Neural Network-Based Autonomous Search Model with Undulatory Locomotion Inspired by Caenorhabditis Elegans
Abstract
:1. Introduction
2. Methodology
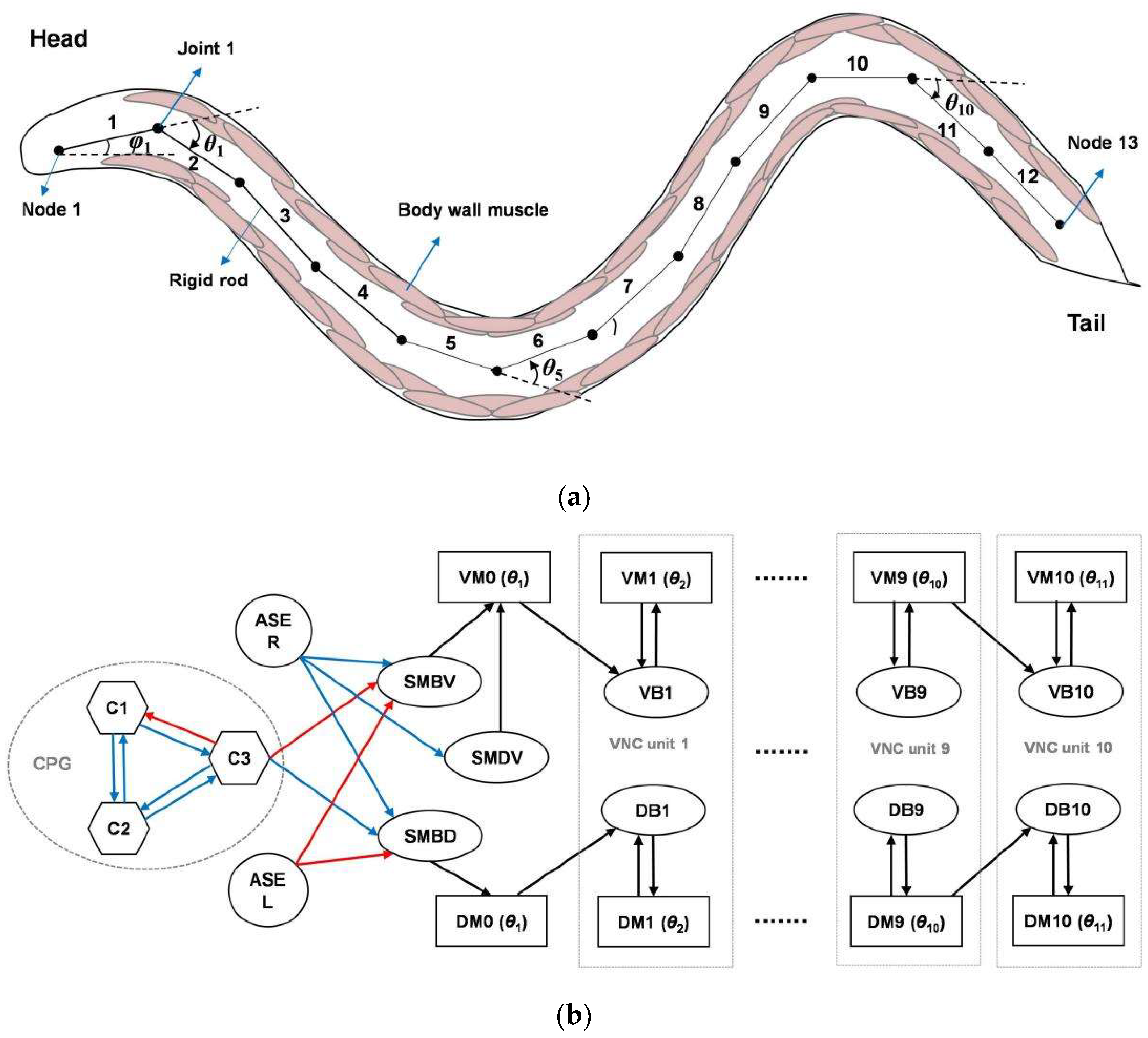
2.1. Overall Model Architecture and Biological Basis
2.2. Definitions
2.3. Undulatory Control Circuit
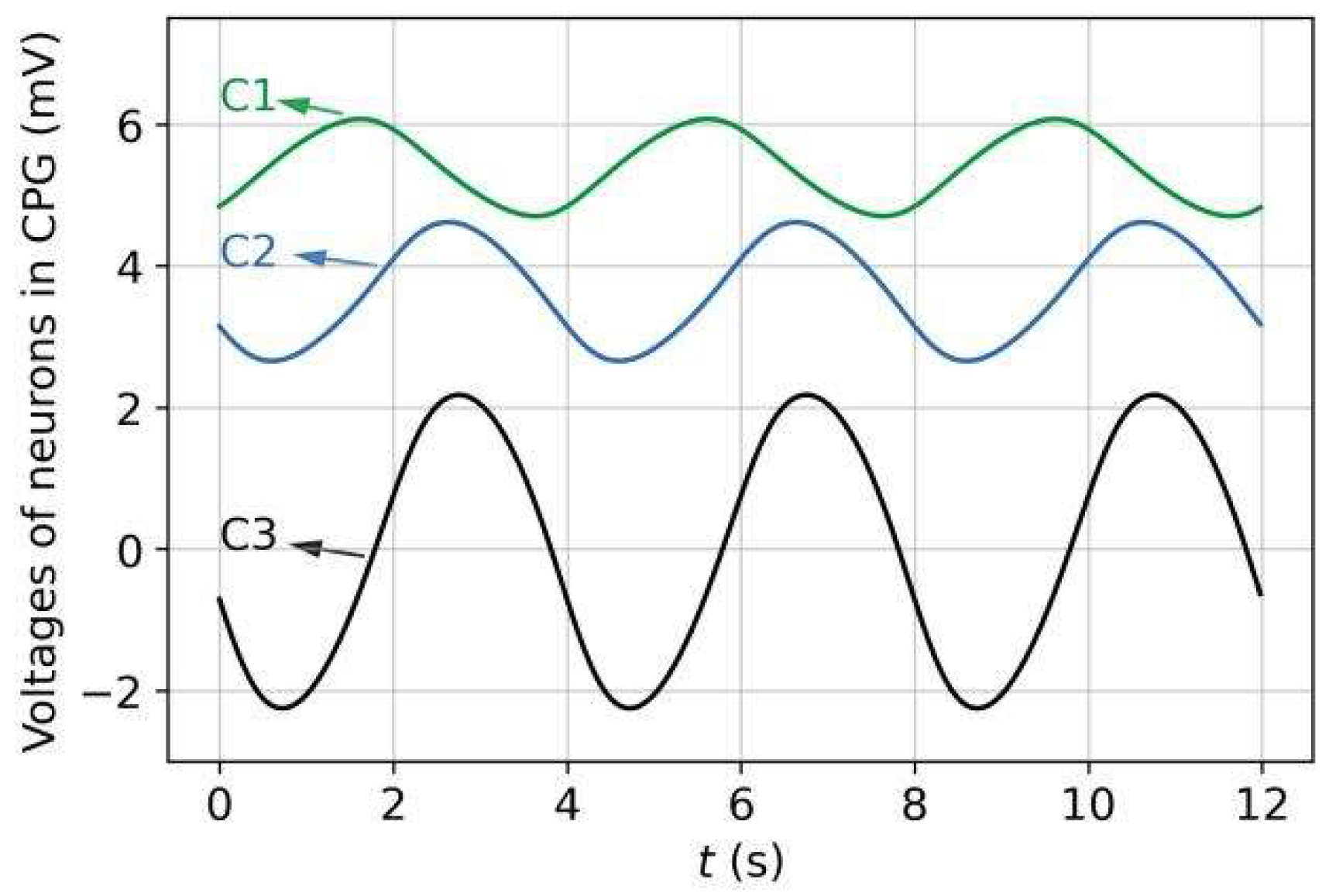
2.3.1. CPG
2.3.2. Undulation Generation and Propagation
2.4. Navigation Control Circuit
2.4.1. Adaptive Sensory Neuron Models
2.4.2. Klinotaxis Control Circuit
2.4.3. Klinokinesis Control Circuit
3. Simulation Results and Discussion
3.1. Rhythmic Patterns of CPG and Joint Angles
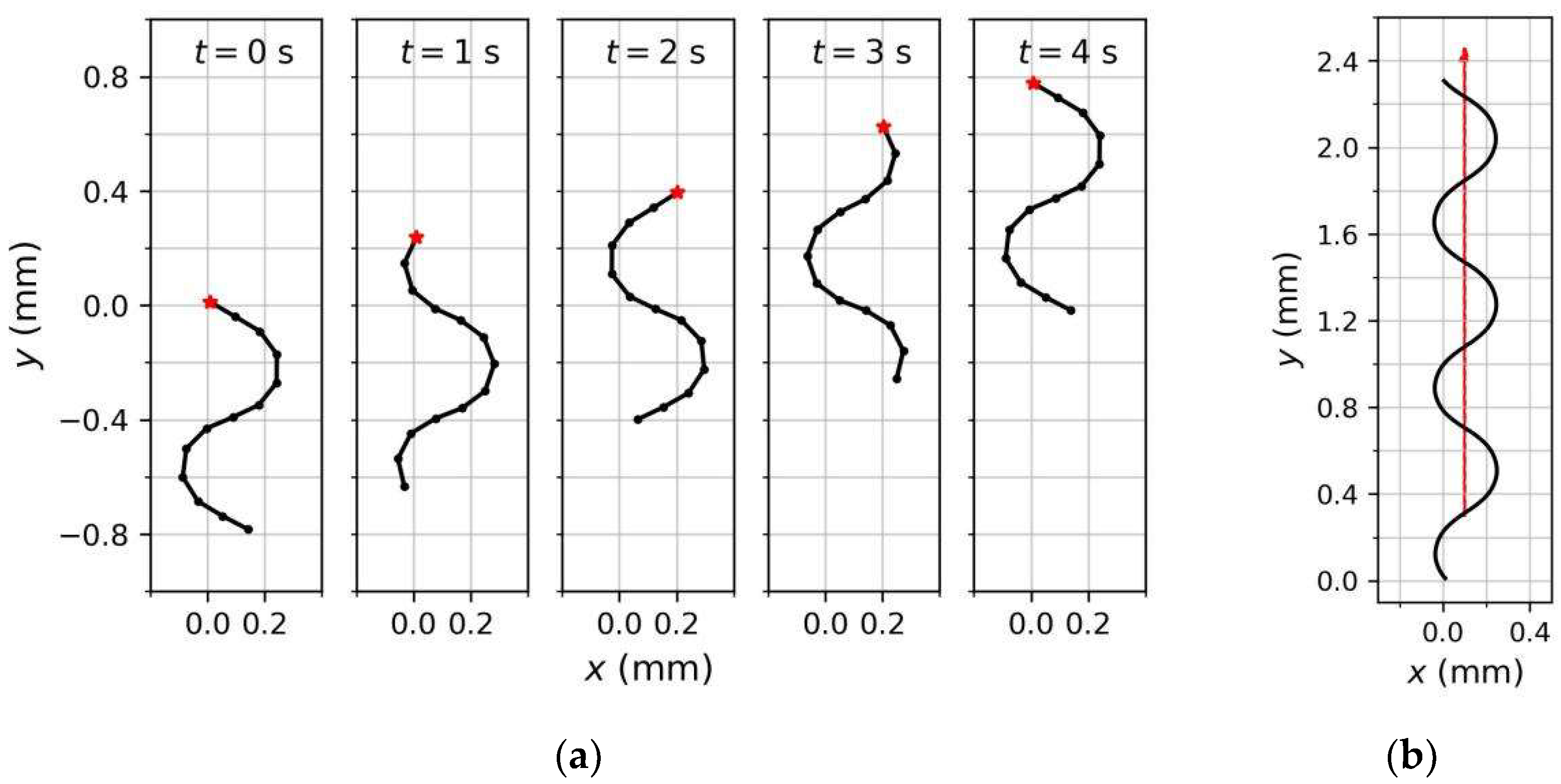
3.2. Forward Undulatory Locomotion Behavior of Model
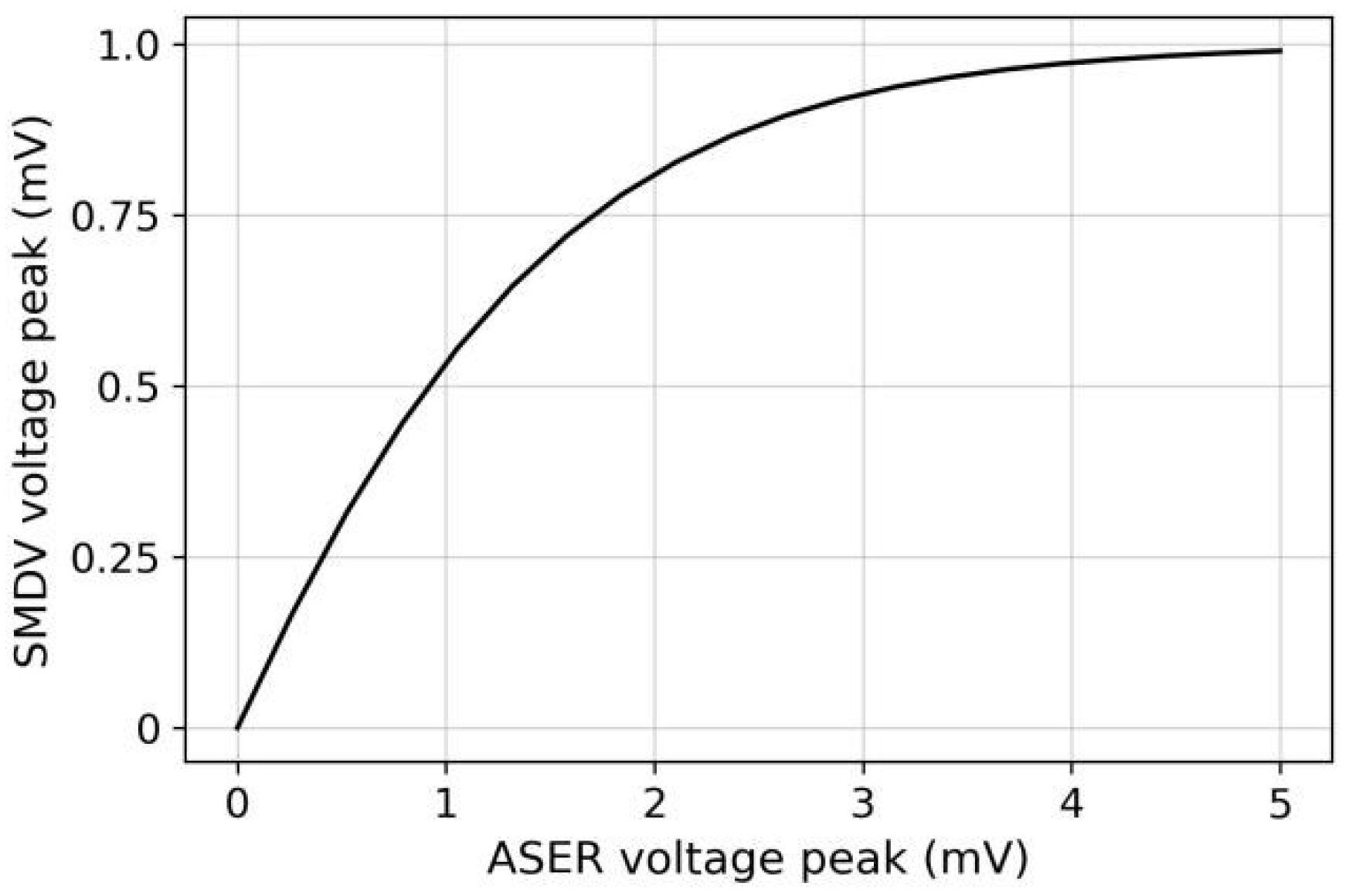
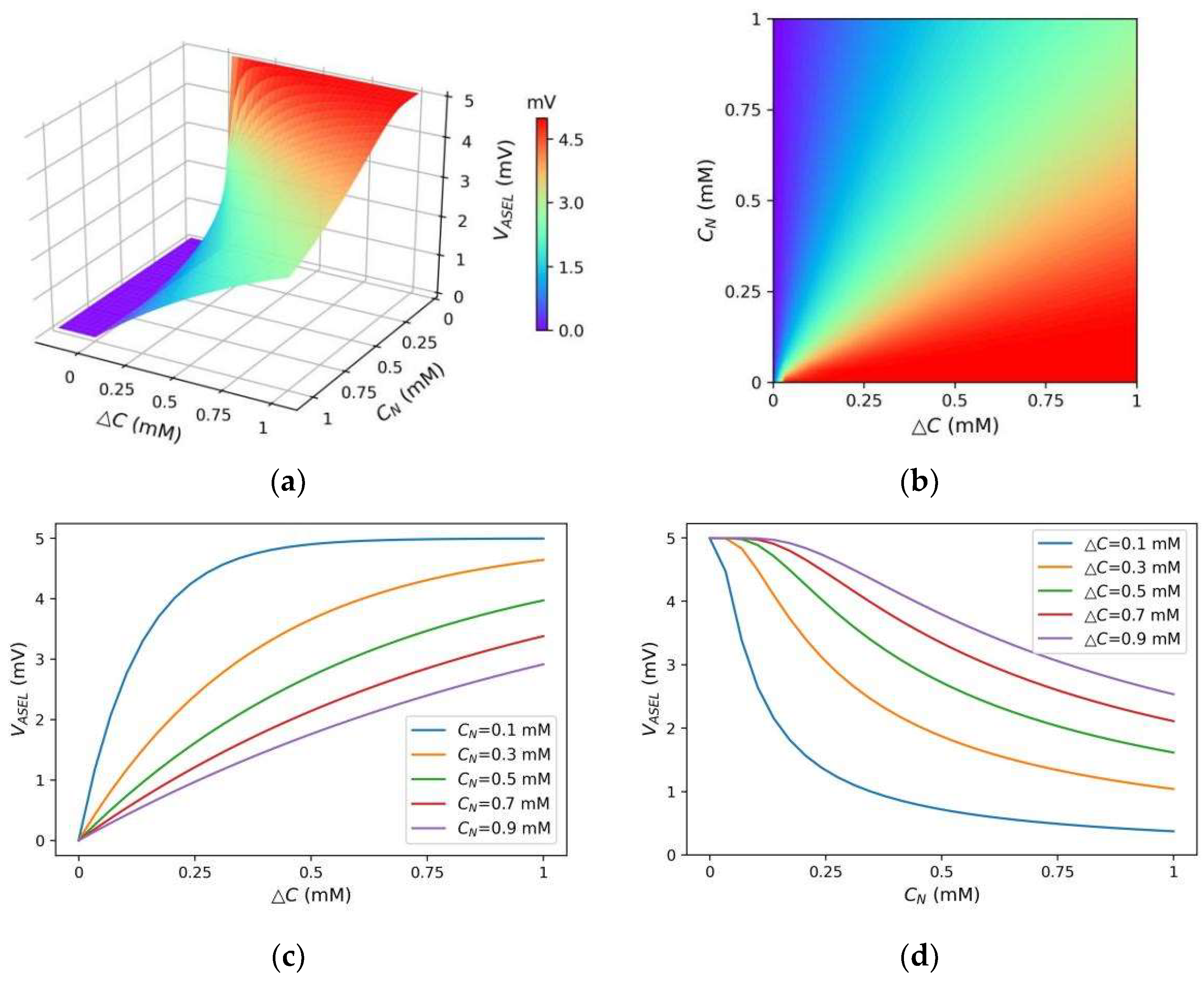
3.3. Adaptive Responses of Sensory Neuron Models
- ASEL responded to concentration increases (ΔC(t) > 0) only, as required in S1;
- When positive ΔC(t) was small, the ASEL response amplitude was small. The ASEL voltage peak increased with the increase in ΔC(t) for any given CN(t) (Figure 10b,c). This indicates that for the local scenario or the scenario with small gradient differences (i.e., the model did not need to re-adapt to the new gradient range), the ASEL response amplitude was proportional to the detected temporal gradient, as required in S2. On this basis, the model can control the steering amplitude by comparing magnitudes of the same-polarity gradients;
- When CN(t) was small, the ASEL response amplitude was large, implying that ASEL was highly sensitive to the gradient. The ASEL voltage peak decreased with the increase in CN(t) for any positive ΔC(t) (Figure 10b,d). This indicates that the ASEL response was adaptive to the local environment. When exposed to large concentration gradients for a period, the sensory neurons became less sensitive to the gradients. Thus, the characteristic in S3 was satisfied, allowing the model to operate effectively across a wide range of gradients.
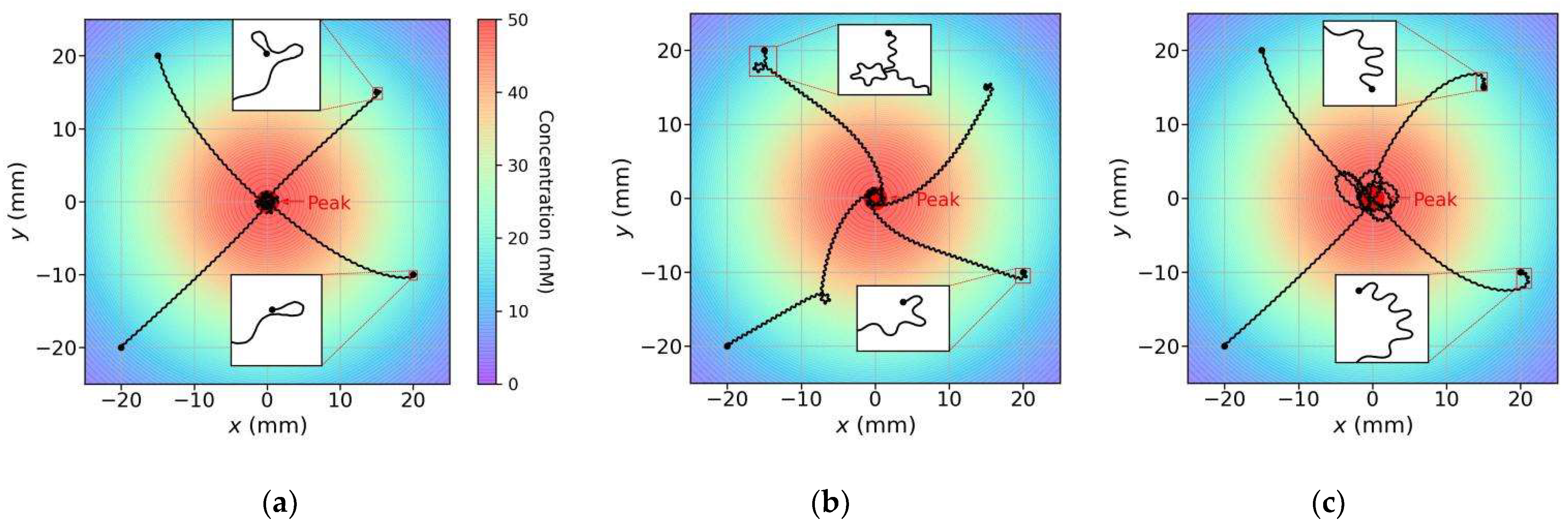
3.4. Autonomous Search Behavior of Model
- For various initial conditions, the three models moved forward in a sinusoidal fashion and successfully reached the concentration peak; however, their search trajectories varied;
- As regards the search trajectories of the klinokinesis-only model (Figure 11b), for the positive gradient direction, the model did not steer, even if there was a deviation from the peak direction. The model corrected the locomotion direction by right turns only when negative gradients were encountered; this behavior is consistent with that of the previous models [29]. In other words, the model could only ensure that it was moving close to the peak, but not that it was using a short search path. In such cases, a large locomotion undulation is advantageous, because a large swing facilitates detection of a negative gradient and adjustment to a more favorable direction. However, a large swing also yields a longer path and may increase the search time;
- As regards the search trajectories of the klinotaxis-only model (Figure 11c), this model continuously and gradually veered toward the side with higher concentration throughout the undulatory locomotion. The orientation adjustment of the model was slightly slower than that of the klinokinesis-only model for negative gradients (compare the beginnings of trajectories whose beginning position are (20, −10) in Figure 11b,c). Because the ASEL responses reduced the Joint-1 oscillation amplitude when the model moved toward positive gradients, the model swing amplitude decreased; hence, the search paths were shortened;
- Our model, which integrates both strategies, yielded significantly shortened search paths (Figure 11a). Regardless of the initial position and orientation, the model reached a peak in a direction close to the steepest gradient. If the search began in a direction away from the peak, the klinokinesis strategy allowed the model to make sharp turns to rapidly correct the direction. Meanwhile, the klinotaxis strategy allowed the model to gradually optimize the locomotion direction according to the deviation between the instantaneous and peak directions.
- The arrival rates of all three models were 1, indicating that the models reached the concentration peaks in all experiments regardless of whether they used single or parallel strategies. In addition, the standard deviations of the SSRs for all models were very small, indicating that the models had robust search performance for different scenarios and initial conditions;
- Compared with the models using a single strategy, the average SSR of the model using parallel strategies was the smallest and close to 1, indicating that the search paths were effectively shortened by simultaneous use of the two complementary strategies, and that the search paths were approximated to the optimal paths although the model moved along a waveform path instead of a straight line;
- The average SSR of the klinokinesis-only model was much larger; this was because it did not optimize the path in the direction of the positive gradients and the swing amplitude of the undulatory locomotion was large (see Figure 11b).
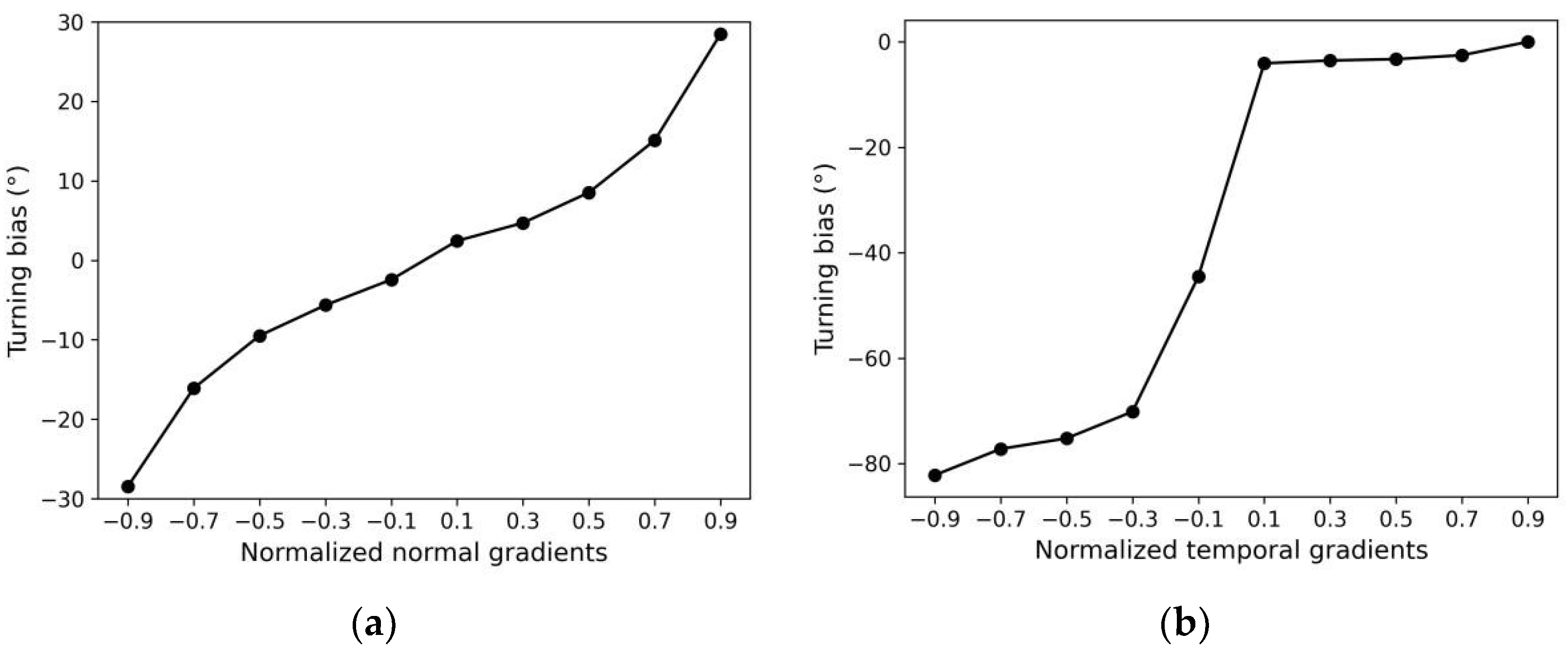
3.5. Analysis of Search Behavior
3.6. Discussion
- (1)
- Previous models typically adopted one strategy to perform navigation tasks. In contrast, our model combines two strategies to improve the search performance;
- (2)
- Our model can realize the klinotaxis behavior with a single sensor by incorporating the state-dependent gating mechanism, whereas previous models that mimic klinotaxis typically require two sensors to obtain the required spatial gradient;
- (3)
- Our model can realize body undulatory locomotion during steering by incorporating a proprioceptive mechanism, similar to the model in [29]. However, the structure of our model is simpler;
- (4)
- Our model exhibits adaptive sensitivity to the concentration gradient to cope with scenarios with various gradient ranges, a function which is absent in the previous models.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, J.G.; Southgate, E.; Tomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [PubMed]
- Cook, S.J.; Jarrell, T.A.; Brittin, C.A.; Wang, Y.; Bloniarz, A.E.; Yakovlev, M.A.; Nguyen, K.; Tang, L.T.; Bayer, E.A.; Duerr, J.S. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 2019, 571, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Yoshida, K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J. Neurosci. 2009, 29, 5370–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce-Shimomura, J.T.; Morse, T.M.; Lockery, S.R. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 1999, 19, 9557–9569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, J.A.; Marques, L.; Costa, E. A comparative study of bio-inspired odour source localisation strategies from the state-actionperspective. Sensors 2019, 19, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-G.; Cao, M.-L.; Meng, Q.-H. Chemical source searching by controlling a wheeled mobile robot to follow an online planned route in outdoor field environments. Sensors 2019, 19, 426. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.; Liu, M.; Neusypin, K.A.; Liu, H.; Guo, M.; Xiao, Y. Autonomous search of radioactive sources through mobile robots. Sensors 2020, 20, 3461. [Google Scholar] [CrossRef]
- Bayat, B.; Crasta, N.; Crespi, A.; Pascoal, A.M.; Ijspeert, A. Environmental monitoring using autonomous vehicles: A survey of recent searching techniques. Curr. Opin. Biotech. 2017, 45, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.J.; Romano, J.M.; Cowan, N.J. Mechanics of precurved-tube continuum robots. IEEE Trans. Robot. 2009, 25, 67–78. [Google Scholar] [CrossRef]
- Iguchi, Y.; Nakajima, M.; Ariizumi, R.; Tanaka, M. Step climbing control of snake robot with prismatic joints. Sensors 2022, 22, 4920. [Google Scholar] [CrossRef]
- Zhao, X.; Dou, L.; Su, Z.; Liu, N. Study of the navigation method for a snake robot based on the kinematics model with MEMS IMU. Sensors 2018, 18, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.M.; Hill, J.J.; Bargmann, C.I. Inaugural Article: A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 3184–3191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalfie, M.; Sulston, J.E.; White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 1985, 5, 956–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef]
- Wen, Q.; Po, M.D.; Hulme, E.; Chen, S.; Liu, X.; Kwok, S.W.; Gershow, M.; Leifer, A.M.; Butler, V.; Fang-Yen, C. Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron 2012, 76, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Fouad, A.D.; Teng, S.; Mark, J.R.; Liu, A.; Alvarez-Illera, P.; Ji, H.; Du, A.; Bhirgoo, P.D.; Cornblath, E.; Guan, S.A. Distributed rhythm generators underlie Caenorhabditis elegans forward locomotion. eLife 2018, 7, e29913. [Google Scholar] [CrossRef]
- Chen, M.; Feng, D.; Su, H.; Su, T.; Wang, M. Neural model generating klinotaxis behavior accompanied by a random walk based on C. elegans connectome. Sci. Rep. 2022, 12, 3043. [Google Scholar] [CrossRef]
- Yu, Y.V.; Xue, W.; Chen, Y. Multisensory integration in Caenorhabditis elegans in comparison to mammals. Brain Sci. 2022, 12, 1368. [Google Scholar] [CrossRef]
- Ferrée, T.C.; Marcotte, B.A.; Lockery, S.R. Neural network models of chemotaxis in the nematode Caenorhabditis elegans. Adv. Neural Inf. Process. Syst. 1996, 9, 55–61. [Google Scholar]
- Ferrée, T.C.; Lockery, S.R. Computational rules for chemotaxis in the nematode C. elegans. J. Comput. Neurosci. 1999, 6, 263–277. [Google Scholar] [CrossRef]
- Dunn, N.A.; Lockery, S.R.; Pierce-Shimomura, J.T.; Conery, J.S. A neural network model of chemotaxis predicts functions of synaptic connections in the nematode Caenorhabditis elegans. J. Comput. Neurosci. 2004, 17, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Morse, T.M.; Ferree, T.C.; Lockery, S.R. Robust spatial navigation in a robot inspired by chemotaxis in Caenorhabditis elegans. Adapt Behav. 1998, 6, 393–410. [Google Scholar] [CrossRef]
- Xu, J.X.; Deng, X.; Ji, D. Biological neural network based chemotaxis behaviors modeling of C. elegans. In Proceedings of the International Joint Conference on Neural Networks, Barcelona, Spain, 18–23 July 2010; pp. 1–6. [Google Scholar]
- Xu, J.X.; Deng, X. Biological modeling of complex chemotaxis behaviors for C. elegans under speed regulation—A dynamic neural networks approach. J. Comput. Neurosci. 2013, 35, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Santurkar, S.; Rajendran, B. C. elegans chemotaxis inspired neuromorphic circuit for contour tracking and obstacle avoidance. In Proceedings of the IEEE International Joint Conference on Neural Networks, Killarney, Ireland, 12–17 July 2015; pp. 1–8. [Google Scholar]
- Shukla, S.; Dutta, S.; Ganguly, U. Design of spiking rate coded logic gates for C. elegans inspired contour tracking. In Proceedings of the 27th International Conference on Artificial Neural Networks, Rhodes, Greece, 4–7 October 2018; pp. 273–283. [Google Scholar]
- Kishore, A.; Saraswat, V.; Ganguly, U. Simplified klinokinesis using spiking neural networks for resource-constrained navigation on the neuromorphic processor Loihi. In Proceedings of the International Joint Conference on Neural Networks, Shenzhen, China, 18–22 July 2021; pp. 1–8. [Google Scholar]
- Deng, X.; Xu, J.X. A 3D undulatory locomotion model inspired by C. elegans through DNN approach. Neurocomputing 2014, 131, 248–264. [Google Scholar] [CrossRef]
- Deng, X.; Xu, J.X.; Wang, J.; Wang, G.; Chen, Q. Biological modeling the undulatory locomotion of C. elegans using dynamic neural network approach. Neurocomputing 2016, 186, 207–217. [Google Scholar] [CrossRef]
- Krieg, M.; Pidde, A.; Das, R. Mechanosensitive body-brain interactions in Caenorhabditis elegans. Curr. Opin. Neurobiol. 2022, 75, 102574. [Google Scholar] [CrossRef]
- Demin, A.V.; Vityaev, E.E. Learning in a virtual model of the C. elegans nematode for locomotion and chemotaxis. Biol. Inspired Cogn. Archit. 2014, 7, 9–14. [Google Scholar] [CrossRef]
- Costalago-Meruelo, A.; Machado, P.; Appiah, K.; Mujika, A.; Leskovsky, P.; Alvarez, R.; Epelde, G.; McGinnity, T.M. Emulation of chemical stimulus triggered head movement in the C. elegans nematode. Neurocomputing 2018, 290, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Thiele, T.R.; Faumont, S.; Ezcurra, M.; Lockery, S.R.; Schafer, W.R. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 2008, 454, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.J.; Lockery, S.R. Evolution and analysis of minimal neural circuits for klinotaxis in Caenorhabditis elegans. J. Neurosci. 2010, 30, 12908–12917. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, E.J.; Beer, R.D. Connecting a connectome to behavior: An ensemble of neuroanatomical models of C. elegans klinotaxis. PLoS Comput. Biol. 2013, 9, e1002890. [Google Scholar] [CrossRef] [PubMed]
- Niebur, E.; Erdös, P. Theory of the locomotion of Nematodes: Dynamics of undulatory progression on a surface. Biophys. J. 1991, 60, 1132–1146. [Google Scholar] [CrossRef] [Green Version]
- Waterston, R. The Nematode C. elegans; Wood, W., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1988; pp. 281–335. [Google Scholar]
- Erdös, P.; Niebur, E. The neural basis of the locomotion of nematodes. Lect. Notes Phys. 1990, 368, 253–267. [Google Scholar]
- Xu, T.; Huo, J.; Shao, S.; Po, M.; Kawano, T.; Lu, Y.; Wu, M.; Zhen, M.; Wen, Q. Descending pathway facilitates undulatory wave propagation in Caenorhabditis elegans through gap junctions. Proc. Natl. Acad. Sci. USA 2018, 115, E4493–E4502. [Google Scholar]
- Mikolajczyk, T.; Mikołajewska, E.; Al-Shuka, H.F.N.; Malinowski, T.; Kłodowski, A.; Pimenov, D.Y.; Paczkowski, T.; Hu, F.; Giasin, K.; Mikołajewski, D.; et al. Recent advances in bipedal walking robots: Review of gait, drive, sensors and control systems. Sensors 2022, 22, 4440. [Google Scholar] [CrossRef]
- Han, Q.; Cao, F.; Yi, P.; Li, T. Motion control of a gecko-like robot based on a central pattern generator. Sensors 2021, 21, 6045. [Google Scholar] [CrossRef]
- Back, T. Evolutionary Algorithm in Theory and Practice; Oxford University Press: New York, NY, USA, 1996; pp. 106–130. [Google Scholar]
- Olivares, E.; Izquierdo, E.J.; Beer, R. A neuromechanical model of multiple network rhythmic pattern generators for forward locomotion in C. elegans. Front. Comput. Neurosci. 2021, 15, 572339. [Google Scholar] [CrossRef]
- Maruyama, I.N. Receptor Guanylyl Cyclases in Sensory Processing. Front. Endocrinol. 2017, 7, 173. [Google Scholar]








| Strategies | Arrival Rate | Average SSR | |
|---|---|---|---|
| Klinokinesis only | 1.0 | 1.4922 ± 0.07309 | |
| Klinotaxis only | 1.0 | 1.1642 ± 0.09253 | |
| Parallel (klinokinesis & klinotaxis) | Adaptive | 1.0 | 1.0964 ± 0.05162 |
| Non-adaptive (a = 15) | 0.8 | 1.2474 ± 0.32701 | |
| Non-adaptive (a = 30) | 1.0 | 1.4388 ± 0.36184 | |
| Non-adaptive (a = 45) | 1.0 | 1.5720 ± 0.43820 | |
 represents the presence of this capability or property in the model).
represents the presence of this capability or property in the model).
 represents the presence of this capability or property in the model).
represents the presence of this capability or property in the model).| Articles | Capabilities | Properties | ||||
|---|---|---|---|---|---|---|
| Klinokinesis | Klinotaxis | Body Undulatory Locomotion | Single Sensor | Adaptive Sensitivity to Gradients | ||
| Xu et al. [23,24] | Model 1 |  |  | |||
| Model 2 |  | |||||
| Santurkar et al. [25] |  |  | ||||
| Shukla et al. [26] |  |  | ||||
| Kishore et al. [27] |  |  | ||||
| Costalago-Meruelo et al. [32] |  |  | ||||
| Deng et al. [28] |  |  |  | |||
| Deng et al. [29] |  |  |  | |||
| Our study |  |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Feng, D.; Su, H.; Wang, M.; Su, T. Neural Network-Based Autonomous Search Model with Undulatory Locomotion Inspired by Caenorhabditis Elegans. Sensors 2022, 22, 8825. https://doi.org/10.3390/s22228825
Chen M, Feng D, Su H, Wang M, Su T. Neural Network-Based Autonomous Search Model with Undulatory Locomotion Inspired by Caenorhabditis Elegans. Sensors. 2022; 22(22):8825. https://doi.org/10.3390/s22228825
Chicago/Turabian StyleChen, Mohan, Dazheng Feng, Hongtao Su, Meng Wang, and Tingting Su. 2022. "Neural Network-Based Autonomous Search Model with Undulatory Locomotion Inspired by Caenorhabditis Elegans" Sensors 22, no. 22: 8825. https://doi.org/10.3390/s22228825
APA StyleChen, M., Feng, D., Su, H., Wang, M., & Su, T. (2022). Neural Network-Based Autonomous Search Model with Undulatory Locomotion Inspired by Caenorhabditis Elegans. Sensors, 22(22), 8825. https://doi.org/10.3390/s22228825







