The Application of Biological Feedback in the Rehabilitation of Patients after Ischemic Stroke
Abstract
:1. Introduction
Biological Feedback
2. Materials and Methods
2.1. Study Group
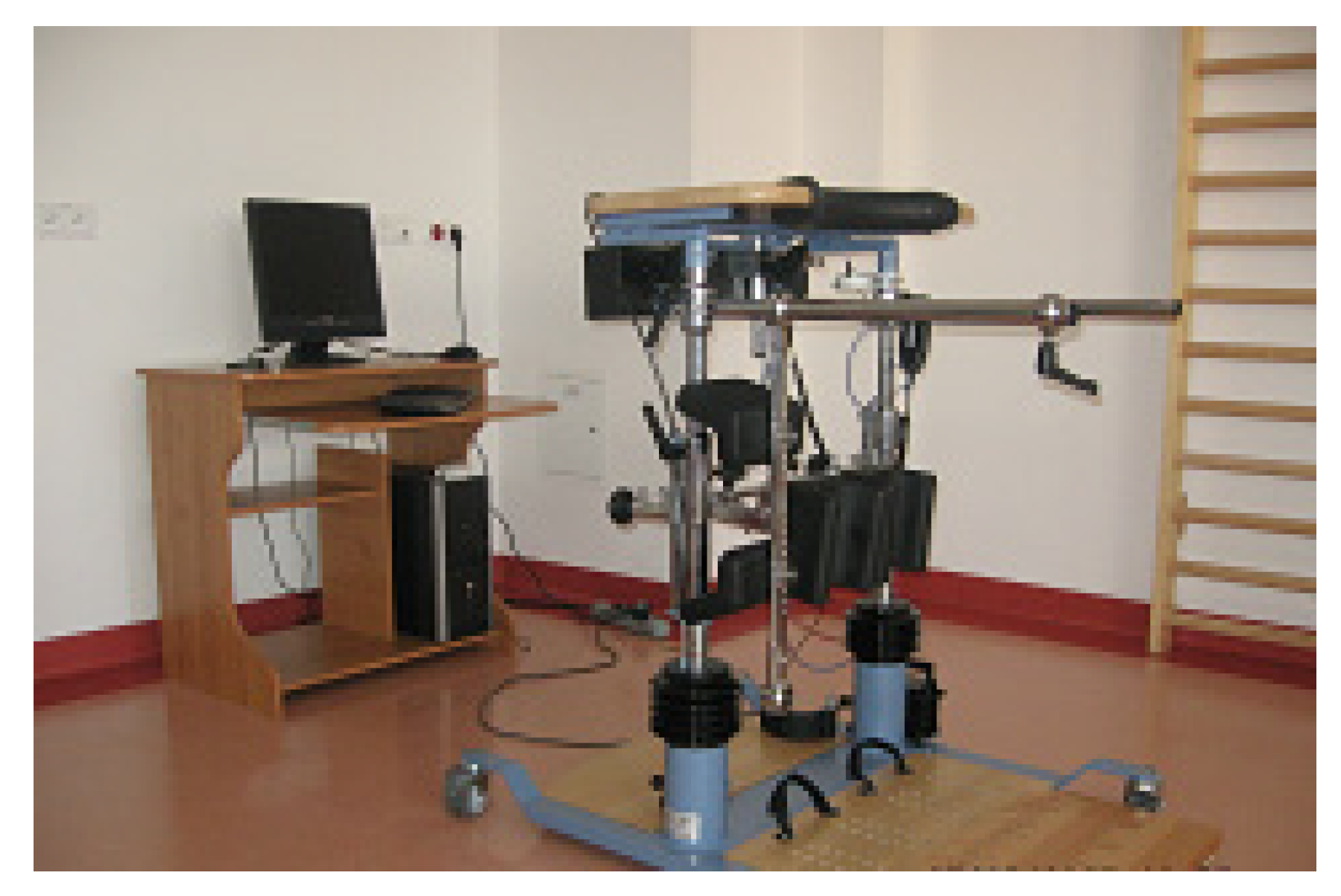
2.2. Methodology of the Study
- Movement on a horizontal track (from right-to-left);
- Movement on a horizontal track (from left-to-right);
- Vertical trajectory (from back-to-front);
- A tilt test (COG).
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nunzio, A.M.; Zucchella, C.; Spicciato, F.; Tortola, P.; Vecchione, C.; Pierelli, F.; Bartolo, M. Biofeedback rehabilitation of posture and weight-bearing distribution in stroke: A center of foot pressure analysis. Funct. Neurol. 2014, 29, 127–134. [Google Scholar]
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [Green Version]
- Hugues, A.; Di Marco, J.; Ribault, S.; Ardaillon, H.; Janiaud, P.; Xue, Y.; Zhu, J.; Pires, J.; Khademi, H.; Rubio, L.; et al. Limited evidence of physical therapy on balance after stroke: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221700. [Google Scholar] [CrossRef] [Green Version]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.; Tallis, R.C. Balance disability after stroke. Phys. Ther. 2006, 86, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef]
- Hasegawa, N.; Asaka, T. Motor learning on postural control using auditory biofeedback training. Impact 2021, 2021, 58–60. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Z.; Wang, J. Robotics in Lower-Limb Rehabilitation after Stroke. Behav. Neurol. 2017, 2017, 3731802. [Google Scholar] [CrossRef] [Green Version]
- Tiseo, C.; Lim, Z.Y.; Shee, C.Y.; Ang, W.T. Mobile Robotic Assistive Balance Trainer—An intelligent compliant and adaptive robotic balance assistant for daily living. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 5300–5303. [Google Scholar] [CrossRef]
- Burgos, P.I.; Lara, O.; Lavado, A.; Rojas-Sepúlveda, I.; Delgado, C.; Bravo, E.; Kamisato, C.; Torres, J.; Castañeda, V.; Cerda, M. Exergames and Telerehabilitation on Smartphones to Improve Balance in Stroke Patients. Brain Sci. 2020, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari Ghoshchi, S.; De Angelis, S.; Morone, G.; Panigazzi, M.; Persechino, B.; Tramontano, M.; Capodaglio, E.; Zoccolotti, P.; Paolucci, S.; Iosa, M. Return to Work and Quality of Life after Stroke in Italy: A Study on the Efficacy of Technologically Assisted Neurorehabilitation. Int. J. Environ. Res. Public Health 2020, 17, 5233. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lledó, L.D.; Díez, J.A.; Bertomeu-Motos, A.; Ezquerro, S.; Badesa, F.J.; Sabater-Navarro, J.M.; García-Aracil, N. A Comparative Analysis of 2D and 3D Tasks for Virtual Reality Therapies Based on Robotic-Assisted Neurorehabilitation for Post-stroke Patients. Front. Aging Neurosci. 2016, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Slater, M.; Sanchez-Vives, M.V. Enhancing our lives with immersive virtual reality. Front. Robot. AI 2016, 3, 74. [Google Scholar] [CrossRef] [Green Version]
- Giggins, O.M.; Persson, U.M.; Caulfield, B. Biofeedback in rehabilitation. J. Neuro. Eng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; Van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11, CD010820. [Google Scholar] [CrossRef]
- Zakharov, A.V.; Bulanov, V.A.; Khivintseva, E.V.; Kolsanov, A.V.; Bushkova, Y.V.; Ivanova, G.E. Stroke Affected Lower Limbs Rehabilitation Combining Virtual Reality With Tactile Feedback. Front. Robot. AI 2020, 7, 81. [Google Scholar] [CrossRef]
- Jeon, H.J.; Hwang, B.Y. Effect of bilateral lower limb strengthening exercise on balance and walking in hemiparetic patients after stroke: A randomized controlled trial. J. Phys. Ther. Sci. 2018, 30, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.S.; Cho, H.Y.; In, T.S. Trunk exercises performed on an unstable surface improve trunk muscle activation, postural control, and gait speed in patients with stroke. J. Phys. Ther. Sci. 2016, 28, 940–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Kim, Y.; Chung, Y.; Hwang, S. Weight-shift training improves trunk control, proprioception, and balance in patients with chronic hemiparetic stroke. Tohoku J. Exp. Med. 2014, 232, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.K.; Kim, J.H. Effects of kinetic chain exercise using EMG-biofeedback on balance and lower extremity muscle activation in stroke patients. J. Phys. Ther. Sci. 2017, 29, 1390–1393. [Google Scholar] [CrossRef] [Green Version]
- Tamburella, F.; Moreno, J.C.; Valenzuela, D.S.H.; Pisotta, I.; Iosa, M.; Cincotti, F.; Mattia, D.; Pons, J.L.; Molinari, M. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: Electromyographic vs joint torque biofeedback. J. Neuroeng. Rehabil. 2019, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.E.; Yoo, J.S.; Kim, K.E.; Cho, S.T.; Jang, W.S.; Cho, K.H.; Lee, W.H. Systematic review of appropriate robotic intervention for gait function in subacute stroke patients. Biomed Res. Int. 2018, 2018, 4085298. [Google Scholar] [CrossRef] [PubMed]
- Leightley, D.; Hoon, M.Y.; Coulson, J.; Piasecki, M.; Cameron, J.; Barnouin, Y.; Tobias, J.; McPhee, J.S. Postural stability during standing balance and sit-to-stand in master athlete runners compared with nonathletic old and young adults. J. Aging Phys. Act. 2017, 25, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winiarska, A.; Ziółkowska, A.; Świtaj, K.; Wojtczak, P. Balance of individuals at different age involved in physical activity—review of publications. J. Educ. Health Sport 2017, 7, 978–985. [Google Scholar] [CrossRef]
- Fung, J. Gait and balance training using virtual reality is more effective for improving gait and balance ability after stroke than conventional training without virtual reality. J. Physiother. 2017, 63, 114. [Google Scholar] [CrossRef]
- Krekora, K.; Czernicki, J. Biofeedback in rehabilitation of stroke patients. Med. Rehabil. 2005, 9, 26–30. [Google Scholar]
- Huang, H.; Wolf, S.L.; He, L. Recent developments in biofeedback for neuromotor rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Przysada, G.; Guzik, A.; Wolan-Nieroda, A.; Walicka-Cupryś, K.; Drużbicki, M. Chosen assessment methods of physiotherapy effects in patients after cerebral stroke treated at a rehabilitation ward. Med. Rev. 2015, 13, 212–222. [Google Scholar] [CrossRef]
- Gałęcki, S.; Walasik, M.; Rokicki, R.; Sikorska, K.; Dudkiewicz, Z. Effectiveness of rehabilitation and exercises on static-dynamic parapodium with biofeedback in relation to body balance in patients after ischaemic stroke. Kwart. Ortop. 2013, 3, 314–325. [Google Scholar]
- Jung, K.W.; Yang, D.H.; Myung, S.J. Biofeedback therapy. In The Encyclopedia of Human Behawior, 2nd ed.; Ramachandran, V.S., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 344–347. [Google Scholar]
- Glick, R.M.; Greco, C.M. Biofeedback and primary care. Prim. Care 2010, 37, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Genthe, K.; Schenck, C.; Eicholtz, S.; Zajac-Cox, L.; Wolf, S.; Kesar, T.M. Effects of real-time gait biofeedback on paretic propulsion and gait biomechanics in individuals post-stroke. Top. Stroke Rehabil. 2018, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Agostini, M.; Luque-Moreno, C.; Tonin, P.; Turolla, A. Reinforced feedback in virtual environment for rehabilitation of upper extremity dysfunction after stroke: Preliminary data from a randomized controlled trial. Biomed Res. Int. 2014, 2014, 752128. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Baba, A.; Agostini, M.; Turolla, A.; Kiper, A.; Nowobilski, R.; Opara, J.; Szczudlik, A. Motor learning and brain plasticity after stroke. State of the art. Rehabil. W Prakt. 2017, 1, 65–68. [Google Scholar]
- Kawato, M. Internal models for motor control and trajectory planning. Curr. Opin. Neurobiol. 1999, 9, 718–727. [Google Scholar] [CrossRef]
- Hung, J.W.; Yu, M.Y.; Chang, K.-C.; Lee, H.-C.; Hsieh, Y.-W.; Chen, P.-C. Feasibility of Using Tetrax Biofeedback Video Games for Balance Training in Patients with Chronic Hemiplegic Stroke. PM&R 2016, 8, 962–970. [Google Scholar] [CrossRef]
- Brennan, L.; Zubiete, E.D.; Caulfield, B. Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review. Sensors 2020, 20, 181. [Google Scholar] [CrossRef] [Green Version]
- Reinkensmeyer, D.; Burdet, E.; Casadio, M.; Krakauer, J.; Kwakkel, G.; Lang, C.; Swinnen, S.; Ward, N.; Schweighofer, N. Computational neurorehabilitation: Modeling plasticity andlearning to predict recovery. J. Neuroeng. Rehabil. 2016, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Bulat, T.; Hart-Hughes, S.; Ahmed, S.; Quigley, P.; Palacios, P.; Werner, D.C.; Foulis, P. Effect of a group-based exercise program on balance in elderly. Clin. Interv. Aging 2007, 2, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciaszek, J. Effects of posturographic platform biofeedback training on the static and dynamic balance of older stroke patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Begg, R. Can Real-time Biofeedback of Foot Clearance Data be used to Assist with Gait Rehabilitation following Stroke? NHMRC. Impact 2018, 2018, 38–40. [Google Scholar] [CrossRef]
- Nagano, H.; Said, C.M.; James, L.; Begg, R.K. Feasibility of Using Foot–Ground Clearance Biofeedback Training in Treadmill Walking for Post-Stroke Gait Rehabilitation. Brain Sci. 2020, 10, 978. [Google Scholar] [CrossRef]
- Skvortsov, D.V.; Kaurkin, S.N.; Ivanova, G.E. A Study of Biofeedback Gait Training in Cerebral Stroke Patients in the Early Recovery Phase with Stance Phase as Target Parameter. Sensors 2021, 21, 7217. [Google Scholar] [CrossRef]
- Yavuzer, G.; Eser, F.; Karakus, D.; Karaoglan, B.; Stam, H.J. The effects of balance training on gait late after stroke: A randomized controlled trial. Clin. Rehabil. 2006, 20, 960–969. [Google Scholar] [CrossRef]
- Srivastava, A.; Taly, A.B.; Gupta, A.; Kumar, S.; Murali, T. Post-stroke balance training: Role of force platform with visual feedback technique. J. Neurol. Sci. 2009, 287, 89–93. [Google Scholar] [CrossRef]
- Kołcz-Trzęsicka, A.; Żurowska, A.; Bidzińska, G.; Piesiewicz-Białas, K.; Kobylańska, M.; Paprocka-Borowicz, M. Use of biofeedback in rehabilitation process of patients after stroke. Med. Sport/Polish J. Sports Med. 2017, 33, 53–60. [Google Scholar] [CrossRef]
- Cabrera-Martos, I.; Ortiz-Rubio, A.; Torres-Sánchez, I.; López-López, L.; Jarrar, M.; Valenza, M.C. The effectiveness of core exercising for postural control in patients with stroke: A systematic review and meta-analysis. PM&R 2020, 12, 1157–1168. [Google Scholar] [CrossRef]
- Pak, N.W.; Lee, J.H. Effects of visual feedback training and visual targets on muscle activation, balancing, and walking ability in adults after hemiplegic stroke: A preliminary, randomized, controlled study. Int. J. Rehabil. Res. 2020, 43, 76–81. [Google Scholar] [CrossRef]
- Ghomashchi, H. Investigating the effects of visual biofeedback therapy on recovery of postural balance in stroke patients using a complexity measure. Top. Stroke Rehabil. 2016, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, E.S.; Kazakov, S.D.; Tolmachev, I.V.; Loonen, A.J.M.; Ivanova, S.A.; Alifirova, V.M. Clinical Evaluation of Different Treatment Strategies for Motor Recovery in Poststroke Rehabilitation during the First 90 Days. J. Clin. Med. 2021, 10, 3718. [Google Scholar] [CrossRef] [PubMed]
- Ordahan, B.; Karahan, A.Y.; Basaran, A.; Turkoglu, G.; Kucuksarac, S.; Cubukcu, M.; Tekin, L.; Polat, A.D.; Kuran, B. Impact of exercises administered to stroke patients with balance trainer on rehabilitation results: A randomized controlled study. Hippokratia 2015, 19, 125–130. [Google Scholar] [PubMed]
- Yanga, Y.; Leeb, J.; Choic, W.; Joob, Y.; Lee, S. Balance trainer training with transcutaneous electrical nerve stimulation improves spasticity and balance in persons with chronic stroke. Phys. Ther. Rehabil. Sci. 2020, 9, 67–73. [Google Scholar] [CrossRef]
- Ventura, A.; Mendes, J.; Caldeira, R.; Pinto, S.; Pereira, Â.M. Benefit of balance retraining after stroke using a force platform biofeedback—Case Study. In Proceedings of the 2nd International Congress of CiiEM: Translational Research and Innovation in Human and Health Science, Egas Moniz, Caparica, Portugal, 11–13 June 2017; Annals of Medicine 2018. Volume 50, p. 164. [Google Scholar] [CrossRef]
- Bałdyga, E.; Białkowska, J. The use of biofeedback in patients with neurological deficits. In Proceedings of the Materiały zjazdowe, Conference V Olsztyński Dzień Fizjoterapii, Olsztyn, Poland, 21 May 2009; pp. 31–41. [Google Scholar]
- Jankowska, A.; Klimkiewicz, P.; Krukowska, S.; Woldańska-Okońska, M. Assessment of the Impact of Training on the Stabilometric Platform Using the Biofeedback Method on Improving Balance and Functional Efficiency of Patients After a Stroke. Acta Balneol. 2021, 62, 15–21. [Google Scholar] [CrossRef]
- Lupo, A.; Cinnera, A.M.; Pucello, A.; Iosa, M.; Coiro, P.; Personeni, S.; Gimigliano, F.; Iolascon, G.; Paolucci, S.; Morone, G. Effects on balance skills and patient compliance of biofeedback training with inertial measurement units and exergaming in subacute stroke: A pilot randomized controlled trial. Funct. Neurol. 2018, 33, 131–136. [Google Scholar]
- Kuczyński, M.; Podbielska, M.L.; Bieć, D.; Paluszak, A.K.K.; Kręcisz, K. The basics of postural control assessment: What, how and why do we need to measure. Acta Biooptica Inf. Med. 2012, 4, 243–249. [Google Scholar]
- Bugajski, M.; Czernicki, J. Evaluation effectiveness of exercises on the balance platform for using biofeedback to walking function in patients after stroke. Medical. Reviev. 2013, 4, 439–447. [Google Scholar]
- de Haart, M.; Geurts, A.C.; Huidekoper, S.C.; Fasotti, L.; van Limbeek, J. Recovery of standing balance in postacute stroke patients: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2004, 85, 886–895. [Google Scholar] [CrossRef]
- Yanohara, R.; Teranishi, T.; Tomita, Y.; Tanino, G.; Ueno, Y.; Sonoda, S. Recovery process of standing postural control in hemiplegia after stroke. J. Phys. Ther. Sci. 2014, 26, 1761–1765. [Google Scholar] [CrossRef] [Green Version]


| Variable | Study Group (n = 92) | Control Group (n = 92) | Study + Control Group (n = 184) |
|---|---|---|---|
| Sex (male/female) | 54/38 | 51/41 | 105/79 |
| * Age (in years) | 62.00 (7.23) | 63.14 (6.51) | 62.57 (6.88) |
| *) | 27.22 (2.23) | 26.20 (1.81) | 26.71 (2.09) |
| Paretic side (left/right) | 36/56 | 71/21 | 107/77 |
| Exercise on a Horizontal Track from Right-to-Left | |||||||
| Group | I | II | III | p * | |||
| X | SD | X | SD | X | SD | ||
| Time (t inside) of COG inside the horizontal track (s) | |||||||
| Study group | 36 | 23.4 | 31.2 | 20.4 | 28.2 | 20.4 | <0.001 |
| Control group | 48 | 27.6 | 36 | 24 | 24.6 | 14.4 | <0.001 |
| p ** | <0.001 | 0.019 | 0.788 | ||||
| Time (t outside) of the COG outside the horizontal track (s) | |||||||
| Study group | 7.2 | 9.6 | 6.6 | 10.2 | 3.6 | 6 | <0.001 |
| Control group | 9 | 9.6 | 6 | 4.8 | 3 | 3 | <0.001 |
| p ** | <0.001 | 0.010 | 0.050 | ||||
| COG distance (cm) | |||||||
| Study group | 28.96 | 10.08 | 27.84 | 10.55 | 22.85 | 6.98 | <0.001 |
| Control group | 35.62 | 14.18 | 35.03 | 13.11 | 27.52 | 8.80 | <0.001 |
| p ** | <0.001 | <0.001 | <0.001 | ||||
| Total time (s) | |||||||
| Study group | 43.2 | 25.2 | 37.2 | 23.4 | 31.8 | 21.6 | <0.001 |
| Control group | 57 | 31.2 | 42 | 25.2 | 27.6 | 15 | <0.001 |
| p ** | <0.001 | 0.119 | 0.936 | ||||
| Exercise on a horizontal track from left-to-right | |||||||
| Time (t inside) of COG inside the horizontal track (s) | |||||||
| Study group | 37.2 | 24.6 | 49.8 | 172.8 | 28.8 | 21.6 | <0.001 |
| Control group | 70.8 | 225 | 38.4 | 22.2 | 25.8 | 17.4 | <0.001 |
| p ** | <0.001 | 0.001 | 0.536 | ||||
| Time (t outside) of the COG outside the horizontal track (s) | |||||||
| Study group | 16.8 | 81 | 13.8 | 81.6 | 12.6 | 69.6 | <0.001 |
| Control group | 12 | 11.4 | 7.2 | 6.6 | 4.2 | 4.2 | <0.001 |
| p ** | 0.002 | 0.001 | 0.108 | ||||
| COG distance (cm) | |||||||
| Study group | 29.98 | 9.552 | 26.15 | 6.872 | 24.66 | 6.608 | <0.001 |
| Control group | 38.16 | 13.72 | 37.36 | 13.08 | 29.37 | 12.17 | <0.001 |
| p ** | <0.001 | <0.001 | 0.001 | ||||
| Total time (s) | |||||||
| Study group | 54.0 | 83.4 | 63.6 | 190.2 | 41.4 | 70.2 | <0.001 |
| Control group | 82.8 | 224.4 | 45.6 | 23.4 | 30.6 | 17.4 | <0.001 |
| p ** | <0.001 | 0.002 | 0.499 | ||||
| Exercise on a Vertical Track | |||||||
| Group | I | II | III | p * | |||
| X | SD | X | SD | X | SD | ||
| Time (t inside) of COG inside the horizontal track (s) | |||||||
| Study group | 48 | 36 | 37.2 | 25.8 | 32.4 | 21.6 | <0.001 |
| Control group | 80.4 | 337.8 | 36.6 | 30.6 | 27 | 24 | <0.001 |
| p ** | 0.921 | 0.894 | 0.097 | ||||
| Time (t outside) of the COG outside the horizontal track (s) | |||||||
| Study group | 7.8 | 13.8 | 16.8 | 112.8 | 13.2 | 88.2 | <0.001 |
| Control group | 6.6 | 9 | 4.2 | 6 | 2.4 | 3.6 | <0.001 |
| p ** | 0.053 | 0.055 | 0.491 | ||||
| COG distance (cm) | |||||||
| Study group | 28.0 | 9.54 | 26.1 | 8.75 | 22.5 | 7.36 | <0.001 |
| Control group | 30.94 | 11.90 | 29.69 | 11.01 | 25.06 | 9.57 | <0.001 |
| p ** | 0.033 | 0.013 | 0.035 | ||||
| Total time (s) | |||||||
| Study group | 55.8 | 39 | 54 | 114.6 | 45.6 | 89.4 | <0.001 |
| Control group | 87 | 337.8 | 40.8 | 31.8 | 28.8 | 24 | <0.001 |
| p ** | 0.611 | 0.433 | 0.013 | ||||
| Brunnström scale, lower limb | |||||||
| Group | I | II | III | p * | |||
| X | SD | X | SD | X | SD | ||
| Study group | 3.48 | 1.02 | 3.92 | 0.85 | 4.37 | 0.75 | <0.001 |
| Control group | 3.12 | 1.00 | 3.39 | 0.95 | 4.67 | 0.71 | <0.001 |
| p ** | 0.037 | 0.001 | 0.009 | ||||
| Ashworth scale, lower limb | |||||||
| Study group | 1.76 | 0.91 | 1.50 | 0.65 | 1.26 | 0.57 | <0.001 |
| Control group | 1.59 | 0.73 | 1.47 | 0.70 | 0.64 | 0.64 | <0.001 |
| p ** | 0.274 | 0.689 | <0.001 | ||||
| Rankin scale | |||||||
| Study group | 2.30 | 1.09 | 1.99 | 0.87 | 1.72 | 1.36 | <0.001 |
| Control group | 2.86 | 0.83 | 2.62 | 0.77 | 1.99 | 0.72 | <0.001 |
| p ** | 0.001 | < 0.001 | 0.003 | ||||
| Barthel scale | |||||||
| Study group | 50.90 | 17.40 | 61.86 | 15.97 | 71.42 | 14.99 | <0.001 |
| Control group | 46.79 | 12.61 | 53.78 | 12.59 | 67.57 | 10.77 | <0.001 |
| p ** | 0.491 | 0.002 | 0.050 | ||||
| VAS | |||||||
| Study group | 7.27 | 1.53 | 6.10 | 1.10 | 4.85 | 0.97 | <0.001 |
| Control group | 7.62 | 1.12 | 6.52 | 1.11 | 3.62 | 1.19 | <0.001 |
| p ** | 0.244 | 0.013 | <0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańdziuk, M.; Krawczyk-Suszek, M.; Maciejewski, R.; Bednarski, J.; Kotyra, A.; Cyganik, W. The Application of Biological Feedback in the Rehabilitation of Patients after Ischemic Stroke. Sensors 2022, 22, 1769. https://doi.org/10.3390/s22051769
Mańdziuk M, Krawczyk-Suszek M, Maciejewski R, Bednarski J, Kotyra A, Cyganik W. The Application of Biological Feedback in the Rehabilitation of Patients after Ischemic Stroke. Sensors. 2022; 22(5):1769. https://doi.org/10.3390/s22051769
Chicago/Turabian StyleMańdziuk, Marzena, Marlena Krawczyk-Suszek, Ryszard Maciejewski, Jerzy Bednarski, Andrzej Kotyra, and Weronika Cyganik. 2022. "The Application of Biological Feedback in the Rehabilitation of Patients after Ischemic Stroke" Sensors 22, no. 5: 1769. https://doi.org/10.3390/s22051769
APA StyleMańdziuk, M., Krawczyk-Suszek, M., Maciejewski, R., Bednarski, J., Kotyra, A., & Cyganik, W. (2022). The Application of Biological Feedback in the Rehabilitation of Patients after Ischemic Stroke. Sensors, 22(5), 1769. https://doi.org/10.3390/s22051769






