Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
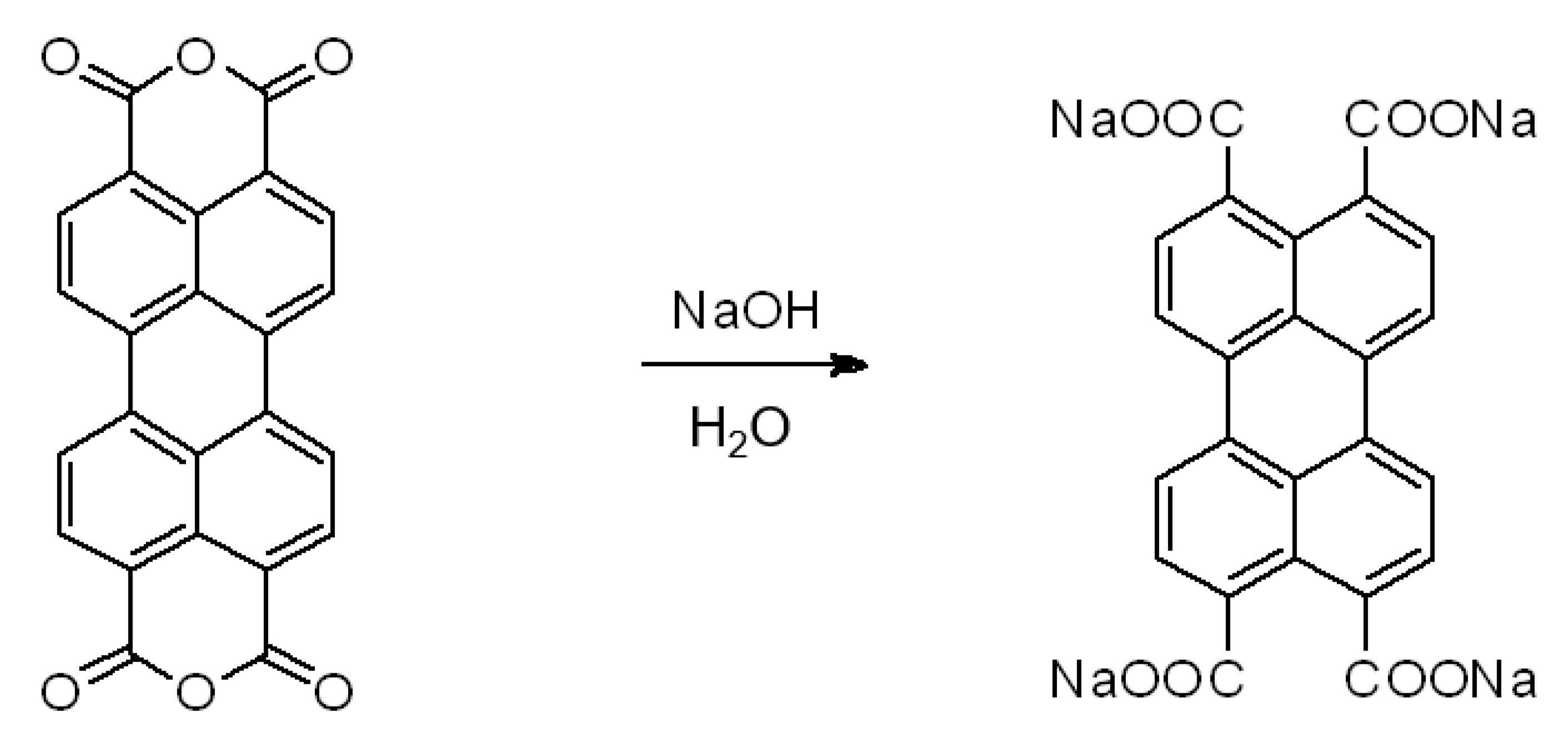
2.1. Synthesis of Sodium-Based Metal–Organic Framework
2.2. Preparation of the Ink and Spray Coating
2.3. Contacting of the Films
2.4. Single Crystal X-ray Diffraction
2.5. Powder XR Diffraction
2.6. X-ray Photoelectron Spectroscopy
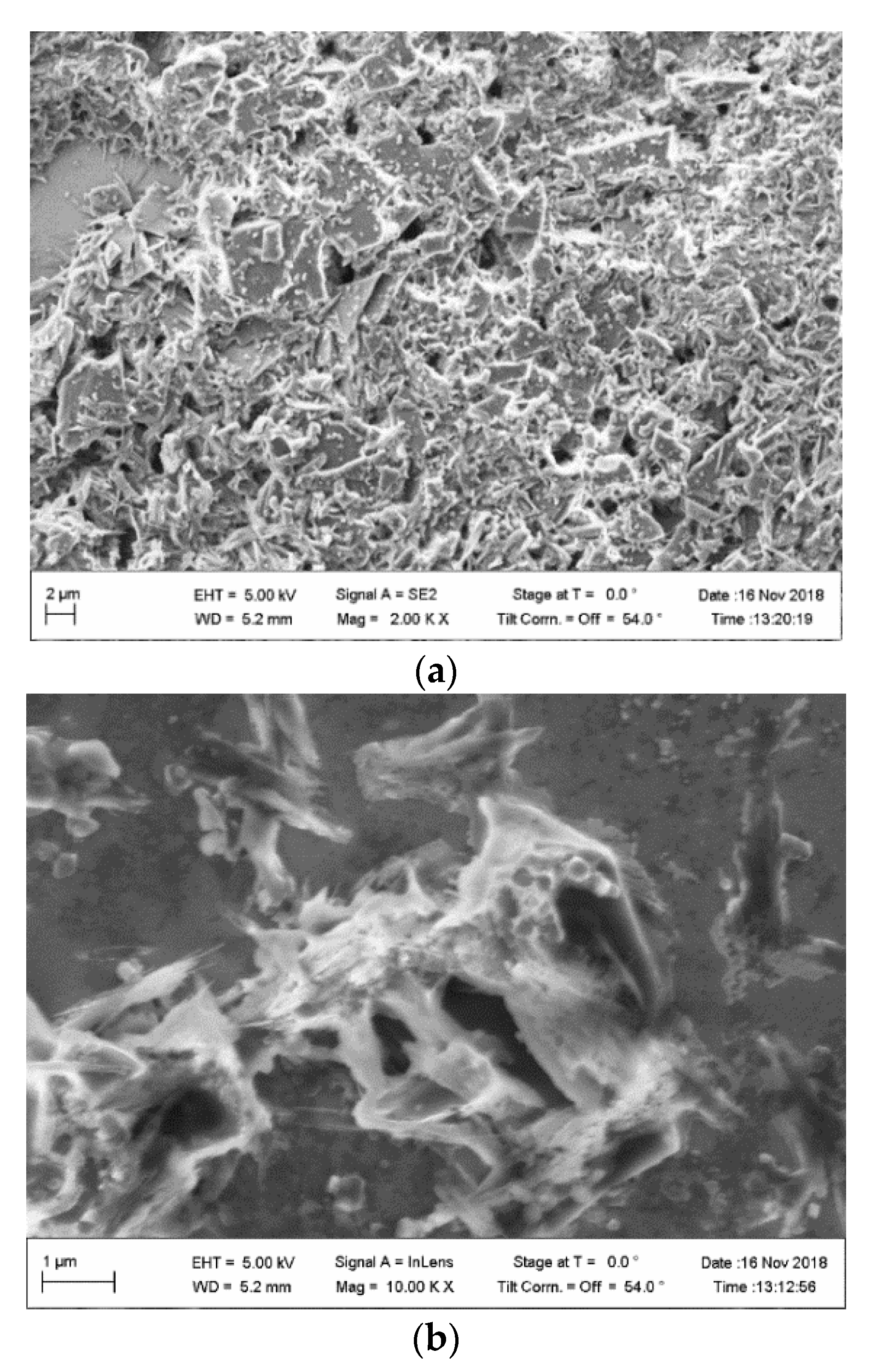
2.7. SEM Imaging
2.8. Device Characterization
3. Results
3.1. Structural Description of the CP
3.2. Characterization of the CP
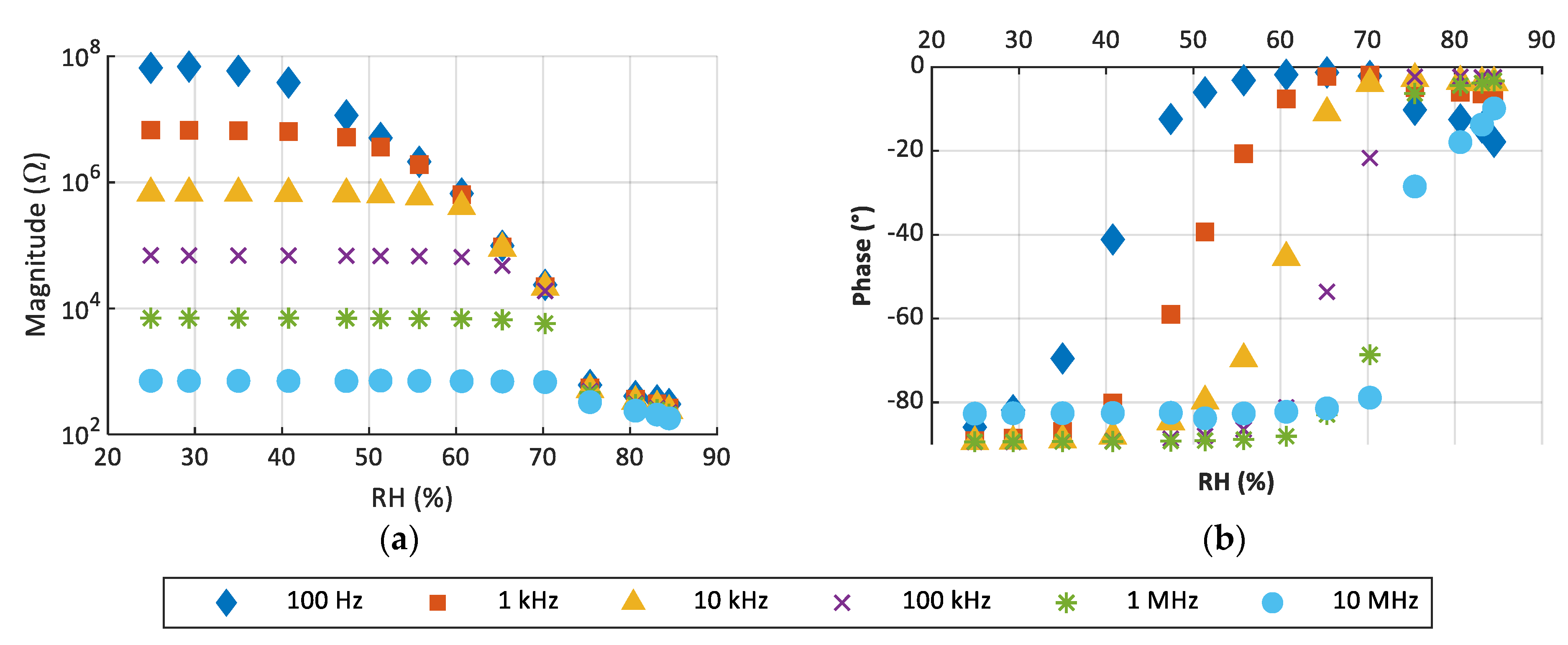
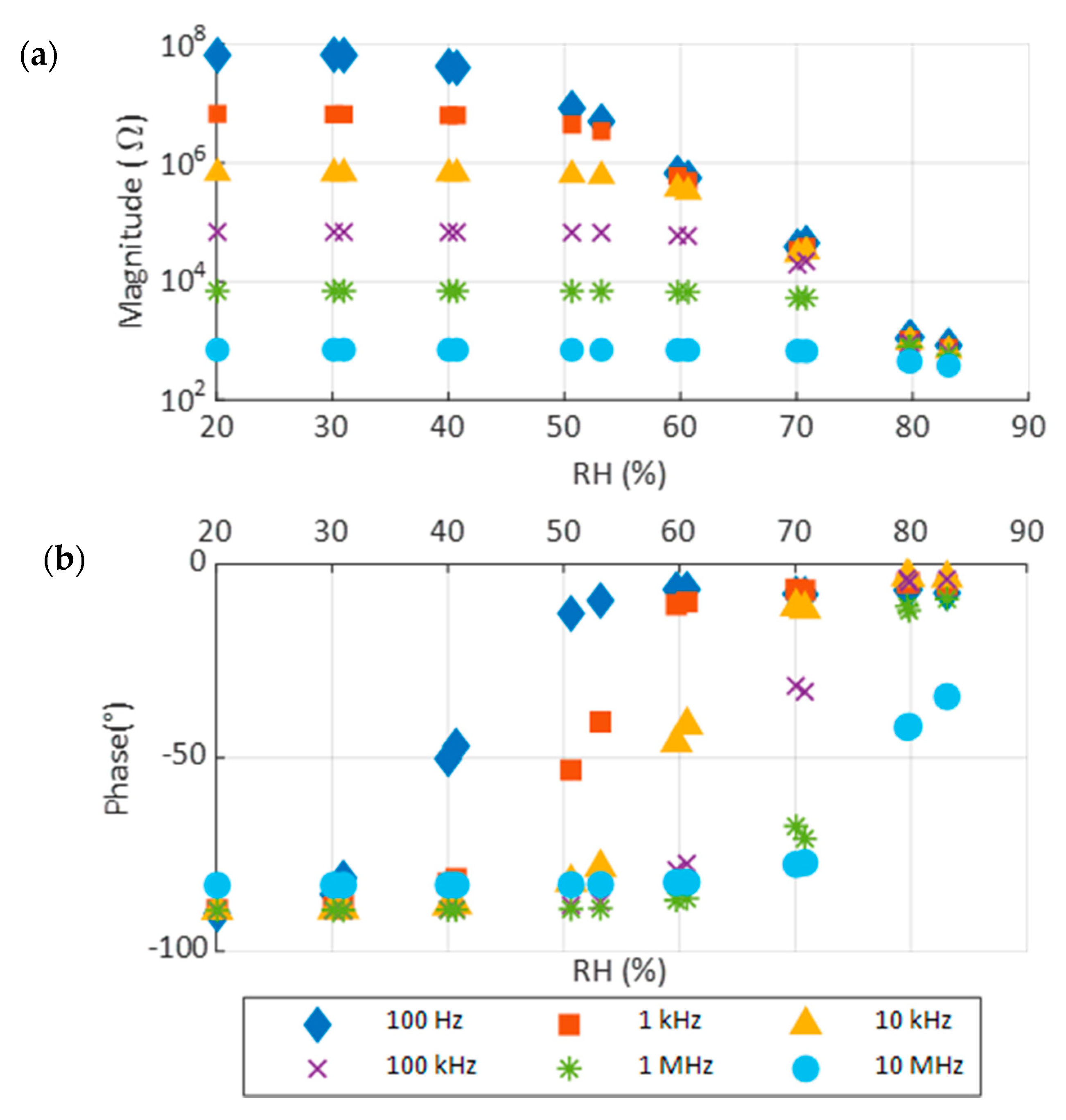
3.3. Response to Moisture Content
3.4. Response to Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Traversa, E. Ceramic Sensors for Humidity Detection: The State-of-the-Art and Future Developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sen. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Kim, J.H.; Park, S.-Y.; Kang, J.-H.; Kim, S.-J.; Choi, Y.-B.; Shin, U.S. Carbon Nanotubes Immobilized on Gold Electrode as an Electrochemical Humidity Sensor. Sens. Actuators B Chem. 2019, 300, 127049. [Google Scholar] [CrossRef]
- Lyuleeva, A.; Helbich, T.; Bobinger, M.; Rieger, B.; Becherer, M.; Lugli, P.; Rivadeneyra, A. Functionalized and Oxidized Silicon Nanosheets: Customized Design for Enhanced Sensitivity towards Relative Humidity. Sens. Actuators B Chem. 2019, 283, 451–457. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–Organic Framework Materials as Catalysts. Chem. Soc. Rev. 2009, 38, 1450. [Google Scholar] [CrossRef]
- Cepeda, J.; Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; Luque, A.; Wright, P.A.; Sneddon, S.; Ashbrook, S.E. Exploiting Synthetic Conditions to Promote Structural Diversity within the Scandium(III)/Pyrimidine-4,6-Dicarboxylate System. Cryst. Growth Des. 2015, 15, 2352–2363. [Google Scholar] [CrossRef]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A Supermolecular Building Approach for the Design and Construction of Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Jing, P.; Yan, N.; Hilbers, M.; Zhang, H.; Rothenberg, G.; Tanase, S. Dual-Mode Humidity Detection Using a Lanthanide-Based Metal–Organic Framework: Towards Multifunctional Humidity Sensors. Chem. Commun. 2017, 53, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Stangl, J.M.; Dietrich, D.; Sedykh, A.E.; Janiak, C.; Müller-Buschbaum, K. Luminescent MOF Polymer Mixed Matrix Membranes for Humidity Sensing in Real Status Analysis. J. Mater. Chem. C 2018, 6, 9248–9257. [Google Scholar] [CrossRef]
- Arazoe, H.; Miyajima, D.; Akaike, K.; Araoka, F.; Sato, E.; Hikima, T.; Kawamoto, M.; Aida, T. An Autonomous Actuator Driven by Fluctuations in Ambient Humidity. Nat. Mater. 2016, 15, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Chen, Y.; Sun, J.; Zhang, H.; Guo, D.; Zhang, H.; Li, Q.; Wang, T.; Wan, Q. Humidity Sensing Properties of a Single Sb Doped SnO2 Nanowire Field Effect Transistor. Sens. Actuators B Chem. 2013, 186, 78–83. [Google Scholar] [CrossRef]
- Lee, S.P.; Park, K.-J. Humidity Sensitive Field Effect Transistors. Sens. Actuators B Chem. 1996, 35, 80–84. [Google Scholar] [CrossRef]
- Subbarao, N.V.V.; Gedda, M.; Iyer, P.K.; Goswami, D.K. Enhanced Environmental Stability Induced by Effective Polarization of a Polar Dielectric Layer in a Trilayer Dielectric System of Organic Field-Effect Transistors: A Quantitative Study. ACS Appl. Mater. Interfaces 2015, 7, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, G.; Xue, Z.; Ge, F.; Zhang, G.; Lu, H.; Qiu, L. Organic Field-Effect Transistors with Macroporous Semiconductor Films as High-Performance Humidity Sensors. ACS Appl. Mater. Interfaces 2017, 9, 14974–14982. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Q.; Abdullah, S.M.; Azmer, M.I.; Najeeb, M.A.; Qadir, K.W.; Sulaiman, K. Influence of Relative Humidity on the Electrical Response of PEDOT:PSS Based Organic Field-Effect Transistor. Sens. Actuators B Chem. 2018, 255, 2652–2656. [Google Scholar] [CrossRef]
- Subbarao, N.V.V.; Gedda, M.; Iyer, P.K.; Goswami, D.K. Organic Field-Effect Transistors as High Performance Humidity Sensors with Rapid Response, Recovery Time and Remarkable Ambient Stability. Org. Electron. 2016, 32, 169–178. [Google Scholar] [CrossRef]
- Weiss, A.; Reimer, N.; Stock, N.; Tiemann, M.; Wagner, T. Surface-Modified CAU-10 MOF Materials as Humidity Sensors: Impedance Spectroscopic Study on Water Uptake. Phys. Chem. Chem. Phys. 2015, 17, 21634–21642. [Google Scholar] [CrossRef] [Green Version]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
- Seco, J.M.; San Sebastian, E.; Cepeda, J.; Biel, B.; Salinas-Castillo, A.; Fernández, B.; Morales, D.P.; Bobinger, M.; Gómez-Ruiz, S.; Loghin, F.C.; et al. A Potassium Metal-Organic Framework Based on Perylene-3,4,9,10-Tetracarboxylate as Sensing Layer for Humidity Actuators. Sci. Rep. 2018, 8, 14414. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Salmeron, J.F.; Becherer, M.; Lugli, P.; Rivadeneyra, A. Screen-Printed Chipless Wireless Temperature Sensor. IEEE Sens. J. 2019, 19, 12011–12015. [Google Scholar] [CrossRef]
- Bruker. V2019.1; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Herrera-Gomez, A.; Bravo-Sanchez, M.; Ceballos-Sanchez, O.; Vazquez-Lepe, M.O. Practical Methods for Background Subtraction in Photoemission Spectra: Practical Methods for Background Subtraction in Photoemission Spectra. Surf. Interface Anal. 2014, 46, 897–905. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Agudo, M.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. Design and Characterization of a Low Thermal Drift Capacitive Humidity Sensor by Inkjet-Printing. Sens. Actuators B Chem. 2014, 195, 123–131. [Google Scholar] [CrossRef]
- Briggs, D.; Marbrow, R.A.; Lambert, R.M. An XPS and UPS Study of the Interaction of Oxygen with Sodium-Dosed Ag(110). Surf. Sci. 1977, 65, 314–324. [Google Scholar] [CrossRef]
- Seifert, M.; Vargas, J.E.B.; Bobinger, M.; Sachsenhauser, M.; Cummings, A.W.; Roche, S.; Garrido, J.A. Role of Grain Boundaries in Tailoring Electronic Properties of Polycrystalline Graphene by Chemical Functionalization. 2D Mater. 2015, 2, 024008. [Google Scholar] [CrossRef]
- Bobinger, M.; Mock, J.; La Torraca, P.; Becherer, M.; Lugli, P.; Larcher, L. Tailoring the Aqueous Synthesis and Deposition of Copper Nanowires for Transparent Electrodes and Heaters. Adv. Mater. Interfaces 2017, 4, 1700568. [Google Scholar] [CrossRef]



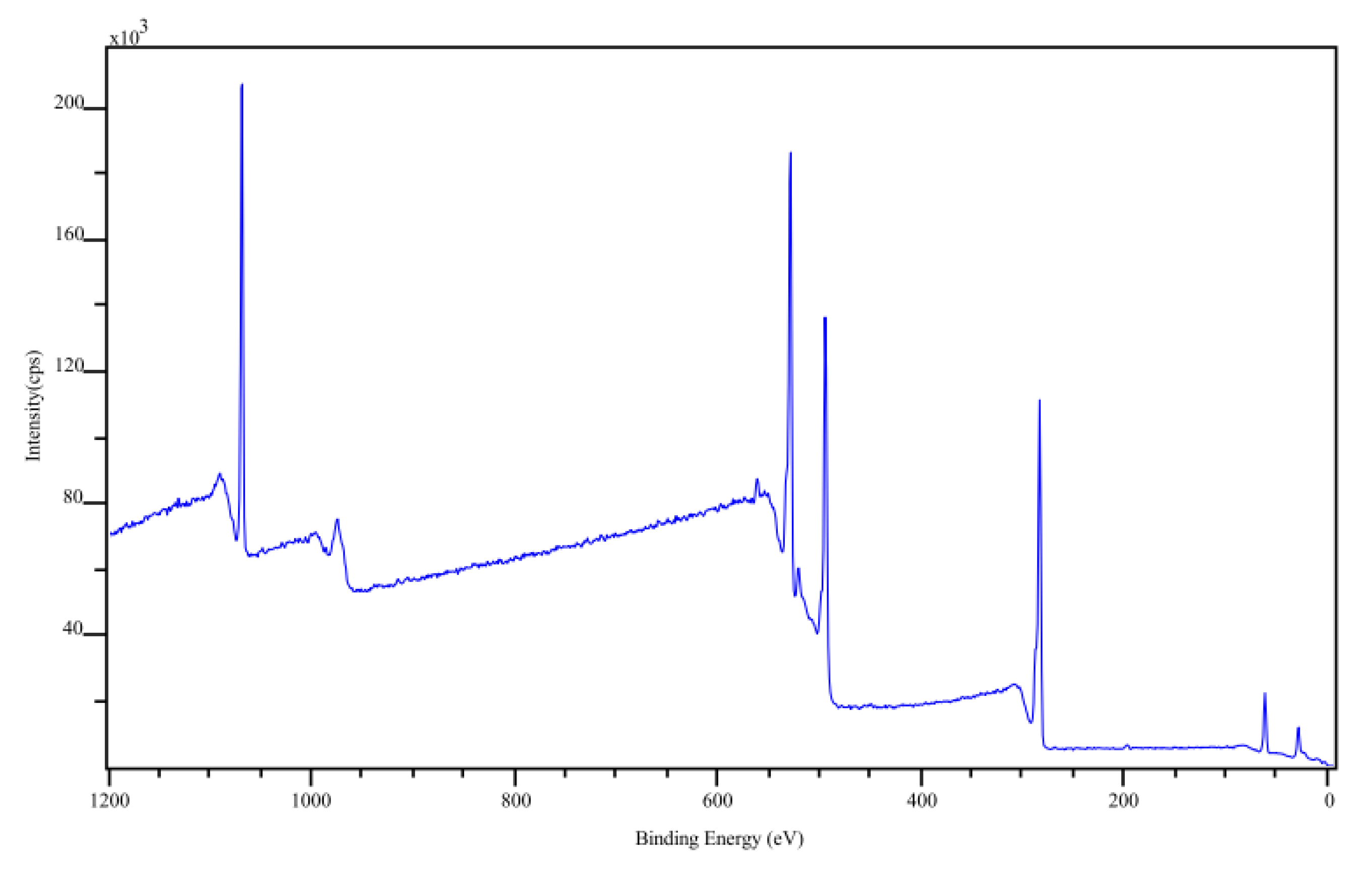
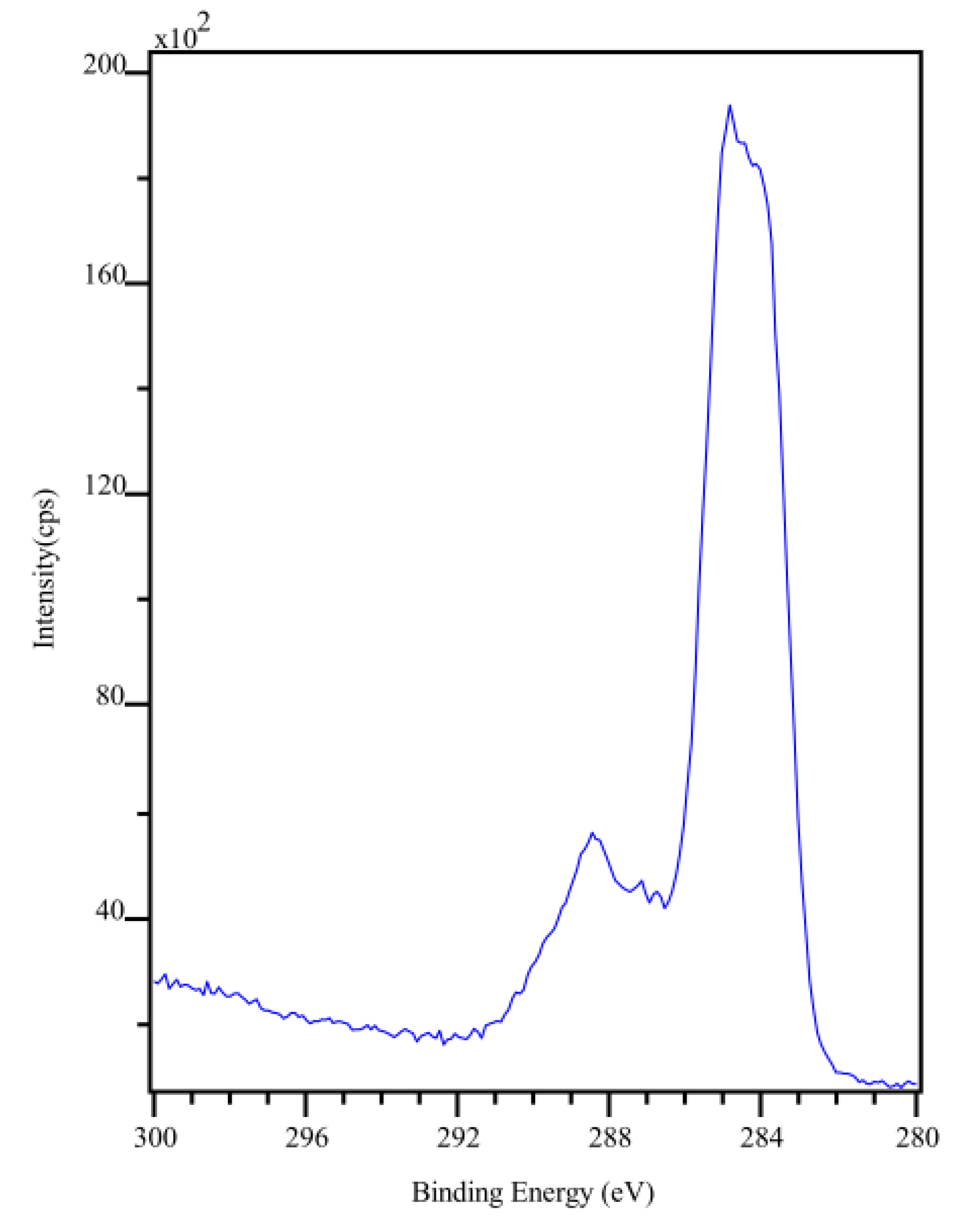


| Element | Atomic Mass | Theoretical Atomic Conc. | Experimental Atomic Conc. |
|---|---|---|---|
| C | 12 | 67% | 60% |
| O | 16 | 22% | 28% |
| Na | 22 | 11% | 12% |
| Frequency | Sensitivity (Ω/%RH) | Range of RH (%) |
|---|---|---|
| 100 Hz | −2.89 × 106 | 29–51 |
| 1 kHz | −2.91 × 105 | 41–61 |
| 10 kHz | −3.90 × 105 | 51–65 |
| 100 kHz | −4.38 × 103 | 61–75 |
| 1 MHz | −610 | 65–75 |
| 10 MHz | −501 | 71–83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, A.; Toral, V.; Quílez del Moral, J.F.; Galisteo Pretel, A.; Morales, D.P.; Salinas-Castillo, A.; Cepeda, J.; Choquesillo-Lazarte, D.; Bobinger, M.; Salmerón, J.F.; et al. Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions. Sensors 2022, 22, 1664. https://doi.org/10.3390/s22041664
García-García A, Toral V, Quílez del Moral JF, Galisteo Pretel A, Morales DP, Salinas-Castillo A, Cepeda J, Choquesillo-Lazarte D, Bobinger M, Salmerón JF, et al. Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions. Sensors. 2022; 22(4):1664. https://doi.org/10.3390/s22041664
Chicago/Turabian StyleGarcía-García, Amalia, Víctor Toral, José F. Quílez del Moral, Alberto Galisteo Pretel, Diego P. Morales, Alfonso Salinas-Castillo, Javier Cepeda, Duane Choquesillo-Lazarte, Marco Bobinger, José F. Salmerón, and et al. 2022. "Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions" Sensors 22, no. 4: 1664. https://doi.org/10.3390/s22041664
APA StyleGarcía-García, A., Toral, V., Quílez del Moral, J. F., Galisteo Pretel, A., Morales, D. P., Salinas-Castillo, A., Cepeda, J., Choquesillo-Lazarte, D., Bobinger, M., Salmerón, J. F., Rivadeneyra, A., & Rodríguez-Diéguez, A. (2022). Selectivity of Relative Humidity Using a CP Based on S-Block Metal Ions. Sensors, 22(4), 1664. https://doi.org/10.3390/s22041664












