Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Shape Modification
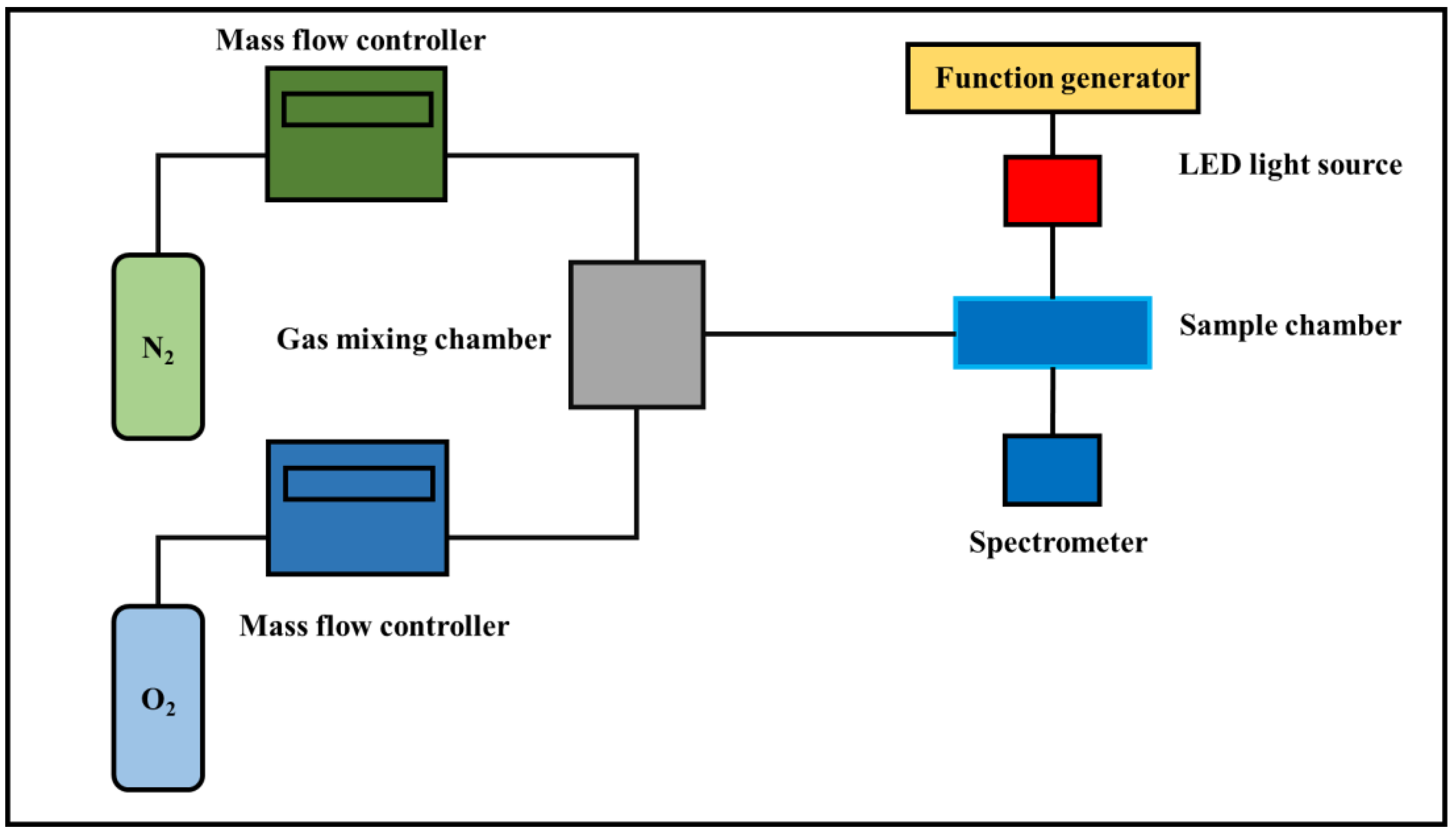
2.3. Device Fabrication and Characterization
2.4. Mechanism of Sensing
3. Results and Discussion
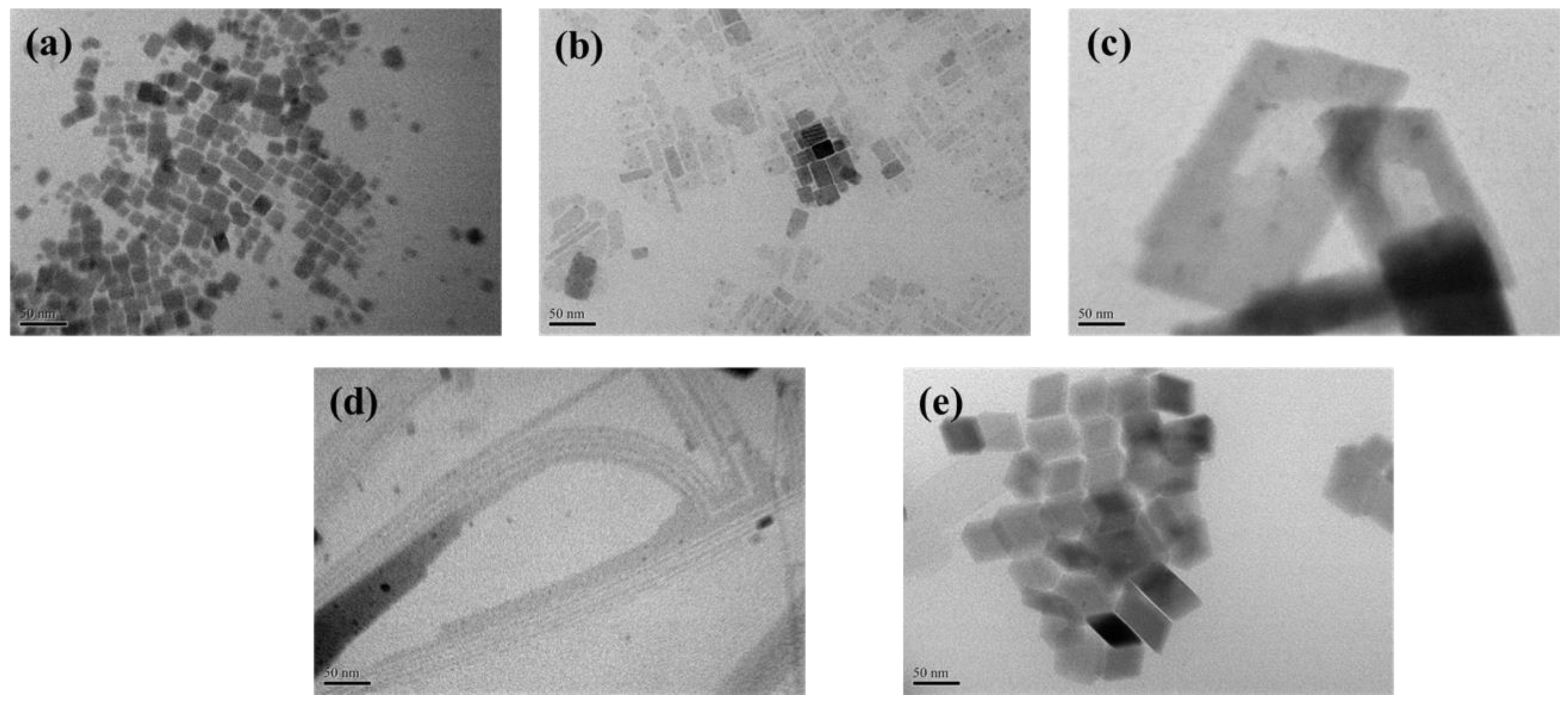
3.1. Effect of Different NC Shapes on Device Response
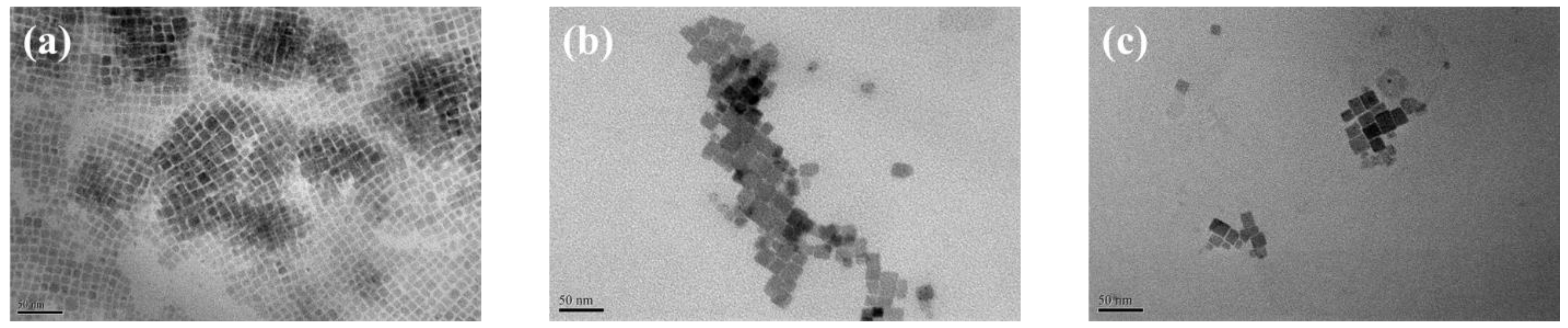
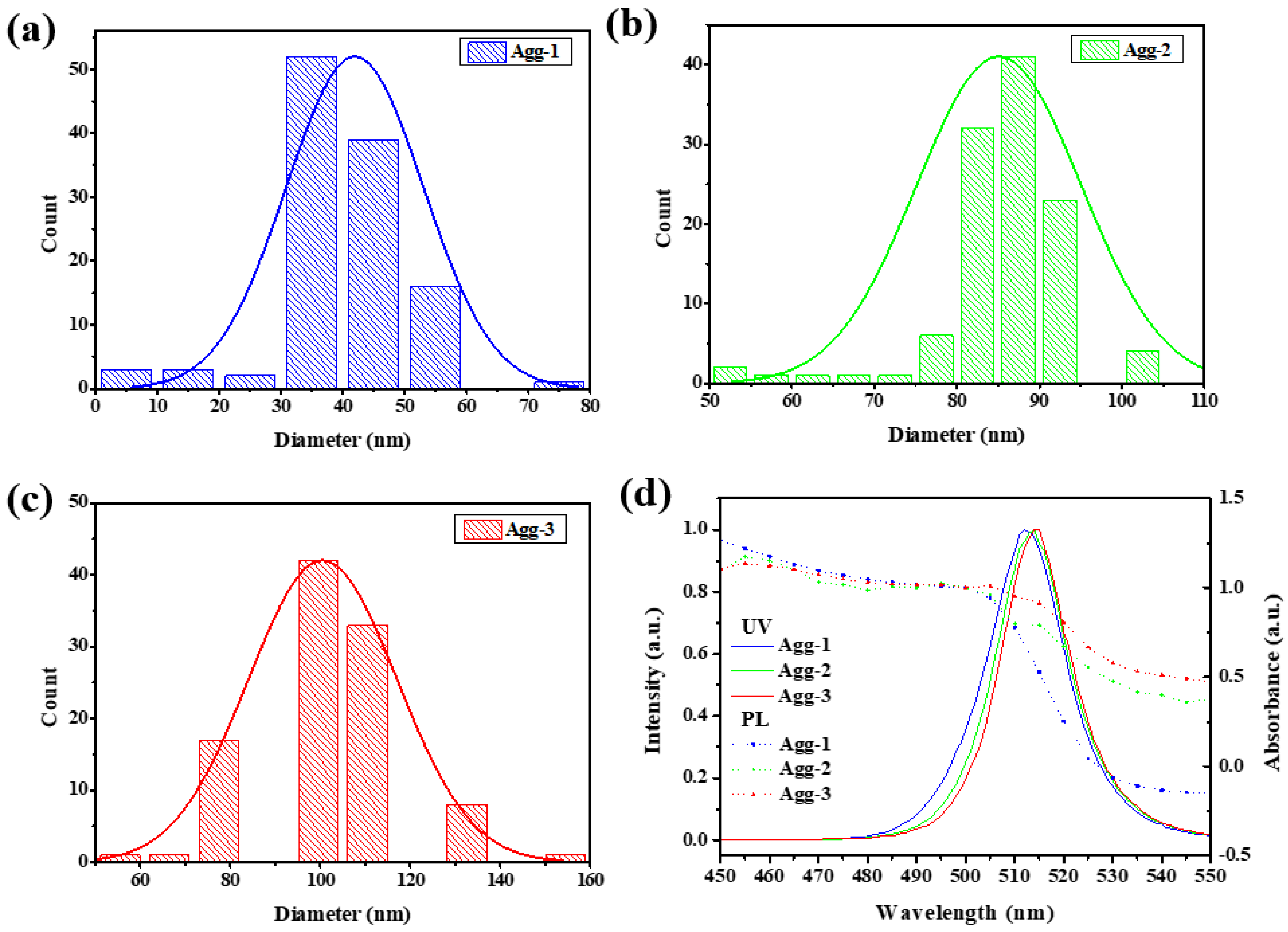
3.2. Effect of Aggregation Size of CPB NCs on Device Response
3.3. Effect of Substrate on Device Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anukunprasert, T.; Saiwan, C.; Traversa, E. The development of gas sensor for carbon monoxide monitoring using nanostructure of Nb–TiO2. Sci. Technol. Adv. Mater. 2005, 6, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Wang, B.; Wu, N.; Han, C.; Wu, C.; Zhang, X.; Tian, Q.; Shen, S.; Li, P.; Wang, Y. Metal-organic frameworks derived porous Co3O4 dodecahedeons with abundant active Co3+ for ppb-level CO gas sensing. Appl. Surf. Sci. 2020, 506, 144900. [Google Scholar] [CrossRef]
- Cui, H.; Feng, Z.; Wang, W.; Peng, X.; Hu, J. Adsorption Behavior of Pd-Doped PtS2 Monolayer Upon SF6 Decomposed Species and the Effect of Applied Electric Field. IEEE Sens. J. 2022, 22, 6764–6771. [Google Scholar] [CrossRef]
- Amirjani, A.; Fatmehsari, D.H. Colorimetric detection of ammonia using smartphones based on localized surface plasmon resonance of silver nanoparticles. Talanta 2018, 176, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Santos, J.P.; Sánchez-Vicente, C. The Effect of Rare Earths on the Response of Photo UV-Activate ZnO Gas Sensors. Sensors 2022, 22, 8150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Yu, P.; Liu, Z. Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor. Sensors 2022, 22, 7949. [Google Scholar] [CrossRef] [PubMed]
- Dhanaji, P.B.; Kim, K.-H.; Mahajan, P.G.; Gore, A.H.; Patil, S.R.; Majhi, S.M.; Naik, G.K.; Liang, T.-T.; Ahemad, J.-M.; Yu, Y.-T.; et al. Fluorescent Chemosensor for Quantitation of Multiple Atmospheric Gases. J. Nanomed. Nanotechnol. 2017, 8, 2. [Google Scholar]
- Jiang, T.; Du, B.; Zhang, H.; Yu, D.; Sun, L.; Zhao, G.; Yang, C.; Sun, Y.; Yu, M.; Ashfold, M.N.R. High-performance photoluminescence-based oxygen sensing with Pr-modified ZnO nanofibers. Appl. Surf. Sci. 2019, 483, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [Green Version]
- Mokoena, T.P.; Swart, H.C.; Hillie, K.T.; Tshabalala, Z.P.; Jozela, M.; Tshilongo, J.; Motaung, D.E. Enhanced propanol gas sensing performance of p-type NiO gas sensor induced by exceptionally large surface area and crystallinity. Appl. Surf. Sci. 2022, 571, 151121. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.D.; Sexton, K. Indoor air pollution: A public health perspective. Science 1983, 221, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, X.; Sorensen, O.T. Oxygen sensors based on semiconducting metal oxides: An overview. Sens. Actuators B Chem. 2000, 65, 2–4. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Liao, L.; Pan, H.; Shen, S.; Chen, J. Phosphorus doped TiO2 as oxygen sensor with low operating temperature and sensing mechanism. Appl. Surf. Sci. 2013, 273, 349–356. [Google Scholar] [CrossRef]
- Baleizao, C.; Nagl, S.; Schaferling, M.; Santos, M.N.B.; Wolfbeis, O.S. Dual Fluorescence Sensor for Trace Oxygen and Temperature with Unmatched Range and Response. Anal. Chem. 2008, 80, 6449–6457. [Google Scholar] [CrossRef]
- Tabari, L.; Farmanzadeh, D. Interesting adsorption behavior of C60O fullerene oxide isomers toward O3 and CO molecules: A DFT study. Appl. Surf. Sci. 2019, 479, 569–575. [Google Scholar] [CrossRef]
- Achatz, D.E.; Meier, R.J.; Fischer, L.H.; Wolfbeis, O.S. Luminescent sensing of oxygen using a quenchable probe and upconverting nanoparticles. Angew. Chem. Int. Ed. Engl. 2011, 50, 260–263. [Google Scholar] [CrossRef]
- Banski, M.; Podhorodecki, A.; Misiewicz, J. NaYF4 nanocrystals with TOPO ligands: Synthesis-dependent structural and luminescent properties. Phys. Chem. Chem. Phys. 2013, 15, 19232–19241. [Google Scholar] [CrossRef]
- Epifani, M.; Comini, E.; Díaz, R.; Force, C.; Siciliano, P.; Faglia, G. TiO2 colloidal nanocrystals surface modification by V2O5 species: Investigation by 47,49Ti MAS-NMR and H2, CO and NO2 sensing properties. Appl. Surf. Sci. 2015, 351, 1169–1173. [Google Scholar] [CrossRef]
- Lei, L.; Dong, Q.; Gundogdu, K.; So, F. Metal Halide Perovskites for Laser Applications. Adv. Funct. Mater. 2021, 31, 2010144. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Wang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A. Enhanced performance of CH3NH3PbI3 perovskite solar cells by excess halide modification. Appl. Surf. Sci. 2021, 564, 150464. [Google Scholar] [CrossRef]
- Ashjari, T.; Roghabadi, F.A.; Ahmadi, V. Facile synthesis of durable perovskite quantum dots film with near unity photoluminescence quantum yield for efficient perovskite light emitting diode. Appl. Surf. Sci. 2020, 510, 145513. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Xiao, Y.; Shen, L.; Ding, L. Advances in perovskite photodetectors. InfoMat 2020, 2, 1247–1256. [Google Scholar] [CrossRef]
- Oh, H.; Kim, H.J.; Kim, S.; Kim, J.A.; Kang, G.; Park, M. Highly flexible and stable perovskite/microbead hybrid photodetectors with improved interfacial light trapping. Appl. Surf. Sci. 2021, 544, 148850. [Google Scholar] [CrossRef]
- Maity, A.; Raychaudhuri, A.K.; Ghosh, B. High sensitivity NH3 gas sensor with electrical readout made on paper with perovskite halide as sensor material. Sci. Rep. 2019, 9, 7777. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hu, H.; Xia, Z.; Gao, W.; Gou, W.; Qu, Y.; Ma, Y. CsPbBr3 perovskite nanocrystals as highly selective and sensitive spectrochemical probes for gaseous HCl detection. J. Mater. Chem. C 2017, 5, 309–313. [Google Scholar] [CrossRef]
- Stoeckel, M.A.; Gobbi, M.; Bonacchi, S.; Liscio, F.; Ferlauto, L.; Orgiu, E.; Samori, P. Reversible, Fast, and Wide-Range Oxygen Sensor Based on Nanostructured Organometal Halide Perovskite. Adv. Mater. 2017, 29, 1702469. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; She, C.; Zhang, Y.; Li, H.; Liu, S.; Yue, F.; Jing, C.; Cheng, Y.; Chu, J. A “Turn-on” fluorescence perovskite sensor based on MAPbBr3/mesoporous TiO2 for NH3 and amine vapor detections. Sens. Actuators B Chem. 2021, 327, 128918. [Google Scholar] [CrossRef]
- Yan, J.; Ke, X.; Chen, Y.; Zhang, A.; Zhang, B. Effect of modulating the molar ratio of organic to inorganic content on morphology, optical absorption and photoluminescence of perovskite CH3NH3PbBr3 films. Appl. Surf. Sci. 2015, 351, 1191–1196. [Google Scholar] [CrossRef]
- Clinckemalie, L.; Valli, D.; Roeffaers, M.B.J.; Hofkens, J.; Pradhan, B.; Debroye, E. Challenges and Opportunities for CsPbBr3 Perovskites in Low- and High-Energy Radiation Detection. ACS Energy Lett. 2021, 6, 1290–1314. [Google Scholar] [CrossRef]
- Gong, C.; Wang, X.; Xia, X.; Yang, X.; Wang, L.; Li, F. In-situ guanidinium bromide passivation treatment of CsPbBr3 perovskite quantum dots exhibiting high photoluminescence and environmental stability. Appl. Surf. Sci. 2021, 559, 149986. [Google Scholar] [CrossRef]
- Yu, J.; Liu, G.; Chen, C.; Li, Y.; Xu, M.; Wang, T.; Zhao, G.; Zhang, L. Perovskite CsPbBr3 crystals: Growth and applications. J. Mater. Chem. C 2020, 8, 6326–6341. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Sun, X.; Wu, Y.; Wang, L.; Meng, G.; Kuang, D.; Guo, X.; Qu, W.; Du, B.; et al. An excellent impedance-type humidity sensor based on halide perovskite CsPbBr3 nanoparticles for human respiration monitoring. Sens. Actuators B Chem. 2021, 337, 129772. [Google Scholar] [CrossRef]
- Kim, J.; Hu, L.; Chen, H.; Guan, X.; Anandan, P.R.; Li, F.; Tang, J.; Lin, C.-H.; Kalantar-Zadeh, K.; Tricoli, A.; et al. P-type Charge Transport and Selective Gas Sensing of All-Inorganic Perovskite Nanocrystals. ACS Mater. Lett. 2020, 2, 1368–1374. [Google Scholar] [CrossRef]
- Lu, W.-G.; Chen, C.; Han, D.; Yao, L.; Han, J.; Zhong, H.; Wang, Y. Nonlinear Optical Properties of Colloidal CH3NH3PbBr3 and CsPbBr3Quantum Dots: A Comparison Study Using Z-Scan Technique. Adv. Opt. Mater. 2016, 4, 1732–1737. [Google Scholar] [CrossRef]
- Fanizza, E.; Cascella, F.; Altamura, D.; Giannini, C.; Panniello, A.; Triggiani, L.; Panzarea, F.; Depalo, N.; Grisorio, R.; Suranna, G.P.; et al. Post-synthesis phase and shape evolution of CsPbBr3 colloidal nanocrystals: The role of ligands. Nano Res. 2019, 12, 1155–1166. [Google Scholar] [CrossRef]
- Liu, C.Y.; Deb, M.; Sadhu, A.S.; Karmakar, R.; Huang, P.T.; Lin, Y.N.; Chu, C.S.; Pal, B.N.; Chang, S.H.; Biring, S. Resolving Cross-Sensitivity Effect in Fluorescence Quenching for Simultaneously Sensing Oxygen and Ammonia Concentrations by an Optical Dual Gas Sensor. Sensors 2021, 21, 6940. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.-S.; Kim, S.-I.; Baek, S.-H.; Chun, D.W.; Jin, C.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.-H.; Layek, M.; Li, C.-F.; Lee, K.-M.; Huang, Y.-C. Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing. Sensors 2022, 22, 8853. https://doi.org/10.3390/s22228853
Huang Z-H, Layek M, Li C-F, Lee K-M, Huang Y-C. Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing. Sensors. 2022; 22(22):8853. https://doi.org/10.3390/s22228853
Chicago/Turabian StyleHuang, Zhi-Hao, Madhuja Layek, Chia-Feng Li, Kun-Mu Lee, and Yu-Ching Huang. 2022. "Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing" Sensors 22, no. 22: 8853. https://doi.org/10.3390/s22228853
APA StyleHuang, Z.-H., Layek, M., Li, C.-F., Lee, K.-M., & Huang, Y.-C. (2022). Cesium Lead Bromide Nanocrystals: Synthesis, Modification, and Application to O2 Sensing. Sensors, 22(22), 8853. https://doi.org/10.3390/s22228853







