Single-Trial Classification of Error-Related Potentials in People with Motor Disabilities: A Study in Cerebral Palsy, Stroke, and Amputees
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Recording
2.3. Experimental Details
2.4. Signal Processing
2.4.1. Pre-Processing
2.4.2. Classification
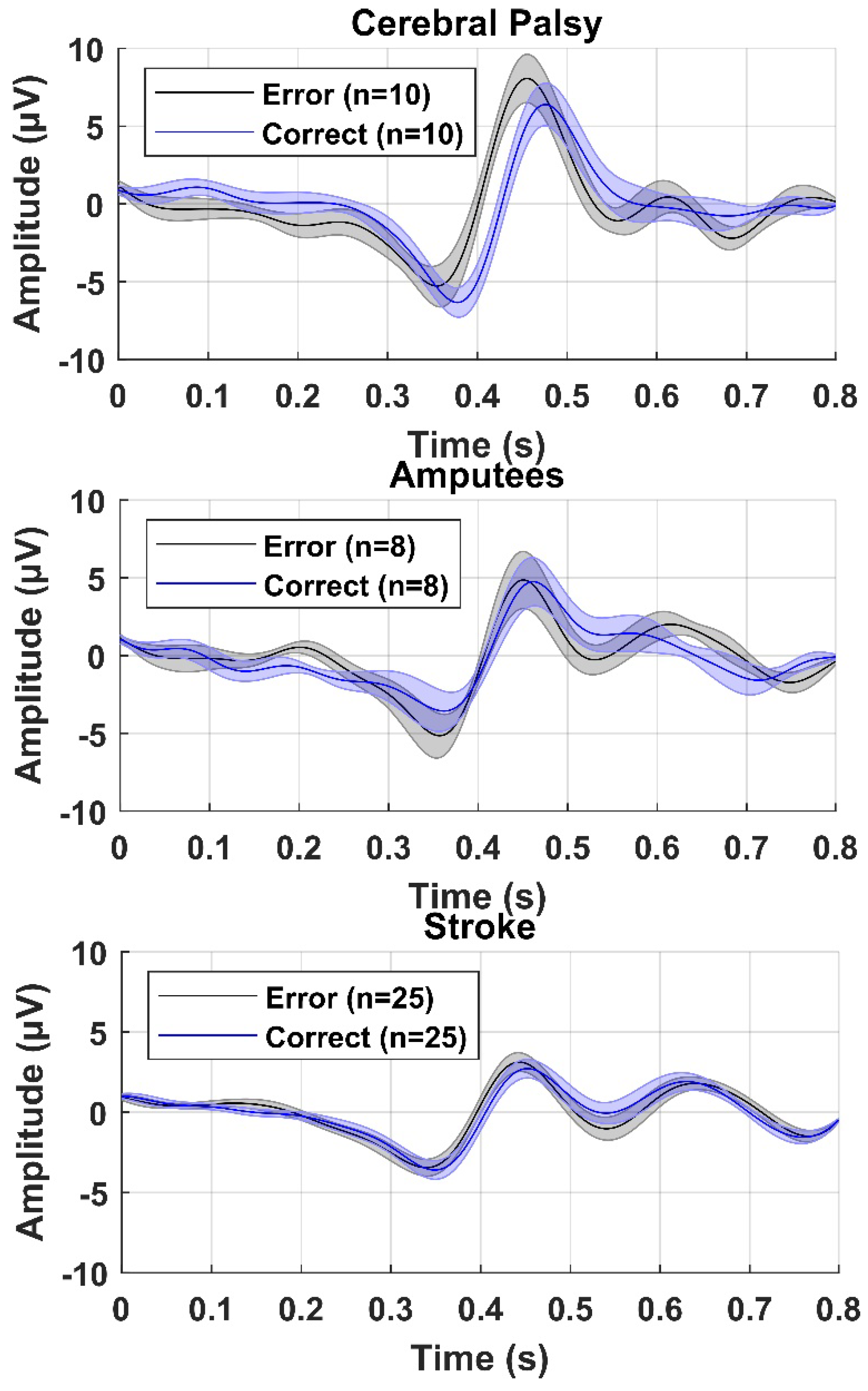
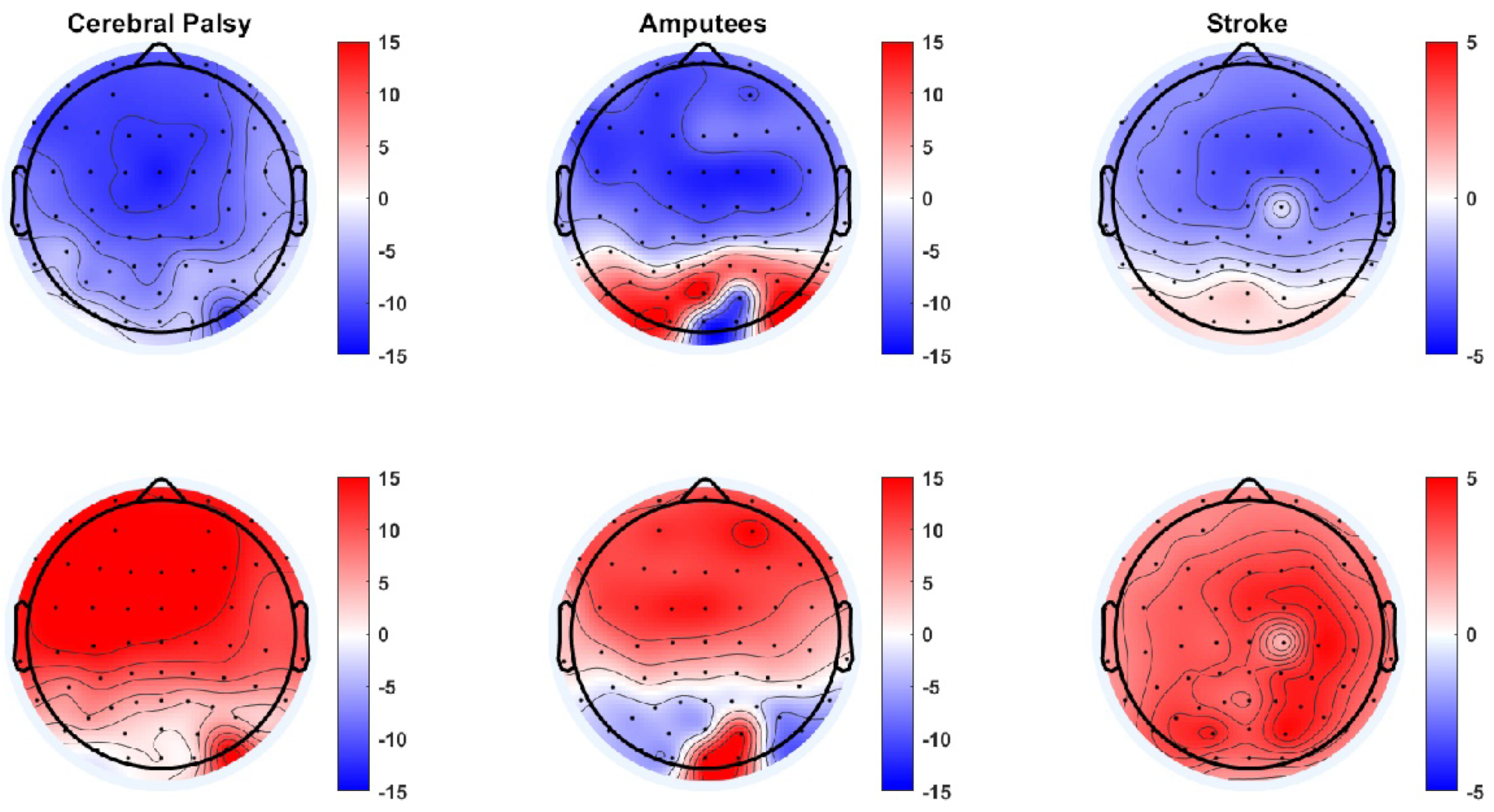
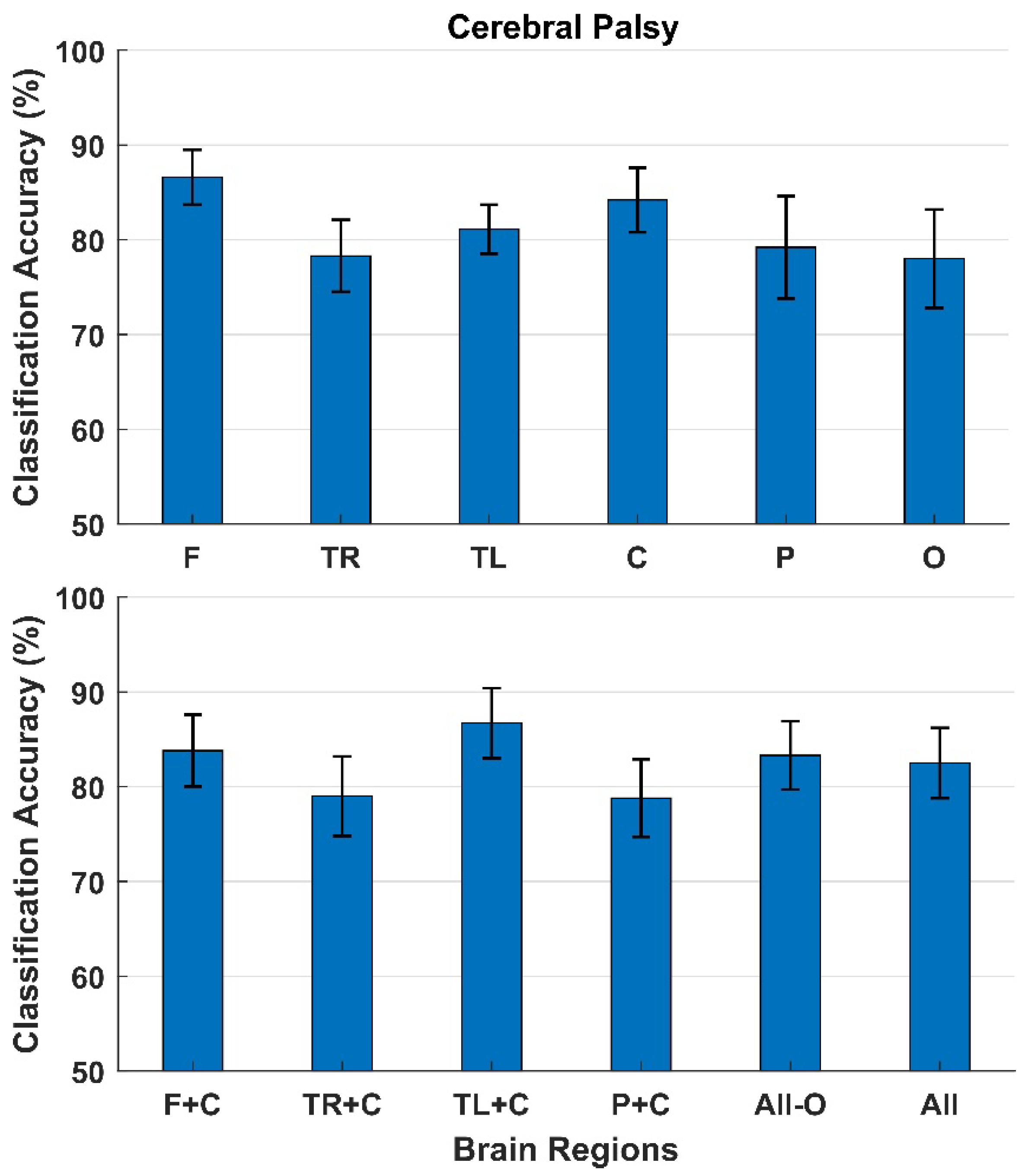
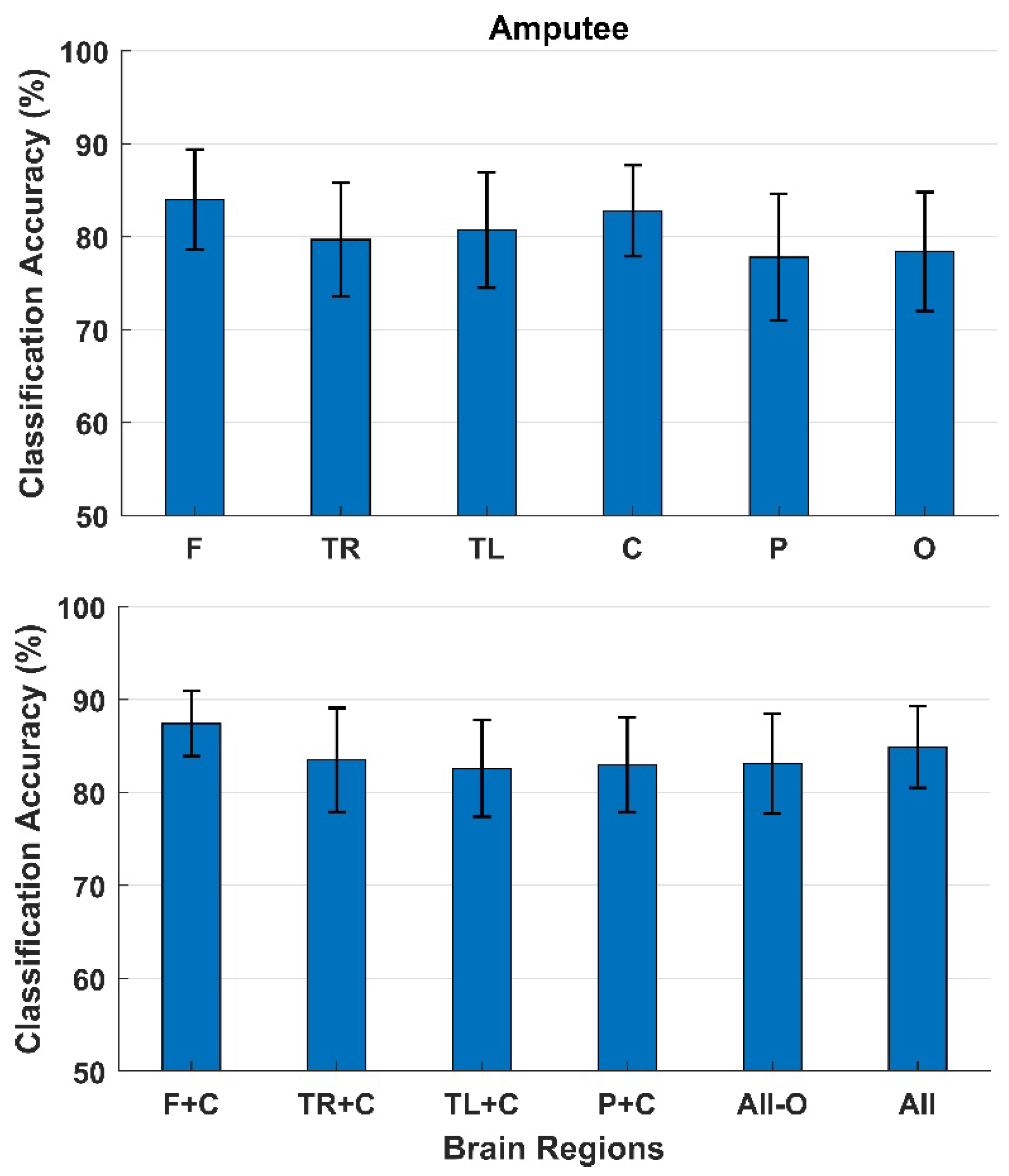
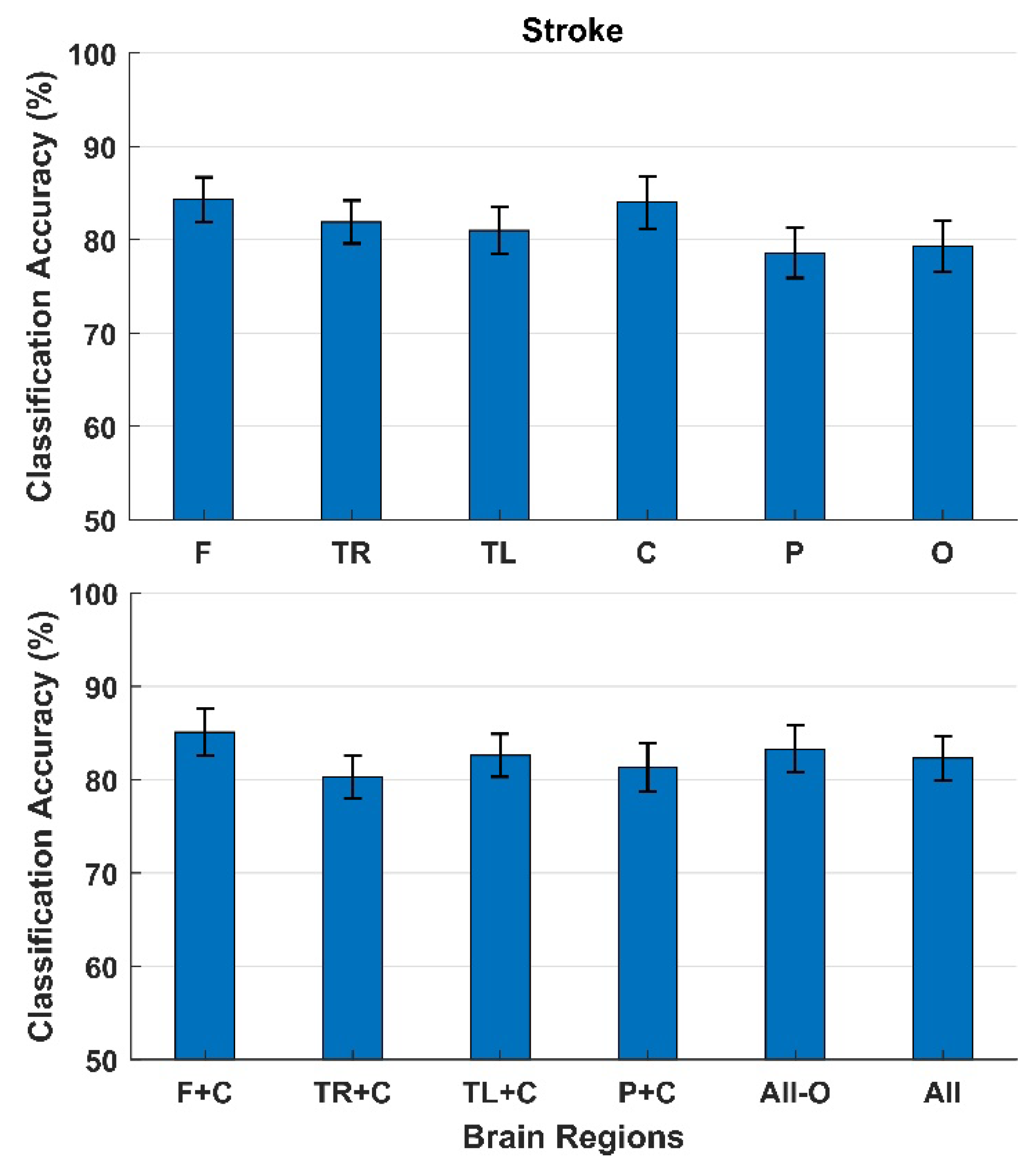
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millán, J.R.; Rupp, R.; Müller-Putz, G.R.; Murray-Smith, R.; Giugliemma, C.; Tangermann, M.; Vidaurre, C.; Cincotti, F.; Kübler, A.; Leeb, R.; et al. Combining brain-computer interfaces and assistive technologies: State-of-the-art and challenges. Front. Neurosci. 2010, 4, 1–15. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Grosse-Wentrup, M.; Mattia, D.; Oweiss, K. Using brain-computer interfaces to induce neural plasticity and restore function. J. Neural Eng. 2011, 8, 025004. [Google Scholar] [CrossRef]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.D.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef]
- Mihajlovic, V.; Grundlehner, B.; Vullers, R.; Penders, J. Wearable, wireless EEG Solutions in daily life applications: What are we missing? IEEE J. Biomed. Health Inform. 2015, 19, 6–21. [Google Scholar] [CrossRef]
- Leeb, R.; Perdikis, S.; Tonin, L.; Biasiucci, A.; Tavella, M.; Creatura, M.; Molina, A.; Al-Khodairy, A.; Carlson, T.; de Millán, J. Transferring brain-computer interfaces beyond the laboratory: Successful application control for motor-disabled users. Artif. Intell. Med. 2013, 59, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chavarriaga, R.; Sobolewski, A.; Millán, J.D.R. Errare machinale est: The use of error-related potentials in brain-machine interfaces. Front. Neurosci. 2014, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gao, L.; Pirogova, E.; Fang, Q. A Review of Error-Related Potential-Based Brain-Computer Interfaces for Motor Impaired People. IEEE Access 2019, 7, 142451–142466. [Google Scholar] [CrossRef]
- Parra, L.C.; Spence, C.D.; Gerson, A.D.; Sajda, P. Response error correction-a demonstration of improved human-machine performance using real-time EEG monitoring. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 173–177. [Google Scholar] [CrossRef]
- Kalaganis, F.P.; Chatzilari, E.; Nikolopoulos, S.; Kompatsiaris, I.; Laskaris, N.A. An error-aware gaze-based keyboard by means of a hybrid BCI system. Sci. Rep. 2018, 8, 13176. [Google Scholar] [CrossRef]
- Cruz, A.; Pires, G.; Nunes, U.J. Double ErrP detection for automatic error correction in an ERP-based BCI speller. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 26, 26–36. [Google Scholar] [CrossRef]
- Kumar, A.; Fang, Q.; Pirogova, E. The influence of psychological and cognitive states on error-related negativity evoked during post-stroke rehabilitation movements. BioMedical Eng. OnLine 2021, 20, 13. [Google Scholar] [CrossRef]
- Kumar, A.; Fang, Q.; Fu, J.; Pirogova, E.; Gu, X. Error-related neural responses recorded by electroencephalography during post-stroke rehabilitation movements. Front. Neurorobotics 2019, 13, 107. [Google Scholar] [CrossRef]
- Usama, N.; Niazi, I.K.; Dremstrup, K.; Jochumsen, M. Detection of Error-Related Potentials in Stroke Patients from EEG Using an Artificial Neural Network. Sensors 2021, 21, 6274. [Google Scholar] [CrossRef]
- Dias, C.L.; Sburlea, A.I.; Breitegger, K.; Wyss, D.; Drescher, H.; Wildburger, R.; Müller-Putz, G.R. Online asynchronous detection of error-related potentials in participants with a spinal cord injury by adapting a pre-trained generic classifier. J. Neural Eng. 2020, 18, 046022. [Google Scholar] [CrossRef]
- Roset, S.A.; Gant, K.; Prasad, A.; Sanchez, J.C. An adaptive brain actuated system for augmenting rehabilitation. Front. Neurosci. 2014, 8, 415. [Google Scholar] [CrossRef][Green Version]
- Spüler, M.; Bensch, M.; Kleih, S.; Rosenstiel, W.; Bogdan, M.; Kübler, A. Online use of error-related potentials in healthy users and people with severe motor impairment increases performance of a P300-BCI. Clin. Neurophysiol. 2012, 123, 1328–1337. [Google Scholar] [CrossRef]
- Milekovic, T.; Ball, T.; Schulze-Bonhage, A.; Aertsen, A.; Mehring, C. Error-related electrocorticographic activity in humans during continuous movements. J. Neural Eng. 2012, 9, 026007. [Google Scholar] [CrossRef]
- Milekovic, T.; Ball, T.; Schulze-Bonhage, A.; Aertsen, A.; Mehring, C. Detection of error related neuronal responses recorded by electrocorticography in humans during continuous movements. PLoS ONE 2013, 8, e55235. [Google Scholar]
- Holroyd, C.B.; Praamstra, P.; Plat, E.; Coles, M.G. Spared error-related potentials in mild to moderate Parkinson’s disease. Neuropsychologia 2002, 40, 2116–2124. [Google Scholar] [CrossRef][Green Version]
- Hakkarainen, E.; Pirilä, S.; Kaartinen, J.; van der Meere, J.J. Error detection and response adjustment in youth with mild spastic cerebral palsy: An event-related brain potential study. J. Child. Neurol. 2013, 28, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Ferrez, P.W.; Millán, J.D.R. Error-related EEG potentials generated during simulated brain-computer interaction. IEEE Trans. Biomed. Eng. 2008, 55, 923–929. [Google Scholar] [CrossRef]
- Chavarriaga, R.; Millán, J.D.R. Learning from EEG error-related potentials in noninvasive brain-computer interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 381–388. [Google Scholar] [CrossRef]
- Dyson, M.; Thomas, E.; Casini, L.; Burle, B. Online extraction and single trial analysis of regions contributing to erroneous feedback detection. Neuroimage 2015, 121, 146–158. [Google Scholar] [CrossRef]
- Shou, G.; Ding, L. Detection of EEG Spatial-Spectral-Temporal Signatures of Errors: A Comparative Study of ICA-Based and Channel-Based Methods. Brain Topogr. 2015, 28, 47–61. [Google Scholar] [CrossRef]
- Shou, G.; Ding, L. EEG-based single-trial detection of errors from multiple error-related brain activity. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Lake Buena Vista, FL, USA, 16–20 August 2016; pp. 2764–2767. [Google Scholar]
- Tong, J.; Lin, Q.; Xiao, R.; Ding, L. Combining multiple features for error detection and its application in brain-computer interface. Biomed. Eng. Online 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Vasios, C.E.; Ventouras, E.M.; Matsopoulos, G.K.; Karanasiou, I.; Asvestas, P.; Uzunoglu, N.K.; van Schie, H.T.; de Bruijn, E.R. Classification of event-related potentials associated with response errors in actors and observers based on autoregressive modeling. Open Med. Inform. J. 2009, 3, 32. [Google Scholar] [CrossRef]
- Ventouras, E.M.; Asvestas, P.; Karanasiou, I.; Matsopoulos, G.K. Classification of Error-Related Negativity (ERN) and Positivity (Pe) potentials using kNN and Support Vector Machines. Comput. Biol. Med. 2011, 41, 98–109. [Google Scholar] [CrossRef]
- Wilson, N.R.; Sarma, D.; Wander, J.D.; Weaver, K.E.; Ojemann, J.G.; Rao, R.P. Cortical topography of error-related high-frequency potentials during erroneous control in a continuous control brain-computer interface. Front. Neurosci. 2019, 13, 502. [Google Scholar] [CrossRef]
- Yazmir, B.; Reiner, M. Neural Signatures of Interface Errors in Remote Agent Manipulation. Neuroscience 2021, in press. [Google Scholar] [CrossRef]
- Yazmir, B.; Reiner, M. I act, therefore I err: EEG correlates of success and failure in a virtual throwing game. Int. J. Psychophysiol. 2017, 122, 32–41. [Google Scholar] [CrossRef]
- Yazmir, B.; Reiner, M. Neural correlates of user-initiated motor success and failure—A brain-computer interface perspective. Neuroscience 2018, 378, 100–112. [Google Scholar] [CrossRef]
- Usama, N.; Leerskov, K.K.; Niazi, I.K.; Dremstrup, K.; Jochumsen, M. Classification of error-related potentials from single-trial EEG in association with executed and imagined movements: A feature and classifier investigation. Med. Biol. Eng. Comput. 2020, 58, 2699–2710. [Google Scholar] [CrossRef]
- Hoffmann, S.; Falkenstein, M. Predictive information processing in the brain: Errors and response monitoring. Int. J. Psychophysiol. 2012, 83, 208–212. [Google Scholar] [CrossRef]
- Müller-Putz, G.R.; Scherer, R.; Brunner, C.; Leeb, R.; Pfurtscheller, G. Better than random? A closer look on BCI results. Int. J. Bioelectromagn. 2008, 10, 52–55. [Google Scholar]
- Iturrate, I.; Montesano, L.; Minguez, J. Task-dependent signal variations in EEG error-related potentials for brain-computer interfaces. J. Neural Eng. 2013, 10, 026024. [Google Scholar] [CrossRef]
- Omedes, J.; Iturrate, I.; Minguez, J.; Montesano, L. Analysis and asynchronous detection of gradually unfolding errors during monitoring tasks. J. Neural Eng. 2015, 12, 056001. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, S.; Zheng, D.; Dai, M. Study on the effect of different electrode channel combinations of motor imagery EEG signals on classification accuracy. J. Eng. 2019, 2019, 8641–8645. [Google Scholar] [CrossRef]
- Buttfield, A.; Ferrez, P.W.; Millan, J.R. Towards a robust BCI: Error potentials and online learning. Neural Syst. Rehabil. Eng. 2006, 14, 164–168. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Konar, A.; Tibarewala, D.N.; Hayashibe, M. A generic transferable EEG decoder for online detection of error potential in target selection. Front. Neurosci. 2017, 11, 226. [Google Scholar] [CrossRef]
- Omedes, J.; Schwarz, A.; Müller-Putz, G.R.; Montesano, L. Factors that affect error potentials during a grasping task: Toward a hybrid natural movement decoding BCI. J. Neural Eng. 2018, 15, 046023. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Blankertz, B.; Treder, M.S. Online detection of error-related potentials boosts the performance of mental typewriters. BMC Neurosci. 2012, 13, 19. [Google Scholar] [CrossRef]
- Spüler, M.; Niethammer, C. Error-related potentials during continuous feedback: Using EEG to detect errors of different type and severity. Front. Hum. Neurosci. 2015, 9, 155. [Google Scholar]
- Gao, C.; Li, Z.; Ora, H.; Miyake, Y. Improving error related potential classification by using generative adversarial networks and deep convolutional neural networks. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Korea, 16–19 December 2020; pp. 2468–2476. [Google Scholar]
- Chavarriaga, R.; Khaliliardali, Z.; Gheorghe, L.; Iturrate, I.; Millán, J.D.R. EEG-based decoding of error-related brain activity in a real-world driving task. J. Neural Eng. 2015, 12, 066028. [Google Scholar]
- Aydarkhanov, R.; Ušćumlić, M.; Chavarriaga, R.; Gheorghe, L.; del Millán, J.R. Spatial covariance improves BCI performance for late ERPs components with high temporal variability. J. Neural Eng. 2020, 17, 036030. [Google Scholar] [CrossRef]
- Abu-Alqumsan, M.; Kapeller, C.; Hintermüller, C.; Guger, C.; Peer, A. Invariance and variability in interaction error-related potentials and their consequences for classification. J. Neural Eng. 2017, 14, 066015. [Google Scholar] [CrossRef]
- Iturrate, I.; Chavarriaga, R.; Montesano, L.; Minguez, J.; Millán, J. Latency correction of event-related potentials between different experimental protocols. J. Neural Eng. 2014, 11, 036005. [Google Scholar] [CrossRef]
- Schönleitner, F.M.; Otter, L.; Ehrlich, S.K.; Cheng, G. Calibration-Free Error-Related Potential Decoding with Adaptive Subject-Independent Models: A Comparative Study. IEEE Trans. Med. Robot. Bionics 2020, 2, 399–409. [Google Scholar] [CrossRef]
- Kim, S.K.; Kirchner, E.A. Handling Few Training Data: Classifier Transfer Between Different Types of Error-Related Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Pezzetta, R.; Nicolardi, V.; Tidoni, E.; Aglioti, S.M. Error, rather than its probability, elicits specific electrocortical signatures: A combined EEG-immersive virtual reality study of action observation. J. Neurophysiol. 2018, 120, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Fan, Z.; Rehman, A.U.; Li, G.; Xiao, G. Motor imagery EEG signals classification based on mode amplitude and frequency components using empirical wavelet transform. IEEE Access 2019, 7, 127678–127692. [Google Scholar] [CrossRef]
- Kevric, J.; Subasi, A. Comparison of signal decomposition methods in classification of EEG signals for motor-imagery BCI system. Biomed. Signal Processing Control. 2017, 31, 398–406. [Google Scholar] [CrossRef]
- Sakhavi, S.; Guan, C.; Yan, S. Parallel convolutional-linear neural network for motor imagery classification. In Proceedings of the 2015 23rd European Signal Processing Conference (EUSIPCO), IEEE, Nice, France, 31 August–4 September 2015; pp. 2736–2740. [Google Scholar]
- Sadiq, M.T.; Yu, X.; Yuan, Z. Exploiting dimensionality reduction and neural network techniques for the development of expert brain-computer interfaces. Expert Syst. Appl. 2021, 164, 114031. [Google Scholar] [CrossRef]
- Nijboer, F.; Plass-Oude Bos, D.; Blokland, Y.; van Wijk, R.; Farquhar, J. Design requirements and potential target users for brain-computer interfaces—Recommendations from rehabilitation professionals. Brain-Comput. Interfaces 2014, 1, 50–61. [Google Scholar] [CrossRef]
- Jochumsen, M.; Knoche, H.; Kidmose, P.; Kjær, T.W.; Dinesen, B.I. Evaluation of EEG Headset Mounting for Brain-Computer Interface-Based Stroke Rehabilitation by Patients, Therapists, and Relatives. Front. Hum. Neurosci. 2020, 14, 13. [Google Scholar] [CrossRef]
| Participant | Gender | Age (Years) | Diagnose | GMFCS |
|---|---|---|---|---|
| 01 | F | 12 | Diplegia | II |
| 02 | F | 10 | Diplegia | II |
| 03 | F | 10 | Hemiplegia-right | II |
| 04 | M | 16 | Hemiplegia-left | II |
| 05 | M | 11 | Hemiplegia-left | I |
| 06 | M | 9 | Hemiplegia-right | II |
| 07 | F | 14 | Hemiplegia-right | II |
| 08 | M | 12 | Hemiplegia-right | III |
| 09 | M | 13 | Diplegia | III |
| 10 | M | 15 | Diplegia | III |
| Participant | Gender | Age (Years) | Time Since Amputation (Years) | Amputation Level | Amputation Side |
|---|---|---|---|---|---|
| 01 | M | 13 | 3 | Hip disarticulation | Left |
| 02 | F | 45 | 2 | Transfemoral | Left |
| 03 | M | 32 | 5 | Wrist disarticulation | Right |
| 04 | M | 27 | 1 | Transfemoral | Right |
| 05 | M | 30 | 2 | Shoulder disarticulation | Left |
| 06 | M | 32 | 5 | Transcredial | Right |
| 07 | M | 53 | 7 | Knee disarticulation | Right |
| 08 | M | 12 | 5 | Hip disarticluation | Left |
| Participant | Gender | Age (Years) | Affected Side | Type of Stroke | Time Since Injury (Days) | Bruunstrom Stage |
|---|---|---|---|---|---|---|
| 01 | M | 48 | Right | Haemorrhage | 91 | II |
| 02 | M | 55 | Right | Ischemic | 172 | V |
| 03 | M | 41 | Left | Ischemic | 70 | III |
| 04 | M | 50 | Left | Haemorrhage | 90 | III |
| 05 | M | 57 | Right | Haemorrhage | 52 | V |
| 06 | M | 52 | Right | Ischemic | 188 | V |
| 07 | M | 24 | Left | Haemorrhage | 180 | IV |
| 08 | F | 32 | Left | Ischemic | 25 | II |
| 09 | F | 26 | Left | Haemorrhage | 20 | I |
| 10 | M | 60 | Right | Ischemic | 87 | IV |
| 11 | M | 54 | Left | Ischemic | 220 | VII |
| 12 | M | 46 | Left | Ischemic | 42 | III |
| 13 | M | 58 | Right | Ischemic | 84 | III |
| 14 | M | 37 | Right | Haemorrhage | 36 | II |
| 15 | M | 42 | Left | Haemorrhage | 118 | V |
| 16 | M | 24 | Left | Haemorrhage | 45 | IV |
| 17 | F | 26 | Right | Ischemic | 12 | I |
| 18 | M | 62 | Right | Haemorrhage | 118 | III |
| 19 | M | 30 | Right | Ischemic | 60 | III |
| 20 | F | 53 | Left | Ischemic | 93 | IV |
| 21 | F | 38 | Right | Haemorrhage | 45 | VI |
| 22 | F | 28 | Left | Ischemic | 27 | V |
| 23 | M | 45 | Left | Ischemic | 90 | IV |
| 24 | M | 35 | Left | Haemorrhage | 17 | II |
| 25 | M | 45 | Right | Haemorrhage | 280 | VI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usama, N.; Niazi, I.K.; Dremstrup, K.; Jochumsen, M. Single-Trial Classification of Error-Related Potentials in People with Motor Disabilities: A Study in Cerebral Palsy, Stroke, and Amputees. Sensors 2022, 22, 1676. https://doi.org/10.3390/s22041676
Usama N, Niazi IK, Dremstrup K, Jochumsen M. Single-Trial Classification of Error-Related Potentials in People with Motor Disabilities: A Study in Cerebral Palsy, Stroke, and Amputees. Sensors. 2022; 22(4):1676. https://doi.org/10.3390/s22041676
Chicago/Turabian StyleUsama, Nayab, Imran Khan Niazi, Kim Dremstrup, and Mads Jochumsen. 2022. "Single-Trial Classification of Error-Related Potentials in People with Motor Disabilities: A Study in Cerebral Palsy, Stroke, and Amputees" Sensors 22, no. 4: 1676. https://doi.org/10.3390/s22041676
APA StyleUsama, N., Niazi, I. K., Dremstrup, K., & Jochumsen, M. (2022). Single-Trial Classification of Error-Related Potentials in People with Motor Disabilities: A Study in Cerebral Palsy, Stroke, and Amputees. Sensors, 22(4), 1676. https://doi.org/10.3390/s22041676







