On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
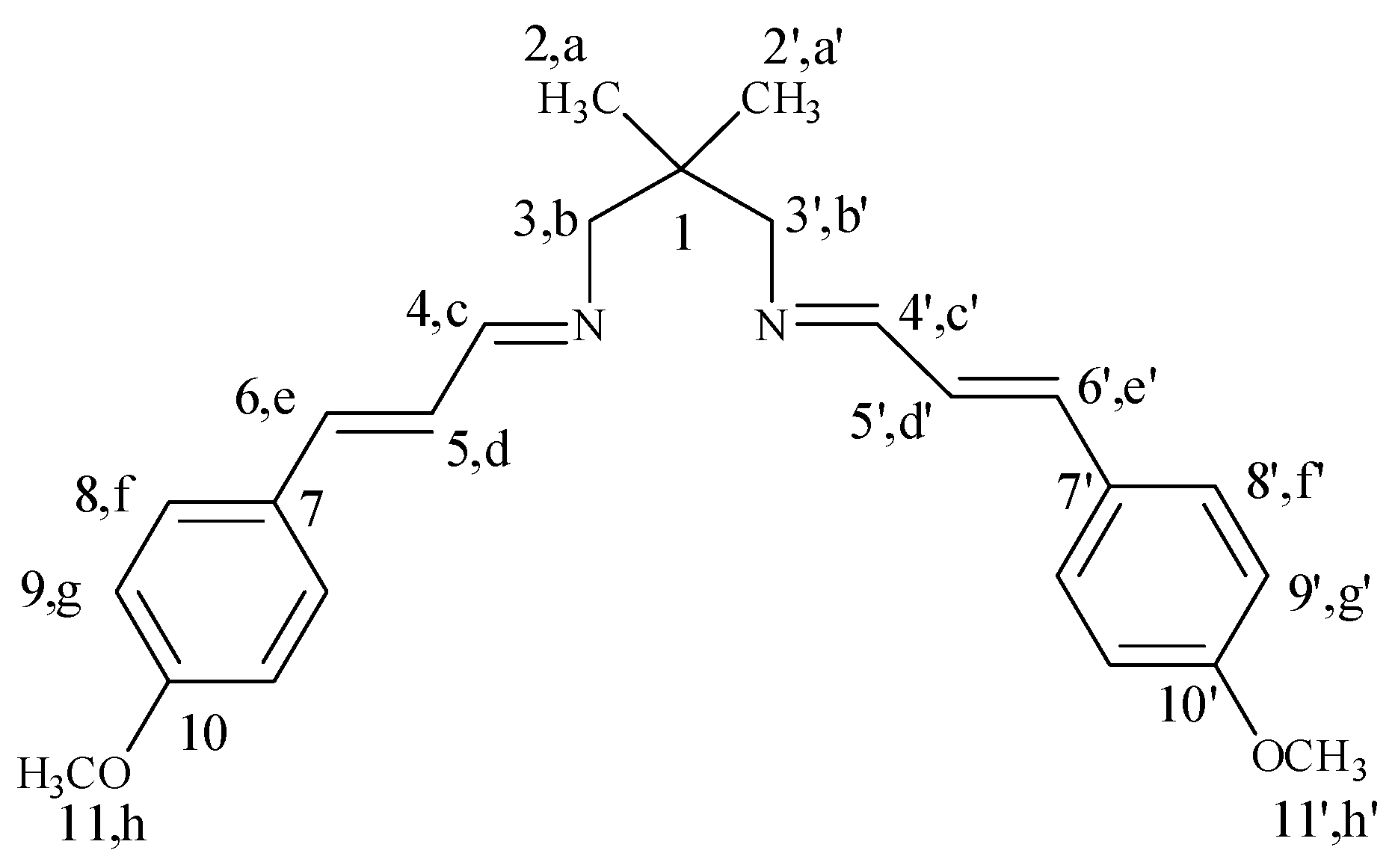
2.2. Synthesis of Bidentate Schiff Base Ligand (L)
2.3. Characterization
2.4. Electrochemical Tests
3. Results
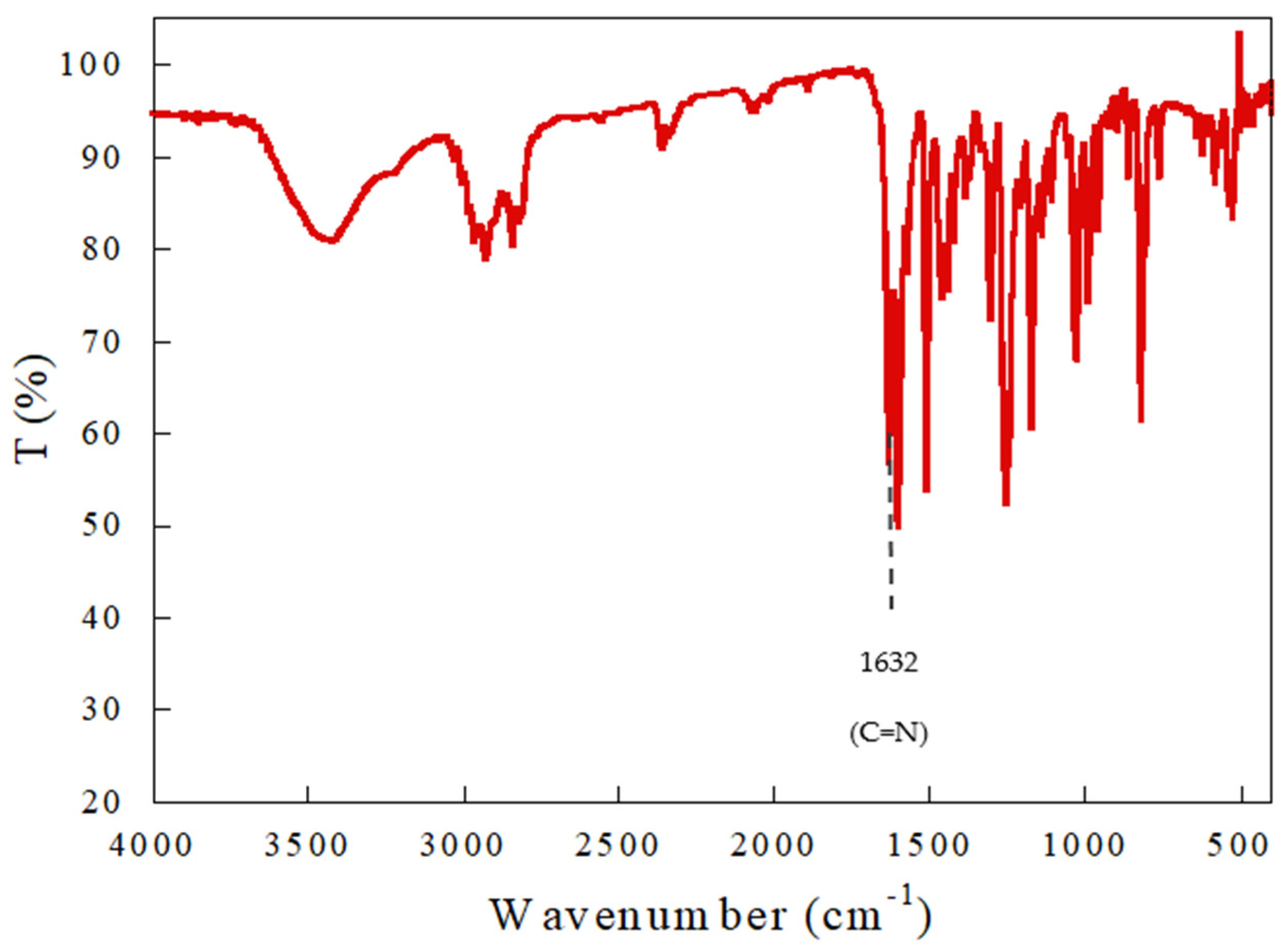
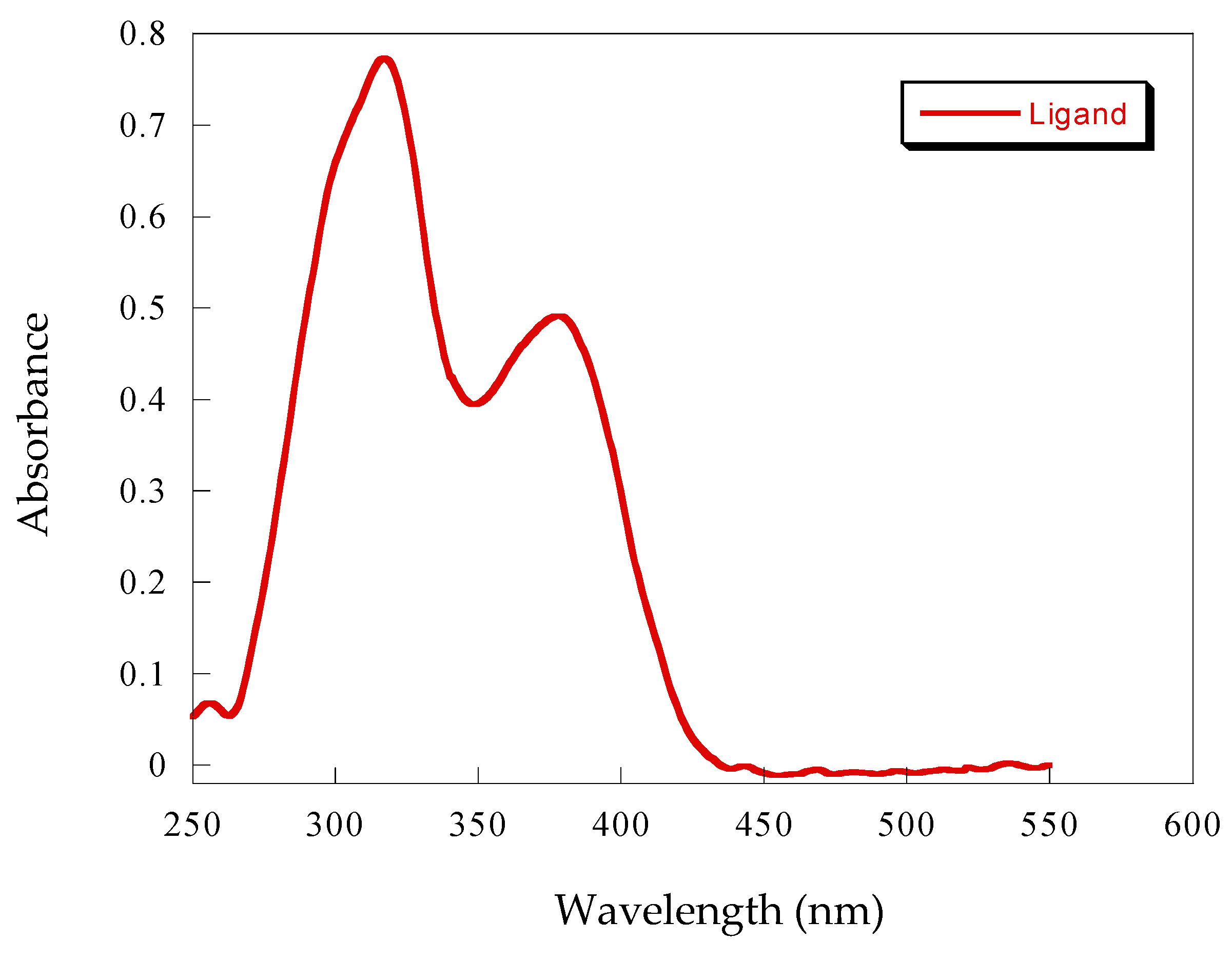
3.1. Characterization of Schiff Base Ligand
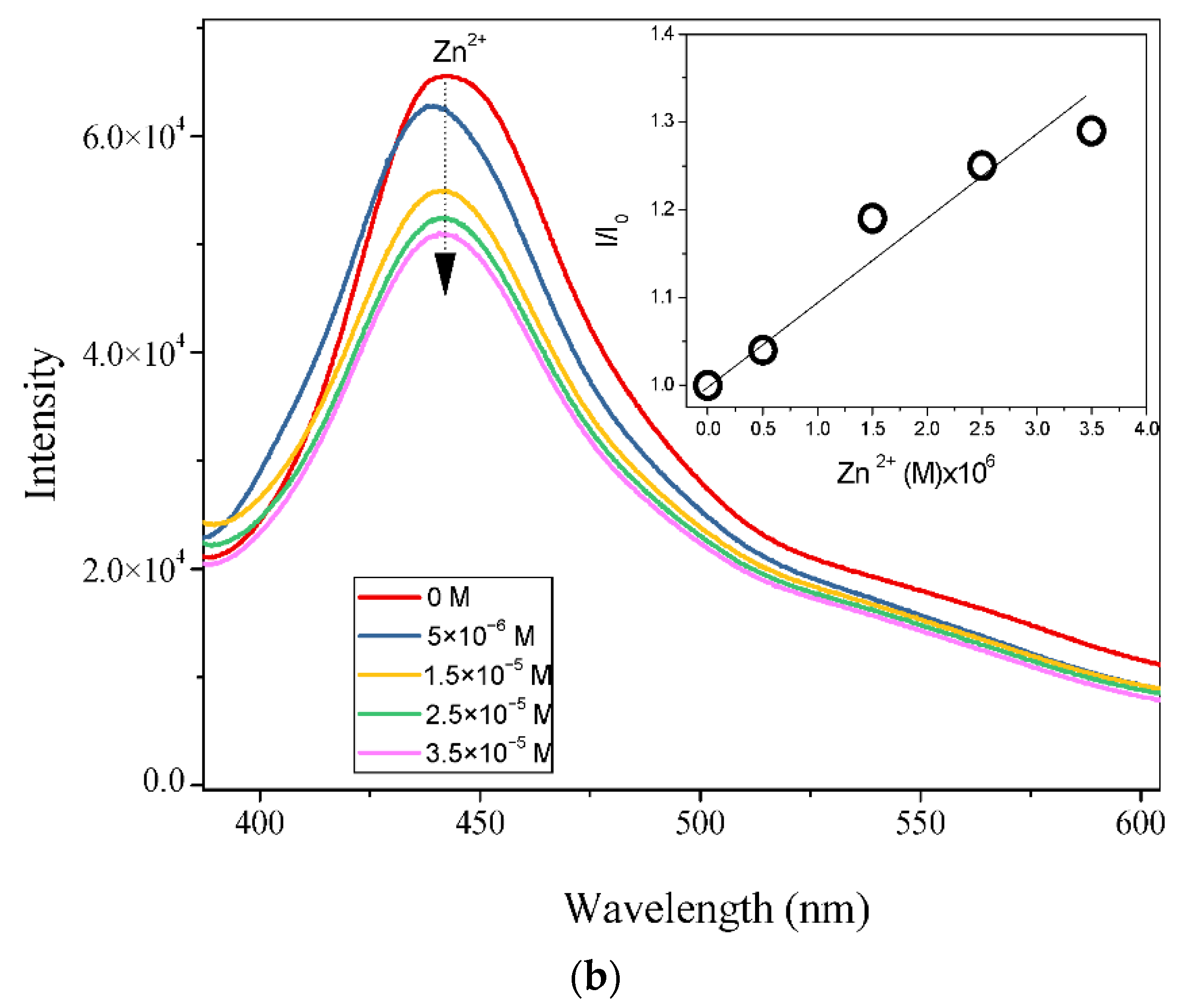
3.2. Fluorescence Studies
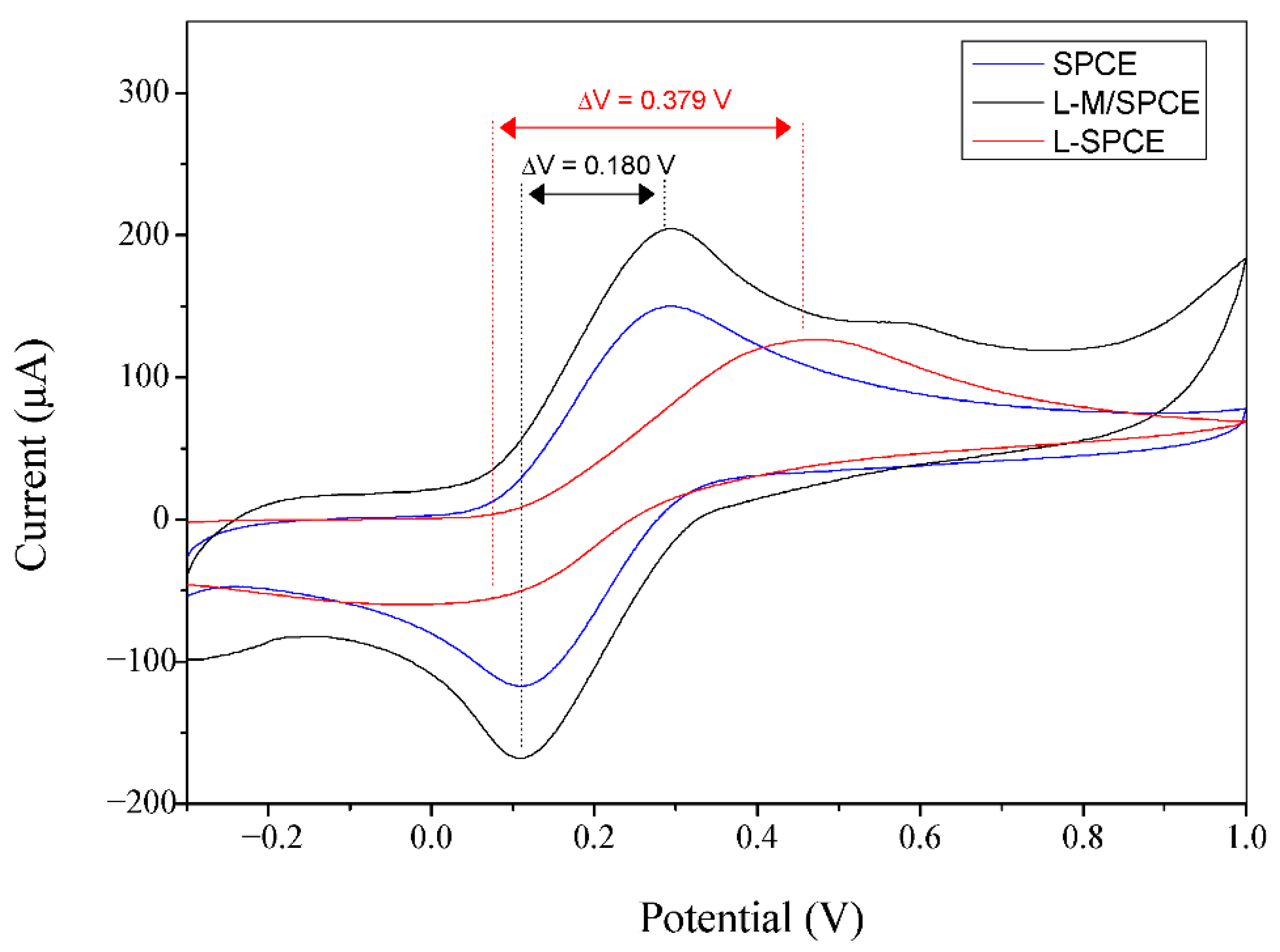
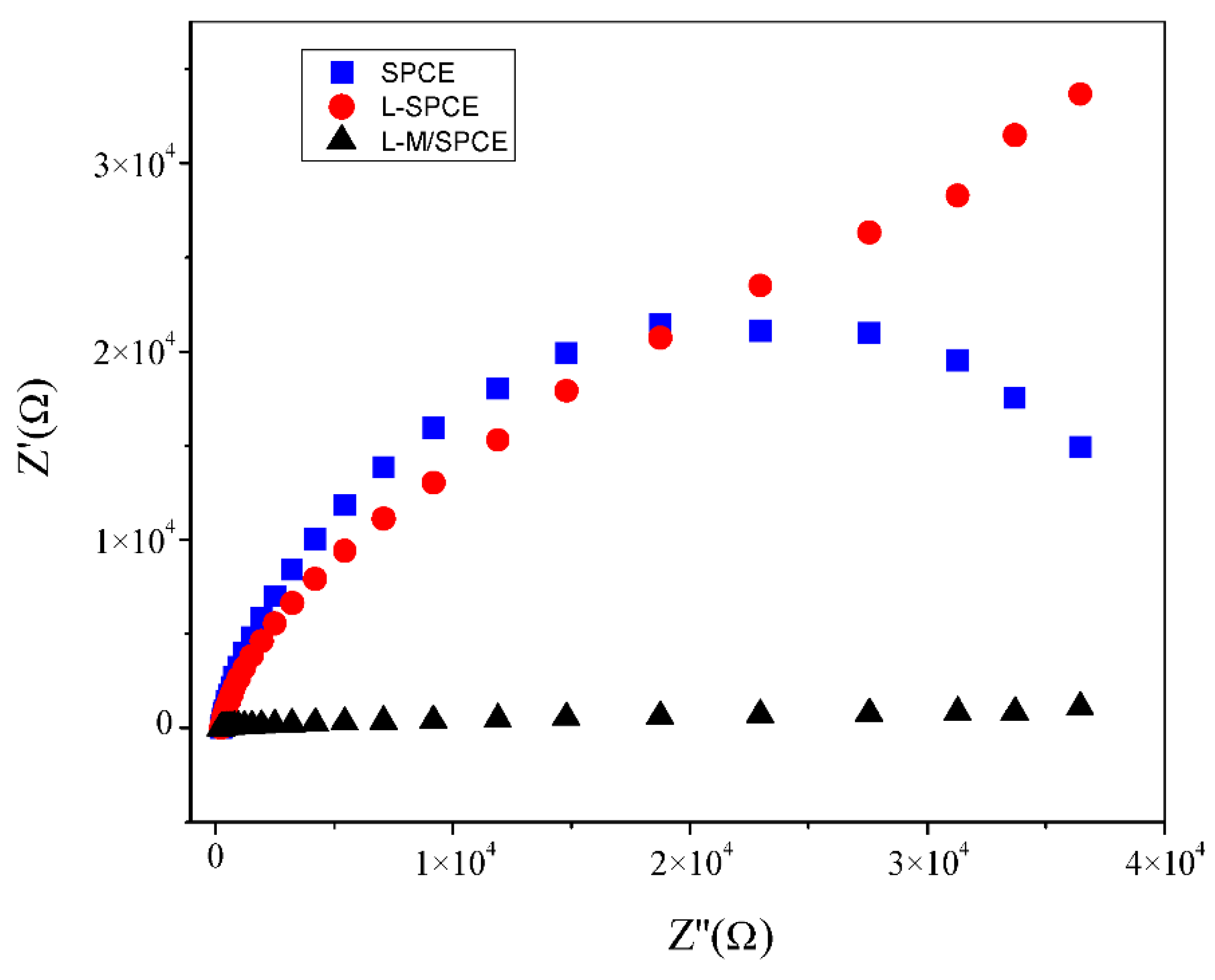
3.3. Electrochemical Studies
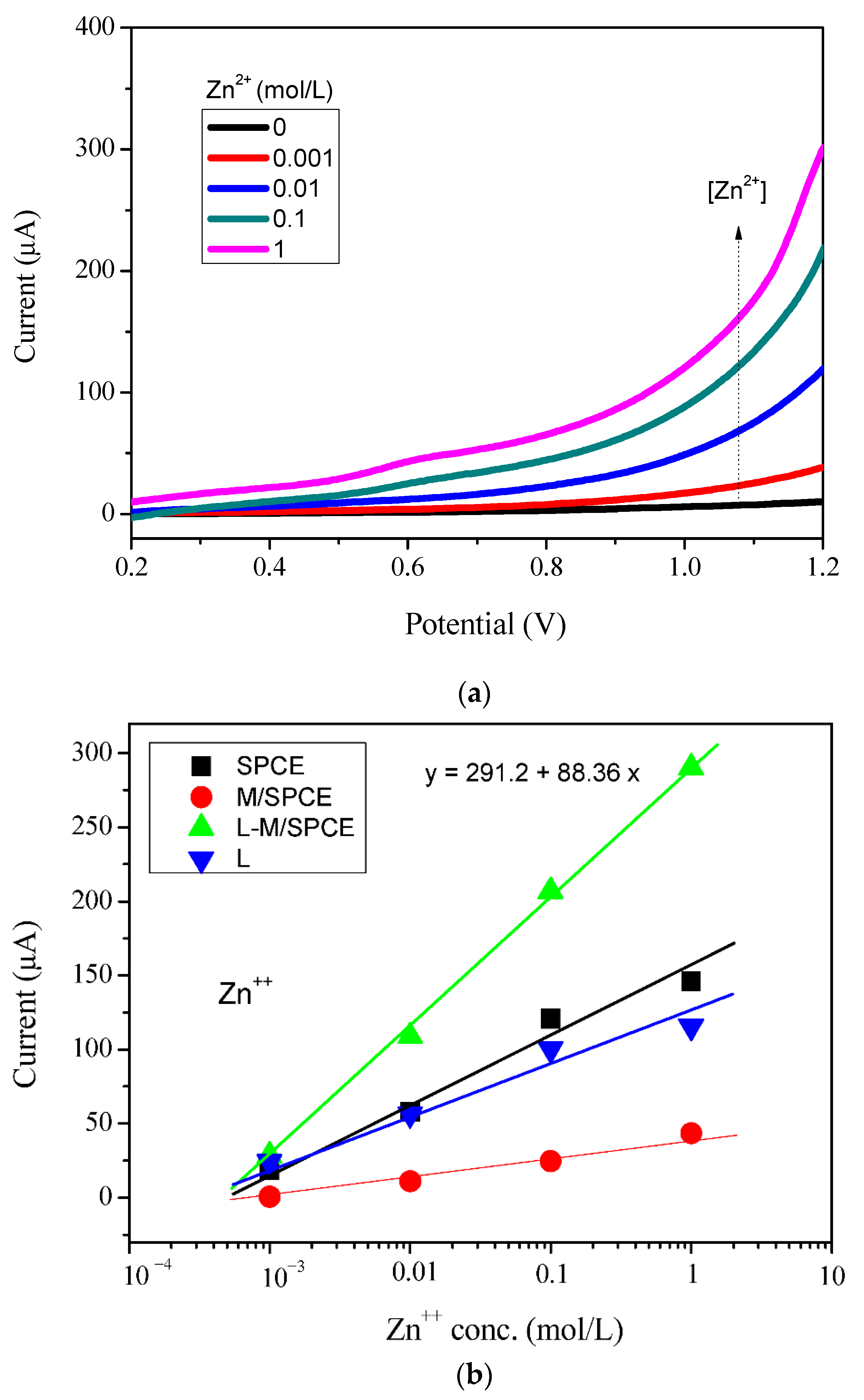
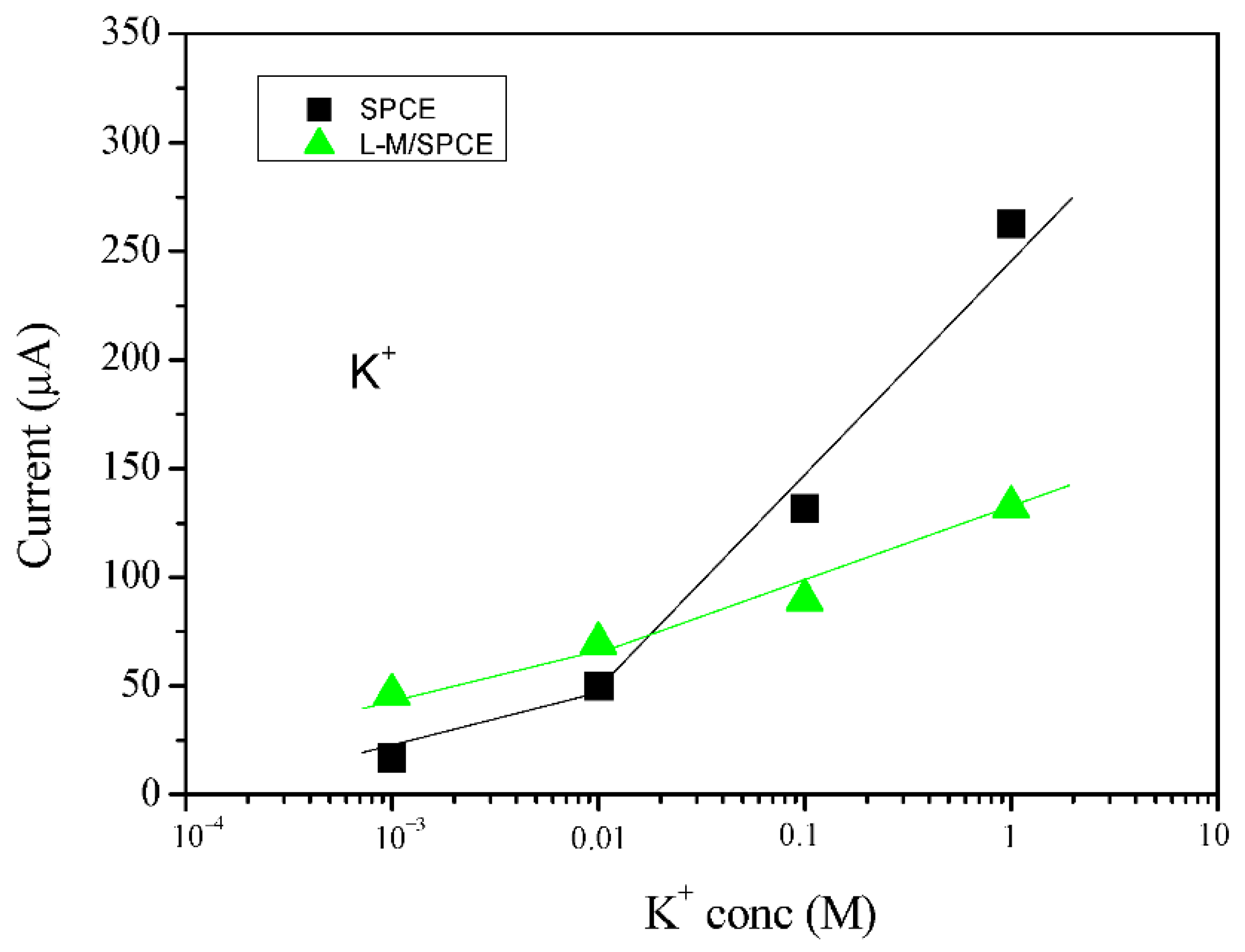
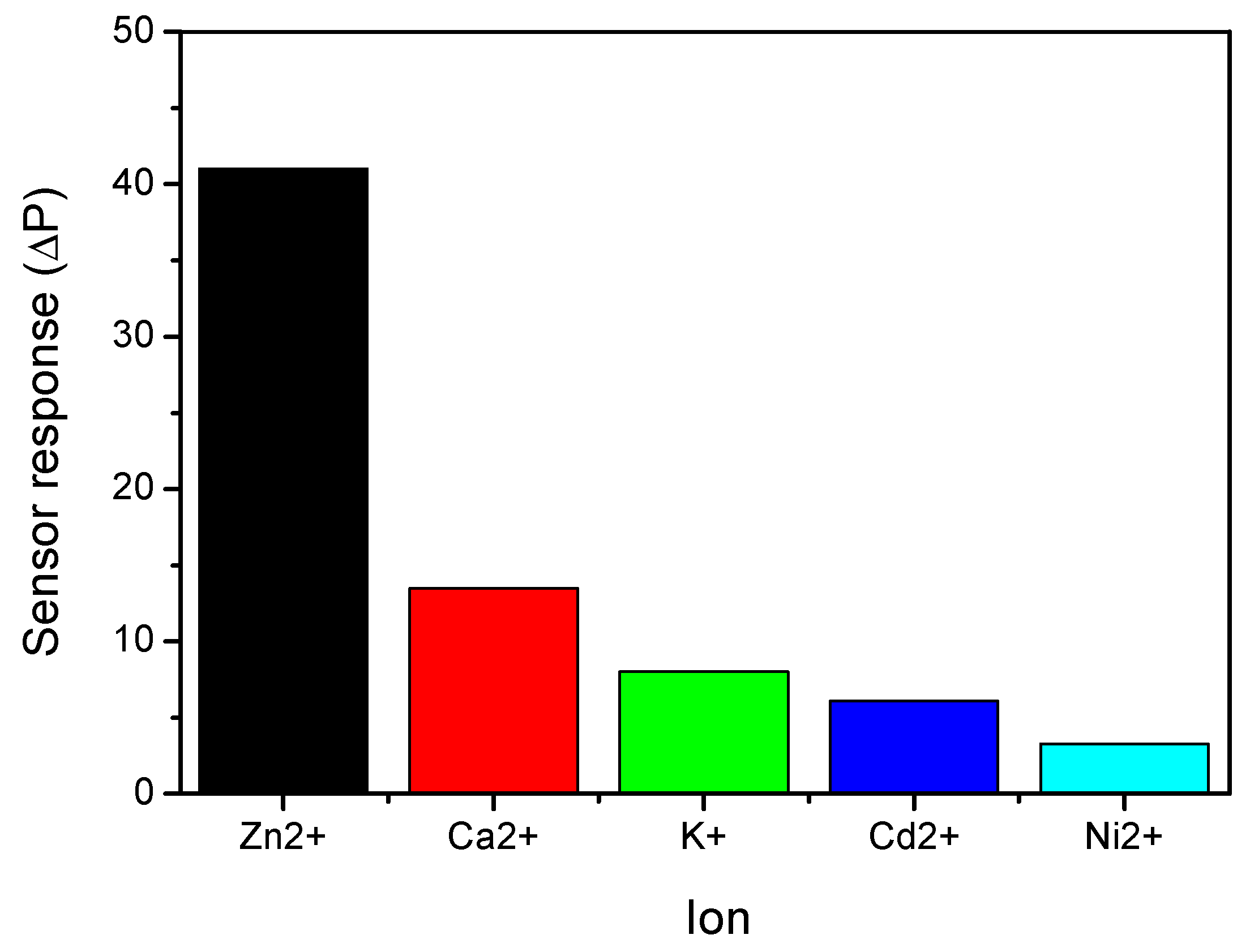
3.4. Electroanalytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.J.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific & Technical: Essex, UK, 1989. [Google Scholar]
- Santhoshkumar, S.; Velmurugan, K.; Prabhu, J.; Radhakrishnan, G.; Nandhakumar, R. A naphthalene derived Schiff base as a selective fluorescent probe for Fe2+. Inorg. Chim. Acta 2016, 439, 1–7. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, B. Colorimetric and fluorescent multi-ion recognition by Anthracene appended di-Schiff base chemosensor. Inorg. Chem. Commun. 2020, 121, 108239. [Google Scholar] [CrossRef]
- Durai, W.A.; Ramu, A.; Dhakshinamoorthy, A. A chromogenic and fluorescence turn-on sensor for the selective and sensitive recognition of Al3+ ions—A new approach by Schiff base derivative as probe. Inorg. Chem. Commun. 2020, 121, 108191. [Google Scholar] [CrossRef]
- Ghosh, S.; Singharoy, D.; Konar, S.; Naskar, J.P.; Bhattacharya, S.C. Solvatochromic behavior of a pyrene-pyrimidine-based Schiff base and detection of heavy metal ions in aqueous media. J. Coord. Chem. 2021, 74, 1272–1283. [Google Scholar] [CrossRef]
- Mohammed, M.Q.; Ismail, H.K.; Alesary, H.; Barton, S.J. Use of a Schiff base-modified conducting polymer electrode for electrochemical assay of Cd(II) and Pb(II) ions by square wave voltammetry. Chem. Pap. 2021, 28, 1–15. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.; Chen, W.; Tao, N. Detection of Heavy Metal Ions in Drinking Water Using a High-Resolution Differential Surface Plasmon Resonance Sensors. Environ. Sci. Technol. 2005, 39, 257–1262. [Google Scholar] [CrossRef]
- Dzianová, P.; Asai, S.; Chrudinová, M.; Kosinová, L.; Potalitsyn, P.; Šácha, P.; Hadravová, R.; Selicharová, I.; Kříž, J.; Turkenburg, J.P.; et al. The efficiency of insulin production and its content in insulin-expressing model β-cells correlate with their Zn2+ levels. Open Biol. 2020, 10, 200137. [Google Scholar] [CrossRef]
- Kambe, T.; Matsunaga, M.; Takeda, T.A. Understanding the contribution of zinc transporters in the function of the early secretory pathway. Int. J. Mol. Sci. 2017, 18, 2179. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef]
- Hsieh, W.H.; Wan, C.F.; Liao, D.J.; Wu, A.T. A turn-on Schiff base fluorescence sensor for zinc ion. Tetrahedron Lett. 2012, 53, 5848–5851. [Google Scholar] [CrossRef]
- Lavado, L.K.; Zhang, M.H.; Patel, K.; Khan, S.; Patel, U.K. Biometals as Potential Predictors of the Neurodegenerative Decline in Alzheimer’s Disease. Cureus 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzaroba, L.; Frizon Alfieri, D.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Bressi, V.; Ferlazzo, A.; Iannazzo, D.; Espro, C. Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives. Nanomaterials 2021, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Israel, Y.; Ofir, T.; Rezek, J. Determination of trace impurities in high-purity reagents by mercury thin-film anodic-stripping voltammetry. Microchim. Acta 1978, 69, 151–163. [Google Scholar] [CrossRef]
- Martinotti, W.; Queirazza, G.; Guarinoni, A.; Mori, G. In-flow speciation of copper, zinc, lead and cadmium in fresh waters by square wave anodic stripping voltammetry Part II. Optimization of measurement step. Anal. Chim. Acta 1995, 305, 183–191. [Google Scholar] [CrossRef]
- Lu, T.; Huang, J.; Sun, I. Perfluorinated anion-exchange polymer mercury film electrode for anodic stripping voltammetric determination of zinc (II): Effect of model organic compounds. Anal. Chim. Acta 2002, 454, 93–100. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Saczk, A.A.; Okumura, L.L.; Fernandes, A.P.; De Moraes, M.; Stradiotto, N.R. Simultaneous determination of zinc, copper, lead, and cadmium in fuel ethanol by anodic stripping voltammetry using a glassy carbon-mercury-film electrode. Anal. Bioanal. Chem. 2004, 380, 135–140. [Google Scholar] [CrossRef]
- Akbari, Z.; Montazerozohori, M.; Bruno, G.; Moulaee, K.; Neri, G. Development of a novel electrochemical nitrite sensor based on Zn-Schiff base complexes. Appl. Organomet. Chem. 2022. [Google Scholar] [CrossRef]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Ion recognition: Application of symmetric and asymmetric Schiff bases and their complexes for the fabrication of cationic and anionic membrane sensors to determine ions in real samples. Comb. Chem. High. Throughput Screen. 2007, 10, 527–546. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Ali, I.A. A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu (II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.S.; Reibenspies, J.; Hancock, R.D. Mechanism of “turn-on” fluorescent sensors for mercury (II) in solution and its implications for ligand design. Inorg. Chem. 2012, 51, 10904–10915. [Google Scholar] [CrossRef]
- Deems, J.C.; Reibenspies, J.H.; Lee, H.S.; Hancock, R.D. Strategies for a fluorescent sensor with receptor and fluorophore designed for the recognition of heavy metal ions. Inorg. Chim. Acta 2020, 499, 119181. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Yan, H.; Lu, J.; Liu, H.; Li, Y.; Wang, S.; Li, D.; Dou, J.; Yang, L.; et al. Multiresponsive luminescent sensitivities of a 3D Cd-CP with visual turn-on and ratiometric sensing toward Al3+ and Cr3+ as well as turn-off sensing toward Fe3+. Inorg. Chem. 2020, 59, 3828–3837. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.K.; Mazzone, G.; Mahmoud, A.M.; Sicilia, E.; Shoeib, T. Novel choline selective electrochemical membrane sensor with application in milk powders and infant formulas. Talanta 2021, 221, 121409. [Google Scholar] [CrossRef]
- Dehabadi, M.; Legin, E.; Legin, A.; Yaghmaei, S.; Nechaev, S.; Babain, V.; Kirsanov, D. Developing potentiometric sensors for scandium. Sens. Actuators B Chem. 2021, 348, 130699. [Google Scholar] [CrossRef]
- Rana, S.; Mittal, S.K.; Kaur, K.; Banks, C.K. Pseudo Cavity of Schiff Base Ionophore Incorporated in Screen Printed Electrode for Sensing of Zn (II). J. Electrochem. Soc. 2019, 166, B464. [Google Scholar] [CrossRef]
- Kavitha, A.; Easwaramoorthy, D. Aminoacid Schiff base Fabricated Glassy carbon Electrode for Efficient Sensing of Zinc, Copper, Mercury ions in Water. IOP Conf. Ser. Mater. Sci. Eng. 2020, 988, 012042. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Rymansai, Z.; Iravani, P. Anodic stripping voltammetric determination of zinc at a 3-D printed carbon nanofiber–graphite–polystyrene electrode using a carbon pseudo-reference electrode. Sens. Actuators B Chem. 2018, 267, 476–482. [Google Scholar] [CrossRef]
- Khairy, M.; Kadara, R.O.; Kampouris, D.K.; Banks, C.E. Disposable Bismuth Oxide Screen Printed Electrodes for the Sensing of Zinc in Seawater. Electroanalysis 2010, 22, 1455–1459. [Google Scholar] [CrossRef]
- Narakathu, B.B.; Abebe, F.A.; Eribal, C.S.; Sinn, E.; Atashbar, M.Z. Detection of Zn2+ Ions Using a Novel Chemosensor Based on Coumarin Schiff-base Derivatives by Electrochemical and Fluorescence Spectroscopy. In Proceedings of the 14th International Meeting on Chemical Sensors 2012, Nuremberg, Germany, 20–23 May 2012. [Google Scholar]
- Hosseini, M.; Vaezi, Z.; Reza Ganjali, M.; Faridbod, F.; Dehghan Abkenar, S.; Alizadeh, K.; Salavati-Niasari, M. Fluorescence “turn-on” chemosensor for the selective detection of zinc ion based on Schiff-base derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Singh, R.; Kaur, P. Dual role of silatranized Schiff base as a fluorimetric probe and a linker to functionalize graphene oxide for the selective detection and adsorption of zinc ions. Inorg. Chim. Acta 2020, 512, 119859. [Google Scholar] [CrossRef]
- Saçmacı, S. Selective back-extraction and preconcentration of zinc (II) from metal-1,3,5-triketone extracts prior to its determination by flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2012, 92, 1626–1637. [Google Scholar] [CrossRef]
- Wang, J.; Dewald, H.D. Determination of trace elements in pharmaceutical tablets using anodic stripping voltammetry. Anal. Lett. 1983, 16, 925–940. [Google Scholar] [CrossRef]
- Lutka, A.; Kokot, Z.; Powidzka, H. Validation of electrochemical determination of zinc in selected pharmaceutical preparations. Acta Pol. Pharm. 2004, 61, 243–247. [Google Scholar]




| Sensing Material | Linear Range | Limit of Detection (LOD) | Ref. |
|---|---|---|---|
| Ionophore (SMS-3) Schiff base moiety modified SPCE | 0.47 to 5.56 μM | 0.92 µM | [28] |
| Aminoacid Schiff base modified GCE | 10 to 150 µM | - | [29] |
| 3-D printed carbon nanofiber–graphite–polystyrene | 12.7 μg/L to 450 μg/L | 8.6 μg/L | [30] |
| Screen printed bismuth oxide | 75 to 600 mg/L | 33 mg/L | [31] |
| Coumarin-based fluorescent sensor | 100 pM to 1 mM | 3 µM | [32] |
| L-SPCE | 1 µM to 100 mM | 3.5 µM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bressi, V.; Akbari, Z.; Montazerozohori, M.; Ferlazzo, A.; Iannazzo, D.; Espro, C.; Neri, G. On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor. Sensors 2022, 22, 900. https://doi.org/10.3390/s22030900
Bressi V, Akbari Z, Montazerozohori M, Ferlazzo A, Iannazzo D, Espro C, Neri G. On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor. Sensors. 2022; 22(3):900. https://doi.org/10.3390/s22030900
Chicago/Turabian StyleBressi, Viviana, Zahra Akbari, Morteza Montazerozohori, Angelo Ferlazzo, Daniela Iannazzo, Claudia Espro, and Giovanni Neri. 2022. "On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor" Sensors 22, no. 3: 900. https://doi.org/10.3390/s22030900
APA StyleBressi, V., Akbari, Z., Montazerozohori, M., Ferlazzo, A., Iannazzo, D., Espro, C., & Neri, G. (2022). On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor. Sensors, 22(3), 900. https://doi.org/10.3390/s22030900










