Near-Infrared Sensors for Onsite and Noninvasive Quantification of Macronutrients in Breast Milk
Abstract
:1. Introduction
2. Materials and Methods
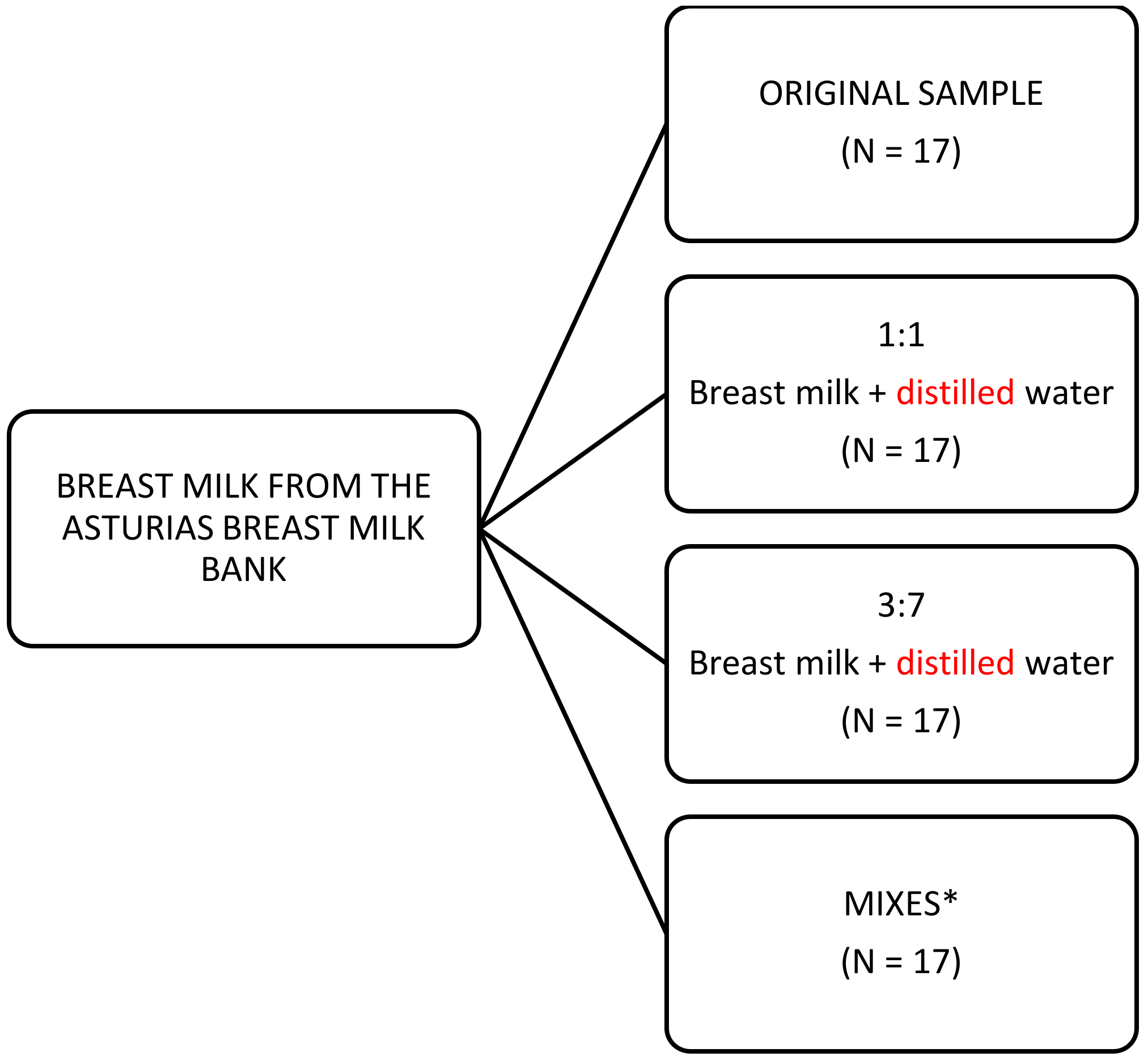
2.1. Milk Samples
2.2. NIRS Spectra Collection
2.3. Data Processing
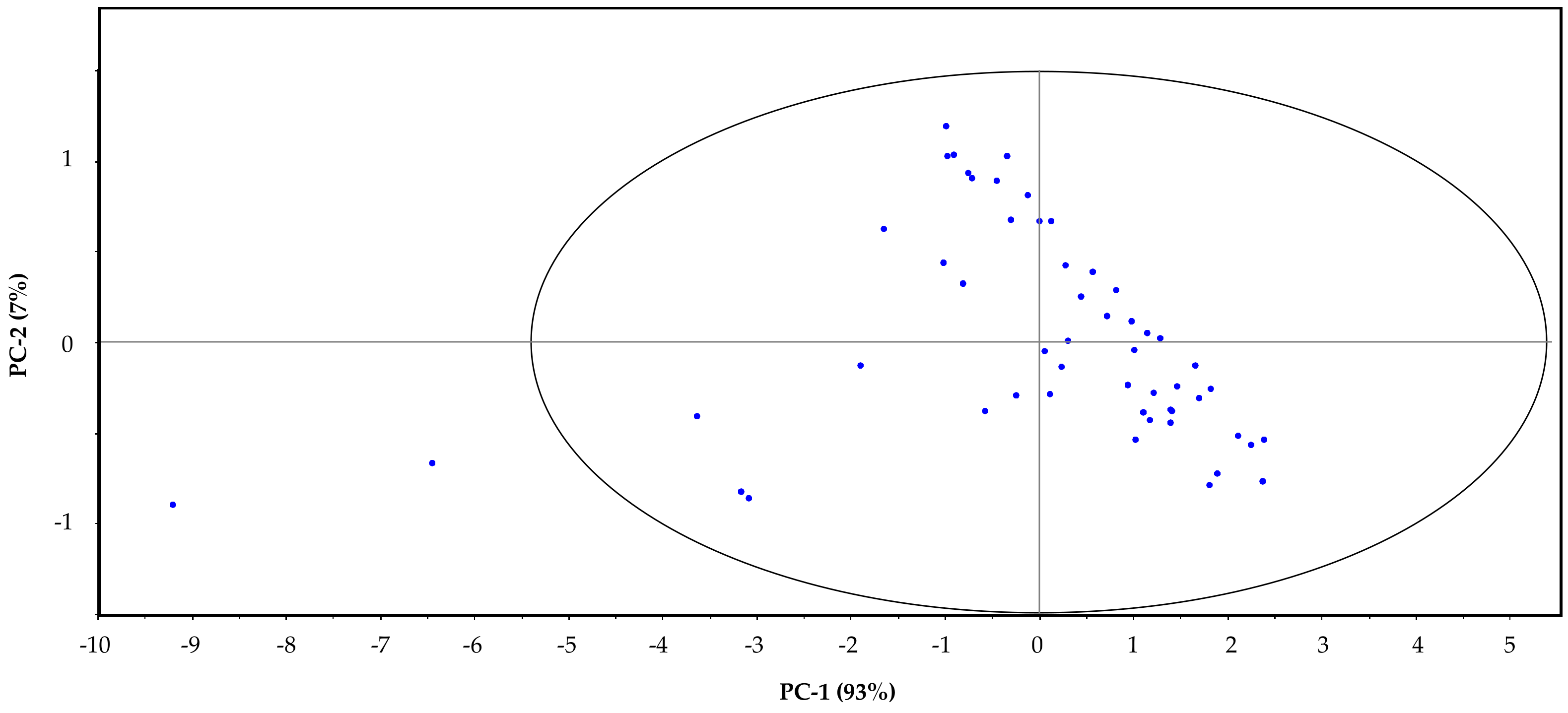
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leghi, G.E.; Middleton, P.F.; Muhlhausler, B.S. A methodological approach to identify the most reliable human milk collection method for compositional analysis: A systematic review protocol. Syst. Rev. 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.T.; Schroder, P.E.; Poindexter, B.B. Macronutrient analysis of target-pooled donor breast milk and corresponding growth in very low birth weight infants. Nutrients 2019, 11, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef]
- Fusch, G.; Kwan, C.; Kotrri, G.; Fusch, C. “Bed Side” human milk analysis in the neonatal intensive care unit: A systematic review. Clin. Perinatol. 2017, 44, 209–267. [Google Scholar] [CrossRef] [PubMed]
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR Spectroscopy to Agricultural Products. In In Handbook of Near Infrared Analysis, 1st ed.; Burns, D.A., Ciurczak, E.W., Eds.; Practical Spectroscopy A Series; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; Volume 13, pp. 347–387. [Google Scholar]
- Bertrand, D.; Dufour, E. Infrared Spectroscopy and Its Analytical Applications; Editions Tec & Doc: Paris, France, 2000. [Google Scholar]
- Murray, I. Forage analysis by near infra-red spectroscopy. In Sward Measurement Handbook; Davies, A., Baker, R.D., Grant, S.A., Laidlaw, A.S., Eds.; NIR Publications: Chichester, UK, 1993; pp. 155–177. [Google Scholar]
- Muncan, J.; Tei, K.; Tsenkova, R. Real Time Monitoring of Yogurt Fermentation Process by Aquaphotomics Near-Infrared Spectroscopy. Sensors 2021, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publ.: Chichester, UK, 2007. [Google Scholar]
- Osborne, B.G.; Fearn, T.; Hindle, P.H. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis, 2nd ed; Longman Scientific and Technica: Essex, UK, 1993; pp. 11–35. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Routine Operation, Calibration Development and Network System Management Manual for Near Infrared Instruments; NIR Systems, Inc.: Silver Spring, MD, USA, 1995; PN IS-0119. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Analysis of Agriculture and Food Products by Near Infrared Reflectance Spectroscopy; Infrasoft International: Port Matilda, PE, USA, 1993; 116p. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI way. In Near infrared Spectroscopy: The Future Waves; Davis, A.M.C., Williams, P., Eds.; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]
- Dos Santos, V.J.; Baqueta, M.R.; Março, P.H.; Valderrama, P. Human Milk Lactation Phases Evaluation Through Handheld Near-Infrared Spectroscopy and Multivariate Classification. Food Anal. Methods 2021, 14, 873–882. [Google Scholar] [CrossRef]
- De la Roza-Delgado, B.; Garrido-Varo, A.; Soldado, A.; González, A.; Cuevas, M.; Maroto, F.; Perez-Marín, D. Matching portable NIRS instruments for in situ monitoring indicators of milk composition. Food Control 2017, 76, 74–81. [Google Scholar] [CrossRef]
- Celia, K.; Fusch, G.; Rochow, N.; Fusch, C. Milk analysis using milk analyzers in a standardized setting (MAMAS) study: A multicentre quality initiative. Clin. Nutr. 2020, 39, 2121–2128. [Google Scholar] [CrossRef]
- Windham, W.R.; Mertens, D.R.; Barton, F.E. Protocol for NIRS calibration: Sample selection and equation development and validation. In Near Infrared Reflectance Spectroscopy (NIRS): Analysis of Forage Quality; US Department of Agriculture: Springfield, MA, USA, 1989; pp. 96–97. [Google Scholar]
- Czosnykowska-Łukacka, M.; Królak-Olejnik, B. Orczyk-Pawiłowicz, M. Breast milk macronutrient components in prolonged lactation. Nutrients 2018, 10, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, H.; Workman, J. Statistics in Spectroscopy; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Camo Analytics Welcome. AspenTech. Available online: https://www.aspentech.com/en/acquisition/camo-analytics (accessed on 3 November 2021).
- Thompson, M.; Wood, R. Using uncertainty functions to predict and specify the performance of analytical methods. Accred. Qual. Assur. 2006, 10, 471–478. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Koletzko, B.; Rodriguez-Palmero, M. Polyunsaturated fatty acids in human milk and their role in early infant development. J. Mammary Gland Biol. Neoplasia 1999, 4, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Savenije, B.; Geesink, G.H.; Van der Palen, J.G.P.; Hemke, G. Prediction of prok quality using visible/near-infrared reflectance spectroscopy. Prediction of pork quality using visible/near-infrared reflectance spectroscopy. Meat. Sci. 2006, 73, 18. [Google Scholar] [CrossRef] [PubMed]
- Bellon-Maurel, V.; Fernández-Ahumada, E.; Palagos, B.; Roger, J.M.; McBratney, A. Critical review of chemometric indicators commonly used for assesing the quality of the prediction of soil attributes. Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Corvaglia, L.; Battistini, B.; Paoletti, V.; Aceti, A.; Faldella, G.; Capretti, M.G. Near-infrared reflectance analysis to evaluate the nitrogen and fat content of human milk in neonatal intensive care units. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F372–F375. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.; Kim, J. Human milk macronutrient analysis using point-of-care near-infrared spectrophotometry. J. Perinatol. 2011, 31, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusch, G.; Rochow, N.; Choi, A.; Fusch, S.; Poeschl, S.; Ubah, A.O.; Lee, S.Y.; Raja, P.; Fusch, C. Rapid measurement of macronutrients in breast milk: How reliable are infrared milk analyzers? Clin. Nutr. 2015, 34, 465–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Calibration Set (N = 53) | Validation Set (N = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Max | Min | SD | Mean | Max | Min | SD | |
| Fat 1 | 2.39 | 5.30 | 0.51 | 1.25 | 2.60 | 4.60 | 0.57 | 1.58 |
| CP 1 | 0.87 | 2.50 | 0.27 | 0.50 | 0.78 | 1.70 | 0.33 | 0.39 |
| RP 1 | 0.72 | 2.00 | 0.21 | 0.40 | 0.69 | 1.40 | 0.27 | 0.34 |
| CH 1 | 5.80 | 8.80 | 2.34 | 2.56 | 5.58 | 8.40 | 2.31 | 2.63 |
| Energy 2 | 49.30 | 86.00 | 15.60 | 22.06 | 50.09 | 81.00 | 15.90 | 26.36 |
| TS 1 | 7.42 | 14.60 | 3.27 | 4.02 | 8.04 | 14.50 | 3.27 | 4.17 |
| Math Pre-Treatment | Parameter | R2 | SEC | r2 | SECV |
|---|---|---|---|---|---|
| 0 0 0 0 | Fat | 0.910 | 0.37 | 0.841 | 0.51 |
| CP | 0.782 | 0.19 | 0.508 | 0.30 | |
| RP | 0.797 | 0.14 | 0.512 | 0.21 | |
| HC | 0.874 | 0.91 | 0.741 | 1.35 | |
| Energy | 0.922 | 6.17 | 0.791 | 10.39 | |
| TS | 0.787 | 1.86 | 0.686 | 2.42 | |
| SNV 0 2 2 2 | Fat | 0.876 | 0.44 | 0.795 | 0.58 |
| CP | 0.725 | 0.25 | 0.498 | 0.35 | |
| RP | 0.580 | 0.22 | 0.411 | 0.27 | |
| HC | 0.860 | 0.94 | 0.593 | 1.65 | |
| Energy | 0.835 | 8.96 | 0.756 | 11.32 | |
| TS | 0.709 | 2.13 | 0.529 | 2.77 | |
| SNV 1 2 2 2 | Fat | 0.826 | 0.52 | 0.779 | 0.61 |
| CP | 0.796 | 0.22 | 0.524 | 0.36 | |
| RP | 0.787 | 0.16 | 0.482 | 0.25 | |
| HC | 0.894 | 0.83 | 0.699 | 1.42 | |
| Energy | 0.927 | 5.94 | 0.830 | 9.60 | |
| TS | 0.929 | 1.07 | 0.685 | 2.20 |
| Math Pre-Treatment | SECV | SEP | SECV/SEP | RPD | Accuracy % | tstudent Reference vs. Predicted | |
|---|---|---|---|---|---|---|---|
| Fat | 0 0 0 0 | 0.510 | 0.579 | 0.881 | 2.7 | 94 | 1.21 |
| CP | SNV 1.2.2.2 | 0.359 | 0.426 | 0.843 | 0.9 | 114 | 0.57 |
| RP | 0 0 0 0 | 0.210 | 0.203 | 1.035 | 1.7 | 92 | 0.69 |
| HC | 0 0 0 0 | 1.347 | 1.630 | 0.826 | 1.6 | 108 | 1.36 |
| Energy | SNV 1.2.2.2 | 9.603 | 11.757 | 0.817 | 2.2 | 94 | 1.74 |
| TS | 0 0 0 0 | 2.420 | 4.541 | 0.533 | 0.9 | 115 | 1.57 |
| Reference | Device | Lab/Portable | Wavelength Range λ (nm) | Sample Size (N) | Analyzed Parameters |
|---|---|---|---|---|---|
| Corvaglia [26] | Fenir 8820, Esetek | Lab | 700–2750 | 124 | Fat and nitrogen contents |
| Sauer [27] | SpectraStar 2400 Near Infrared Analyzer, Unity Scientific | Lab | 1200–2400 | 52 | Fat, protein, and carbohydrates |
| Fusch [28] | SpectraStar 2400 Near Infrared Analyzer, Unity Scientific | Lab | 1200–2400 | 1188 | Fat, protein, and carbohydrates |
| dos Santos [14] | MicroNIR™ 1700, JDS Uniphase Corporation | Portable | 910–1676 | 198 | Qualitative (colostrum, transition milk, and mature milk) |
| Present study | MicroPHAZIR Mod. 1624, Thermo Fisher Scientific Inc. | Portable | 1396–2396 | 68 | Fat, crude protein, raw protein, carbohydrates, total solids, and energy |
| Portable | Laboratory | Portable | Laboratory | ||
|---|---|---|---|---|---|
| Linear Regression | r2 | Sy/x | r2 | ||
| Fat | y = 0.69x + 0.72 | y = 0.55x + 1.25 | 0.85 | 0.547 | 0.79 |
| RP | y = 0.77x + 0.16 | y = 0.55x + 0.54 | 0.67 | 0.154 | 0.76 |
| HC | y = 0.95x + 0.60 | y = 0.02x + 5.69 | 0.01 | 0.904 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melendreras, C.; Forcada, S.; Fernández-Sánchez, M.L.; Fernández-Colomer, B.; Costa-Fernández, J.M.; López, A.; Ferrero, F.; Soldado, A. Near-Infrared Sensors for Onsite and Noninvasive Quantification of Macronutrients in Breast Milk. Sensors 2022, 22, 1311. https://doi.org/10.3390/s22041311
Melendreras C, Forcada S, Fernández-Sánchez ML, Fernández-Colomer B, Costa-Fernández JM, López A, Ferrero F, Soldado A. Near-Infrared Sensors for Onsite and Noninvasive Quantification of Macronutrients in Breast Milk. Sensors. 2022; 22(4):1311. https://doi.org/10.3390/s22041311
Chicago/Turabian StyleMelendreras, Candela, Sergio Forcada, María Luisa Fernández-Sánchez, Belén Fernández-Colomer, José M. Costa-Fernández, Alberto López, Francisco Ferrero, and Ana Soldado. 2022. "Near-Infrared Sensors for Onsite and Noninvasive Quantification of Macronutrients in Breast Milk" Sensors 22, no. 4: 1311. https://doi.org/10.3390/s22041311
APA StyleMelendreras, C., Forcada, S., Fernández-Sánchez, M. L., Fernández-Colomer, B., Costa-Fernández, J. M., López, A., Ferrero, F., & Soldado, A. (2022). Near-Infrared Sensors for Onsite and Noninvasive Quantification of Macronutrients in Breast Milk. Sensors, 22(4), 1311. https://doi.org/10.3390/s22041311










