrolyte and the electrode. After heating, the contact between the electrode material and the electrolyte was effectively improved. Jingang Zheng et al. [
10] introduced a double ionic–electronic transfer interface layer at the interface between the electrolyte and electrode by polymerizing 2,2′-bithiophene in polyethylene oxide electrolyte. The formation of the conductive polythiophene layer with superior interfacial stability and contact properties at the interface resulted in a sevenfold reduction in interface resistance. Zhuo Li and Xin Guo [
11] reduced the interface resistance between the composite solid electrolyte and the cathode by creating an integrated interfacial structure. Polyethylene oxide, lithium bis(trifluoromethylsulfonyl)imide, and ionic liquids were used to form a viscoelastic interface. As a result, an interface between the solid electrolyte and the cathode with tight contact, low interface resistance, and fast ion transport was achieved. Zelin Yang et al. [
12] used an interface adhesion strategy to reduce the interface resistance between the solid electrolyte and the cathode. A thin layer of adhesive PEO was used as an interfacial binder to connect the dense Li
1.3Al
0.3Ti
1.7(PO
4)
3 ceramic electrolyte to the solid electrode. Additionally, the interface was enhanced by using a PEO adhesive instead of a poly(1,1-difluoroethylene) (PVDF) adhesive to bond the cathode assembly. The results showed that the interface resistance between the solid electrolyte and the electrode was reduced by two orders of magnitude.
In recent years, the ultrasonic vibration-assisted processing method has become a hot research topic. This processing method is applied to bond thermoplastic composites, and its mechanism involves the frictional heating of molecules using low-amplitude, high-frequency ultrasonic vibration [
13,
14]. Irene Fernandez Villegas and Regis van Moorleghem [
15] demonstrated that high-frequency ultrasonic vibrations at tens of thousands of times per second could produce partial high temperatures that caused thermoplastics to melt. When two pieces of plastic were placed together, the interface could melt, and the materials could be fused. Yibo Sun et al. [
16] established an experimental platform to study vibration transmission and investigated the interface fusion process of thermoplastic polymers under ultrasonic vibration. The results showed that the mobility of the polymer was further enhanced with the effect of ultrasonic vibration. The experimental results indicated that the ultrasonic vibration promoted the formation of the fused interface and reduced the generation of defects. Habibi et al. [
17] investigated the effect of ultrasonic vibration on the wetting of the poly(3,4-ethylenedioxythiophene): poly(styrene sulfonic acid) (PEDOT: PSS) films used in solar cells. The results showed that ultrasonic vibration improved surface wetting by promoting droplet spreading and repairing the dewetted areas. Weibing Guo et al. [
18] used the molecular dynamics (MD) simulation method to analyze the effect of ultrasonic vibration on the wetting of Pb droplets on the Al (1 0 0) surface. It was found that the interaction between Pb and Al was small in the absence of ultrasonic vibration. Under ultrasonic vibration, aluminum atoms dissolved in the Pb droplets, and Pb atoms diffused into the aluminum lattice, producing violent surface alloying.
To solve the problem of poor compatibility between the solid electrolyte and the cathode, an ultrasonic fusion method is proposed in this paper to promote the contact between the solid electrolyte and the cathode and reduce the interface resistance. Firstly, the polymer electrolytes and LiFePO4 cathodes were prepared, and ultrasonic vibration was applied to promote the fusion of the solid electrolytes and the cathodes. Then, the ultrasonic vibration parameters were optimized in orthogonal experiments, and the effect of the ultrasonic fusion method on reducing the interface resistance was analyzed and verified. Finally, the mechanism of the ultrasonic fusion method on reducing the interface resistance was studied.
2. Materials and Methods
2.1. Materials
The matrix material used for the solid polymer electrolyte (SPE) is PEO (Mw = 106 g/mol, Shanghai Kaiyuan Chemical Technology, Shanghai, China). The lithium salt is lithium bis(trifluoromethane)sulfonimide (LiTFSI, 99%, Shanghai Aichun Biological Technology, Shanghai, China). The solvents are acetonitrile (ACN, 99.8%, Hefei Jiankun Chemical, Hefei, China) and N-Methylpyrrolidone (NMP, 99.5%, Wuxi Yatai United Chemical, Wuxi, China). LiFePO4 (Shenzhen Kejing Zhida Technology, Shenzhen, China) and SUPER-P (Shenzhen Kejing Zhida Technology, Shenzhen, China) are used for the active cathode material and conductive agent. The binder is poly(1,1-difluoroethylene) (PVDF, 99.8%, Sinopharm Chemical Reagent, Shanghai, China). The cathode current collector is aluminum foil (Taizhou Yajun Battery Material, Taizhou, China).
2.2. Experimental Method
2.2.1. Preparation of Solid Polymer Electrolyte
The PEO-based solid polymer electrolyte was prepared using the solution casting method. Before preparation, PEO and LiTFSI were dried in a vacuum drying oven at 60 °C for 24 h to remove residual moisture. In an argon-filled glove box (O2 < 0.01 ppm, H2O < 0.01 ppm), 1.25 g PEO and 0.45 g LiTFSI (EO:Li = 18:1) were weighed and slowly added into 30 mL acetonitrile in turn, and the solution was magnetically stirred for 24 h until PEO and LiTFSI were wholly dissolved. The electrolyte solution was poured into a polytetrafluoroethylene (PTFE) mold. Then, the mold was placed horizontally in the glove box for 6 h to allow the acetonitrile solvent to evaporate naturally. After that, it was dried in a vacuum drying oven at 50 °C for 5 h to remove the residual acetonitrile solvent from the electrolyte. Finally, the electrolyte films were slowly removed from the PTFE mold and measured with a digital micrometer. The prepared electrolyte had a uniform thickness of about 100 μm. The electrolyte films were cut into disks with a diameter of 16 mm (the size of the diaphragm for a button cell) using a slicer and placed in the glove box.
2.2.2. Preparation of LiFePO4 Cathode
The cathode is made up of active materials, conductive agents, a binder, and current collectors. The active material used for the cathode was LiFePO4, the conductive agent was SUPER-P, the binder was PVDF, and the current collector was aluminum foil. Here, 0.15 g PVDF was weighed and added into 1.35 g NMP solvent, and the solution was magnetically stirred until the PVDF was completely dissolved. A binder solution with a mass fraction of 10% was obtained. Then, 0.8 g LiFePO4 and 0.1 g SUPER-P were weighed and added to a mortar, and the mixture was ground for 1 h. After that, 1 g of the binder solution was added to the mixture and ground for 1 h to obtain a uniformly mixed slurry. A 100 μm doctor blade was used to coat the slurry on aluminum foil evenly. The cathodes were dried in a vacuum drying oven at 60 °C for 6 h to allow the solvent to evaporate, and were then vacuum dried at 120 °C for 4 h to remove residual solvent. A manual microtome was applied to cut the cathode into disks with a diameter of 13 mm and placed in the glove box for later use.
2.2.3. Ultrasonic Fusion Method
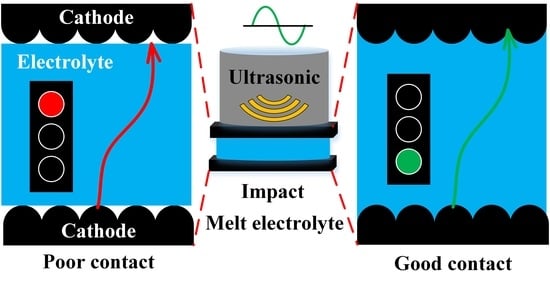
The traditional method used pressure to combine the solid electrolyte and the cathode, resulting in poor contact at the solid–solid interface. In this study, the ultrasonic fusion method applied ultrasonic vibration to impact the electrolyte/cathode structure to form a good contact interface between the solid electrolyte and the cathode. The impact between the solid electrolyte and the cathode generated a high enough temperature to melt the electrolyte. Thus, the cathode was wetted by molten electrolyte, and the solid–solid interface was substituted with the solid–liquid interface during the formation. Furthermore, the impact promoted the penetration of the molten electrolyte into the cathode. The contact area was enhanced between the solid electrolyte and the cathode. Therefore, a good contact interface was achieved.
The equipment used to fuse the solid electrolyte and the cathode was the Taiwan MAXWIND® ME-1800 ultrasonic vibration platform. The ultrasonic vibration platform consisted of an ultrasonic generator, a transducer, an amplitude transformer, and a sonotrode. When the ultrasonic equipment works, the generator converts the low-frequency electric signal into a high-frequency electric signal matched with the transducer. Then, the transducer converts the high-frequency electric signal into ultrasonic vibration. The amplitude of the generated ultrasonic vibration is so minimal that it is not large enough to achieve the fusion of the solid electrolyte and the cathode. Therefore, the amplitude transformer is applied to increase the amplitude of the ultrasonic vibration. Finally, the ultrasonic vibration is transmitted to the position to be fused through the sonotrode. In addition, the ultrasonic vibration platform can pressurize the cylinder using an air pump, and the ultrasonic pressure can be changed by adjusting the pressure in the cylinder.
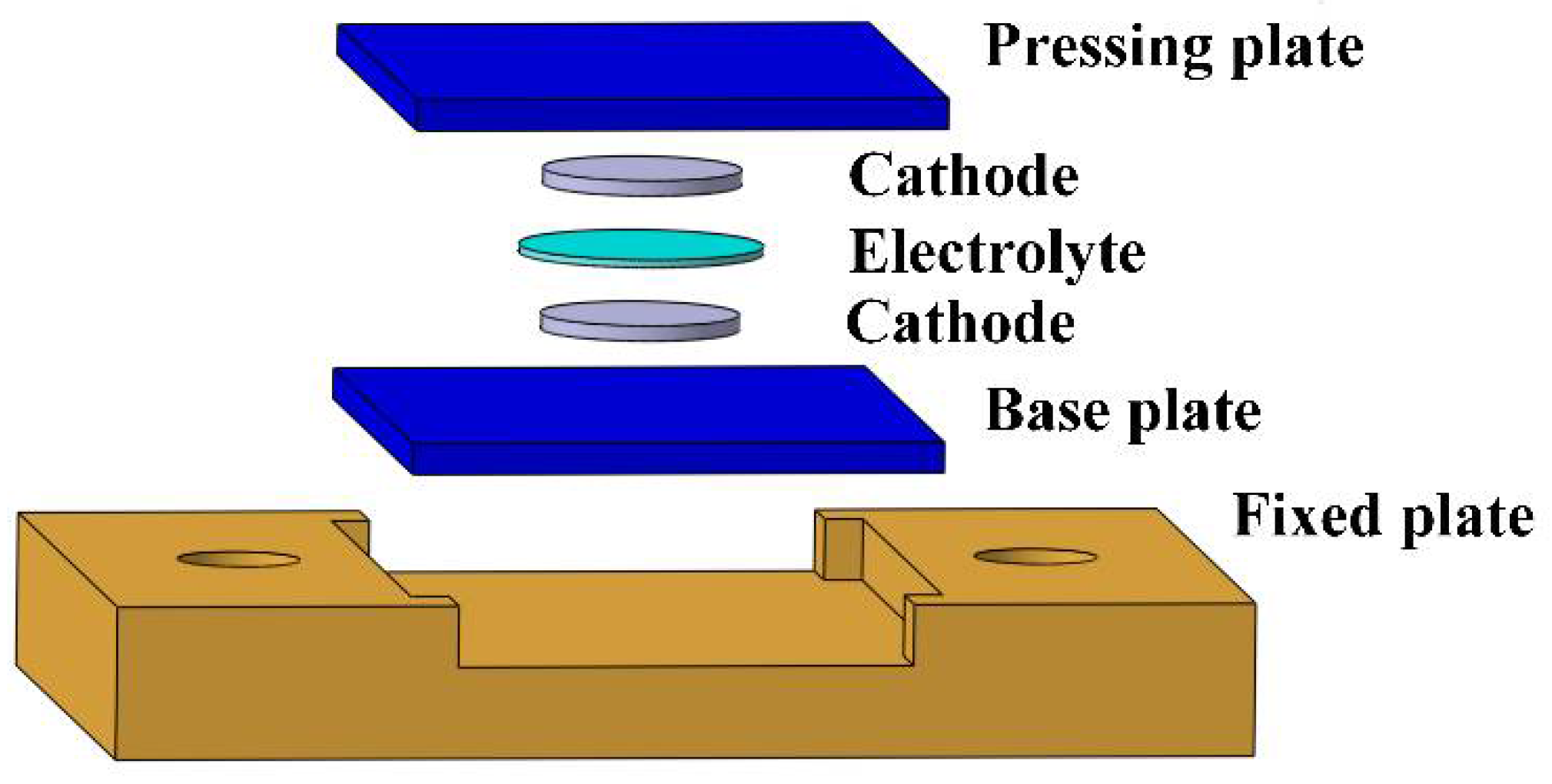
An ultrasonic fusion mold was applied to prevent the solid electrolyte and the cathode from being damaged during the high-frequency vibration, as shown in
Figure 1. Both the pressing plate and the base plate were carbon fiber-reinforced plastic (CFRP) laminates, which could effectively transmit the ultrasonic vibration and avoid rigid contact. The fixed plate was made of a 7075 aluminum alloy and was bolted to the experimental platform to prevent horizontal movement during ultrasonic fusion from tearing the solid electrolyte and the cathode.
The cathode symmetric batteries, with the cathode on both sides of the electrolyte, were applied to study the interface resistance between the solid electrolyte and the cathode. Firstly, the electrolyte and the cathode were put in the ultrasonic fusion mold, as shown in
Figure 1. Next, the ultrasonic frequency was set to 20 kHz. A pulse mode was applied to avoid damaging the electrolytes by overheating them. In one cycle on this mode, the ultrasonic vibration operated for 4 s and was suspended for 4 s. The ultrasonic time (ultrasonic vibration operating time) was 4~16 s, the ultrasonic amplitude was 14~17 μm, and the ultrasonic pressure was 0.08~0.32 MPa. The processing parameters were optimized via an orthogonal experiment, conducted as follows. The sonotrode was pushed down to touch the pressing plate, and the ultrasonic generator was turned on. The ultrasonic vibration was applied to the interface between the electrolyte and the cathode. Finally, the sonotrode was pushed up, and the fused cathode symmetric battery was removed from the mold. The cathode symmetric batteries made using the traditional method were recorded as the Reference Group, and those with the ultrasonic fusion method were recorded as the Ultrasonic Group. In the traditional method, the cathode symmetric battery was assembled with a pressure of 5 MPa.
2.3. Orthogonal Experimental Design
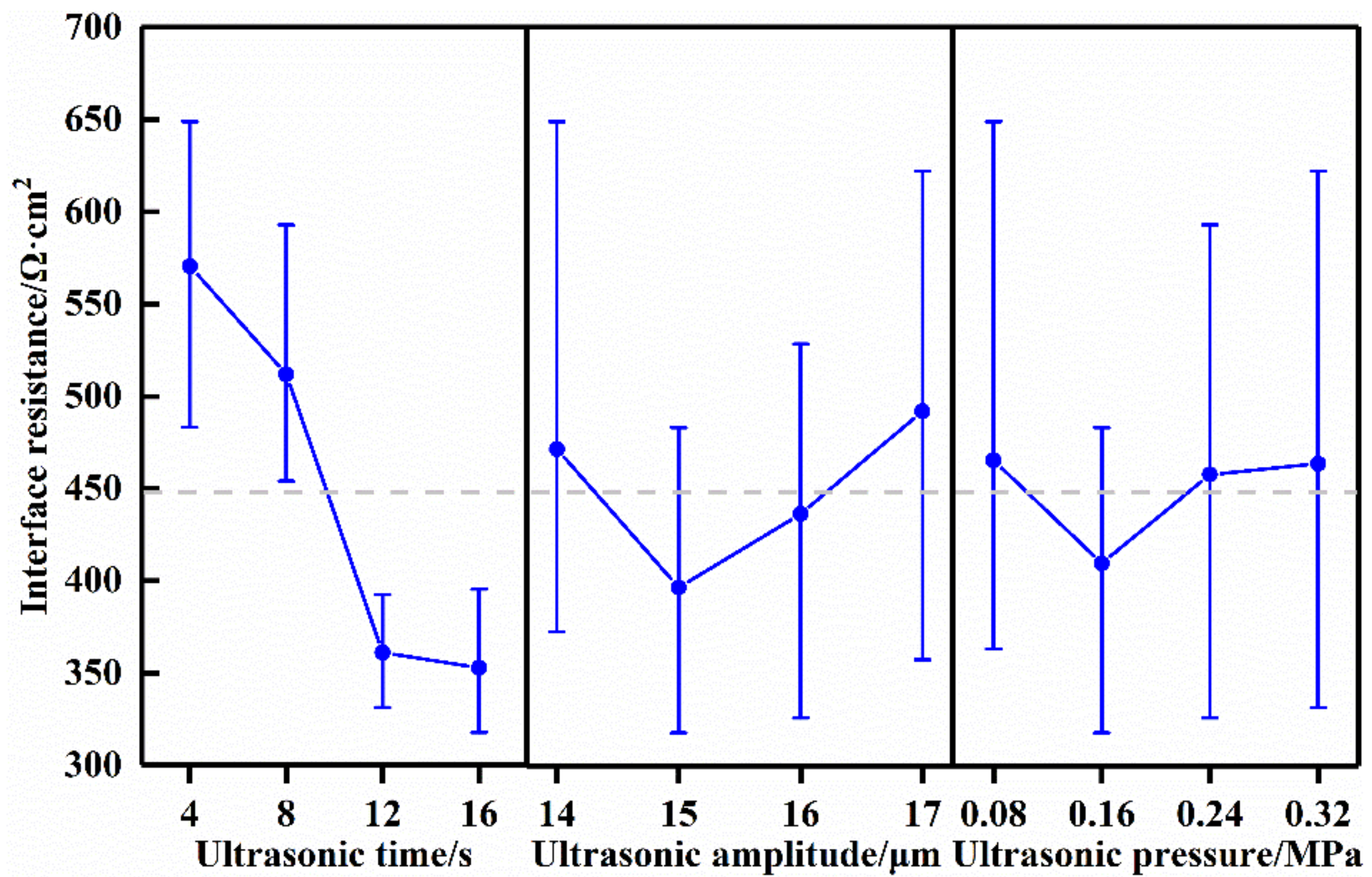
The orthogonal experiment method was applied to obtain the optimal processing parameters for the reduction of the interface resistance between the solid electrolyte and the cathode. The main processing parameters include the ultrasonic time, ultrasonic amplitude, and ultrasonic pressure. Based on preliminary trials, we observed the influence of the parameters on the sample. The orthogonal experiment was designed using Minitab (V17.1) software, and the orthogonal L16 (4
3) table was obtained, as shown in
Table 1. The orthogonal experiment was carried out according to the table. In order to ensure the validity of the data, the experiments were repeated five times, and the mean values were recorded.
2.4. Characterization
2.4.1. Direct Current (DC) Polarization
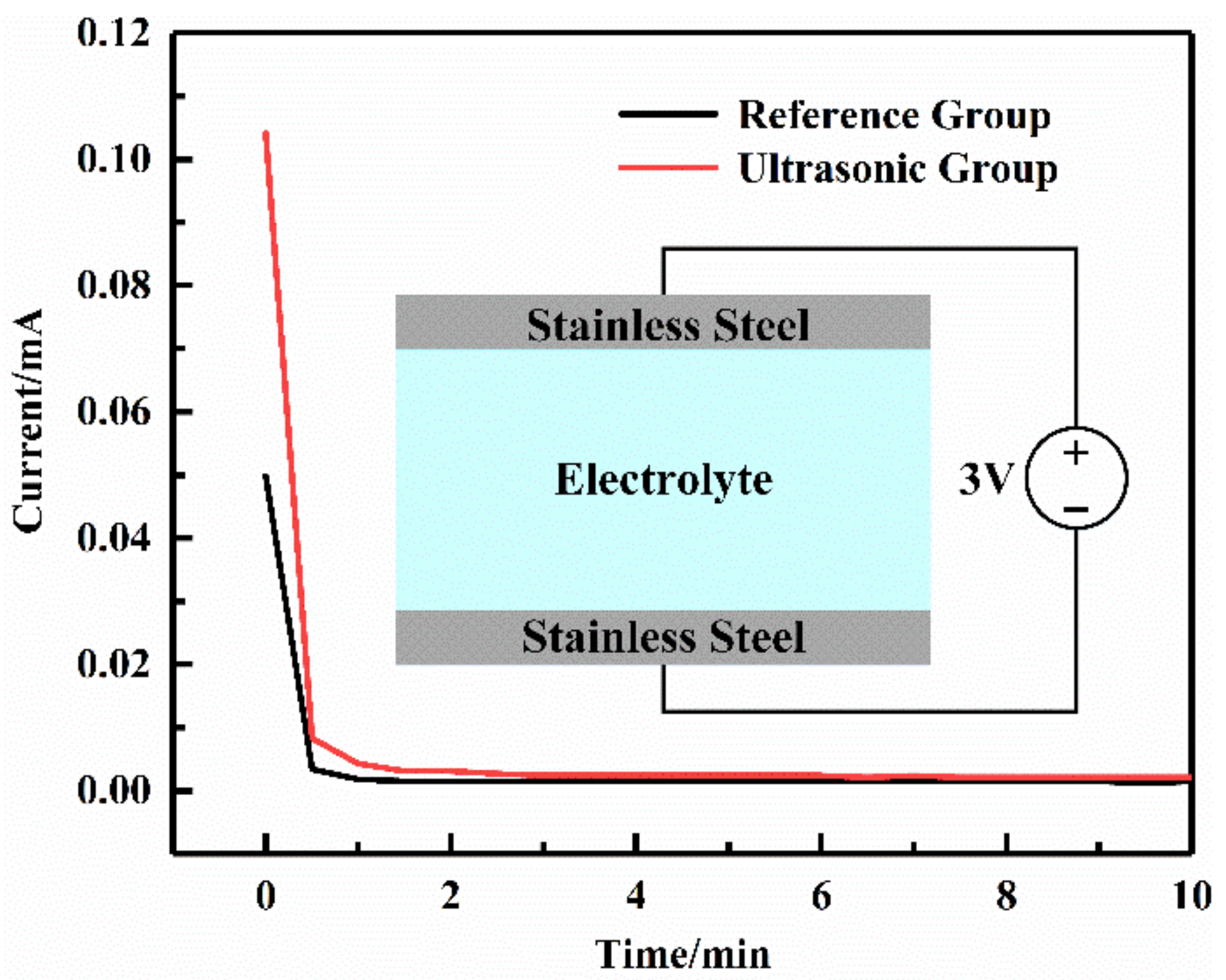
In order to prevent the battery from short circuiting, one of the basic requirements for solid electrolytes is that the electrolyte cannot conduct electrons. The ability of PEO-based solid polymer electrolyte films to conduct electrons can be analyzed with the DC polarization test. The SS (stainless steel)/SPE/SS structure was tested using the Shanghai Chenhua CHI760E electrochemical workstation at room temperature. The polarization voltage was set at 3 V, and the test time was 300 min.
2.4.2. Alternating Current (AC) Impedance
AC impedance spectroscopy of the cathode symmetric battery was measured by placing the prepared electrolyte film between two LiFePO4 cathodes. The test instrument was the Shanghai Chenhua CHI760E electrochemical workstation, the test frequency range was from 0.1 Hz to 1 MHz, and the signal amplitude was 5 mV.
2.4.3. Linear Sweep Voltammetry (LSV)
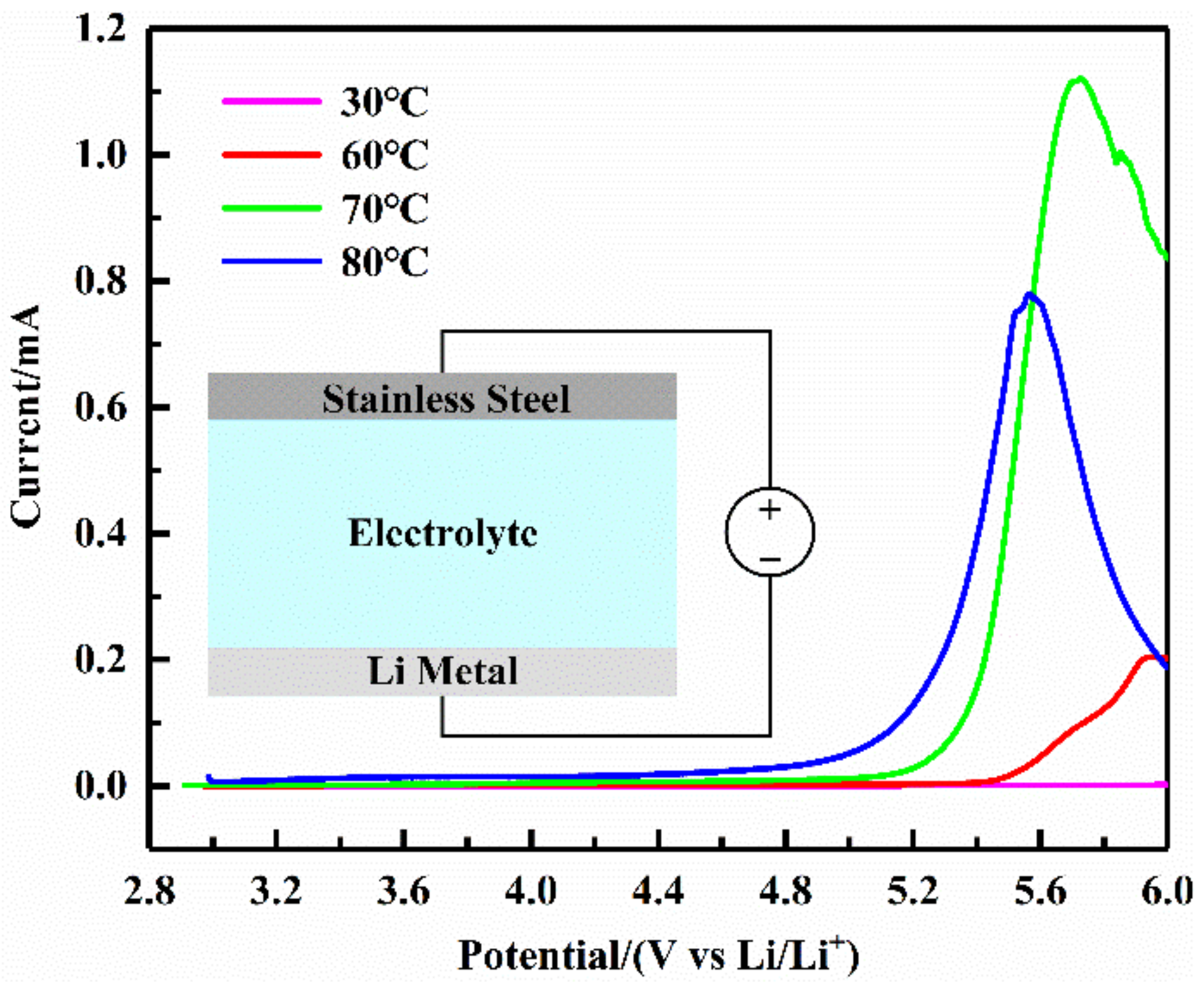
The electrochemical window is an essential indicator of the stability of solid electrolytes. The electrochemical window of the SS/SPE/Li structure was examined by linear sweep voltammetry. The test instrument was the Shanghai Chenhua CHI760E electrochemical workstation. The sweep voltage was increased from open circuit potential to 6 V at a rate of 0.5 mV/s.
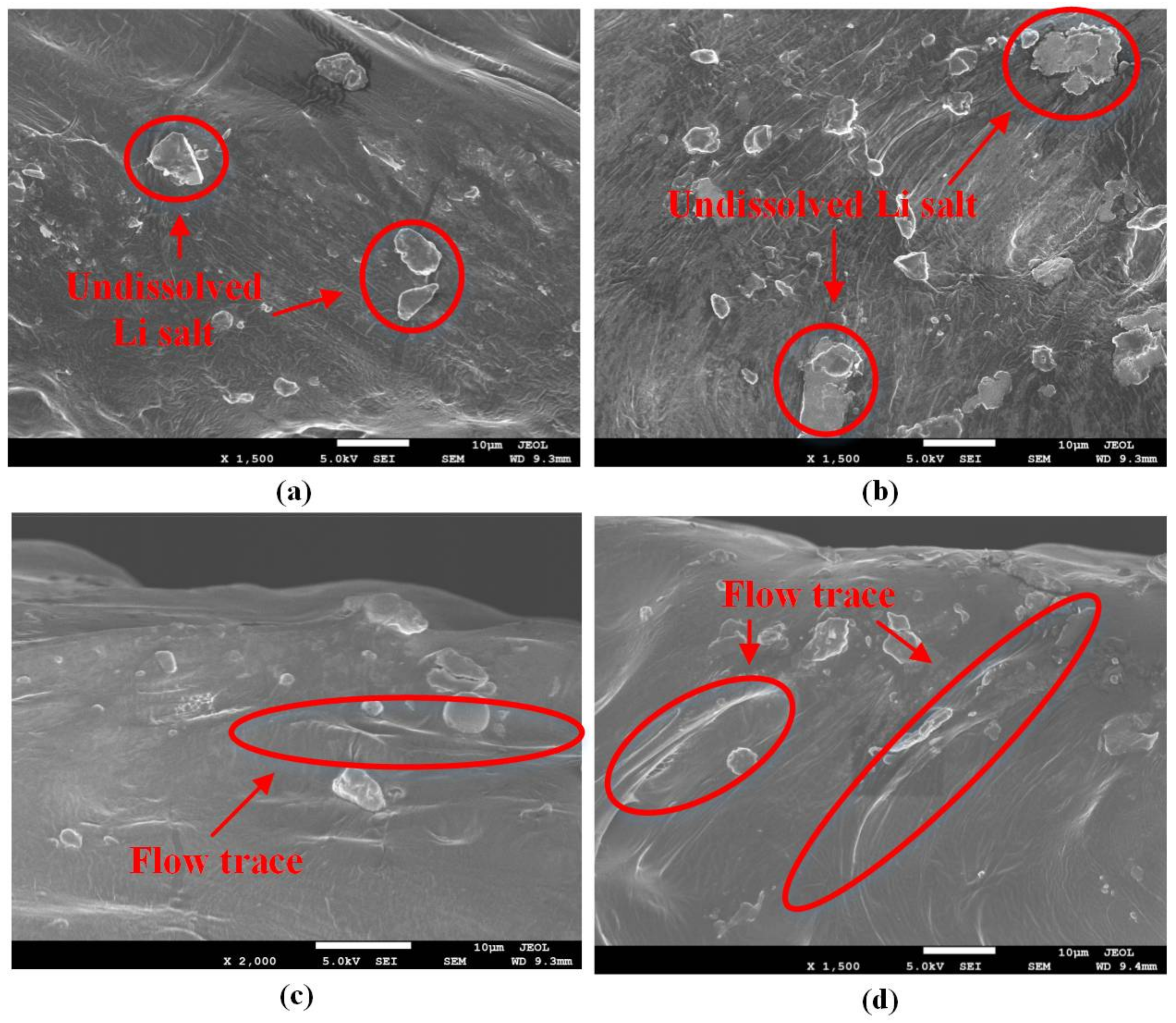
2.4.4. Scanning Electron Microscope (SEM)
The microstructure of the interface between the electrolyte and the cathode was observed using the TESCAN MIRA4 field emission scanning electron microscope. Before the observation, the electrolyte/cathode structure was fractured in liquid nitrogen. After gold coating, the fracture surface was observed.
2.4.5. Fourier Transform Infrared (FTIR) Spectroscopy
The change of functional groups at the interface was observed using the Nicolet 6700 Fourier Transform Infrared Spectrometer produced by Thermo Fisher Scientific. Powder was achieved by scraping at the interface, and this was used to prepare the KBr pellet to perform the infrared scanning test. The wave number was from 400 cm−1 to 4000 cm−1.