1. Introduction
In humans, oxygen plays a crucial role in providing energy and sustaining life and functionality [
1]. A variety of diseases and defects correlate with a lack of oxygen supply (hypoxia) that can lead to severe impairment and even death [
2]. In the late eighteenth century, a therapy was developed, which counteracts the effects of hypoxia and decompression sickness: hyperbaric oxygen therapy (HBOT) [
2]. The treatment involves the intake of pure oxygen under increased ambient pressure, hence the term hyperbaric, thereby increasing oxygen intake (hyperoxia) and enhancing oxygen tissue tension throughout the body. The therapy is now widely used in medical treatment of various maladies such as wound healing disorders, carbon monoxide poisoning, or decompression sickness [
3,
4] and the understanding of its working mechanisms continues to improve [
2]. The duration and utilized pressure in HBOT depends on the diagnosis and intended therapeutic result. For the most prevalent application in the treatment of wound healing disorders, the standard parameters are HBOT given at 2.0 to 2.4 ATA for 90 minutes daily for 30 to 40 days [
5,
6,
7,
8].
In order to avoid the Paul Bert effect in patients, gas- or air-break episodes are introduced.
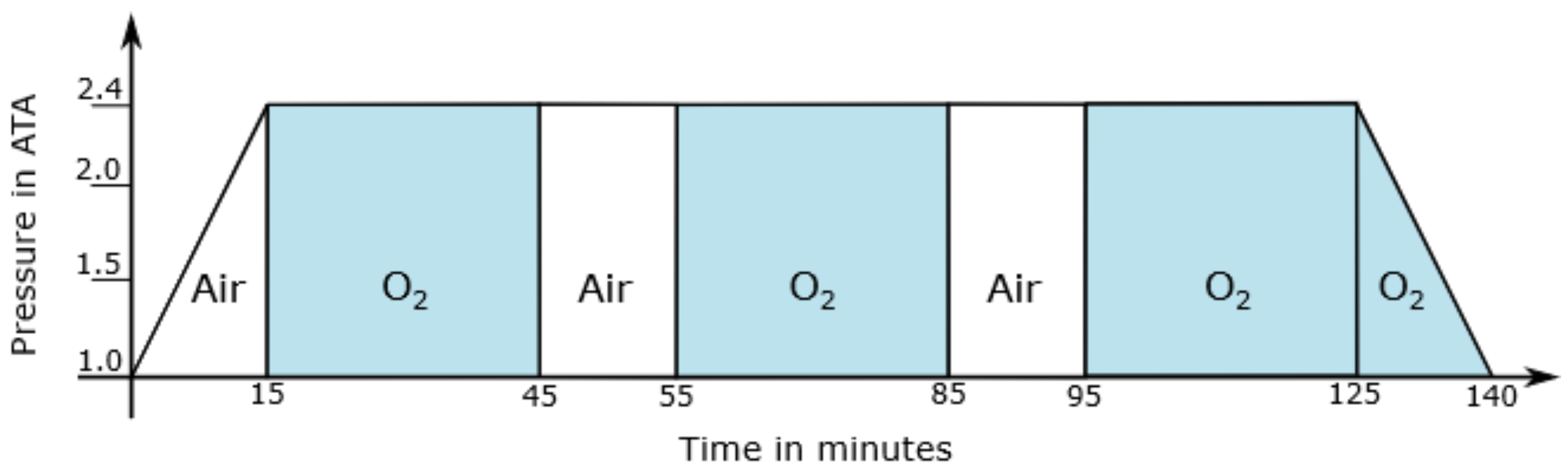
Figure 1 illustrates the therapy scheme TS 240-90 [
3] that is divided into different sections:
Compression to Atmospheres Absolute (ATA);
Isopressure phase during which pure oxygen is administered for 30 via respiratory masks with a subsequent 10 ambient air break, each repeated twice;
Isopressure phase during which pure oxygen is administered for 30 via respiratory masks with subsequent decompression back to normal ambient air (1 ATA) with continued oxygen exposure
It is commonly assumed that hyperbaric oxygen (HBO) has a vasoconstrictive effect on human arteries [
1,
2,
9,
10,
11,
12]. Vasoconstrictive effects can be detected by studying changes in the Pulse Transit Time (PTT). The propagation of the pulse pressure wave in an artery depends, inter alia, on the diameter
D and the elasticity
E of the vessel, both of which are affected by vasoconstriction. The Pulse Wave Velocity (PWV ) [
13,
14,
15,
16] as described by the Moens–Korteweg equation
increases as a result to HBO exposure, as the diameter
D decreases and elasticity
E increases, and can thus be used as an indicator for the cardiovascular response to HBO. Here,
refers to the Pulse Wave Velocity,
E to Young’s modulus of elasticity,
h to a vessel’s wall thickness,
D to the diameter of the artery, and
to the blood density.
The PWV affects the time period required for the pulse-generated pressure wave to travel between two arterial sites, e.g., between heart and finger, within the same cardiac cycle. This is referred to as the PTT and it is directly proportional to the state of the cardiovascular system. Consequently, arterial stiffness, vasoconstriction and systemic factors, such as age or cardiovascular health, which affect the elasticity of the arteries, produce changes in PTT. We can therefore predict a shortened PTT as a result of HBO exposure [
9,
10,
11,
12,
17,
18]. Most studies on the effect of hyperbaric oxygen on the vascular system focus either on young healthy volunteers or have very limited participant counts. While devices to measure the PTT are commercially available, most hyperbaric chambers are equipped with one such device, if at all, due to cost limits. The measuring unit presented in this article, named SPECTRE 3.0, is designed as an affordable tool to study the above described effects of HBO on the vascular system on a larger patient number. SPECTRE 3.0 is currently in use in a clinical trial at the HBO-center of the University Clinic of Duesseldorf. The results of this ongoing study are to be presented at a later time and are thus not considered in this article.
2. Materials and Methods
The PTT is typically measured by detecting the time of the R-peak of an Electrocardiogram (ECG) as starting point, which is closely related to the opening of the aortic valve [
17], and measuring the arrival of the pulse wave at a peripheral site, e.g., the finger, using a Photoplethysmogram (PPG) [
19].
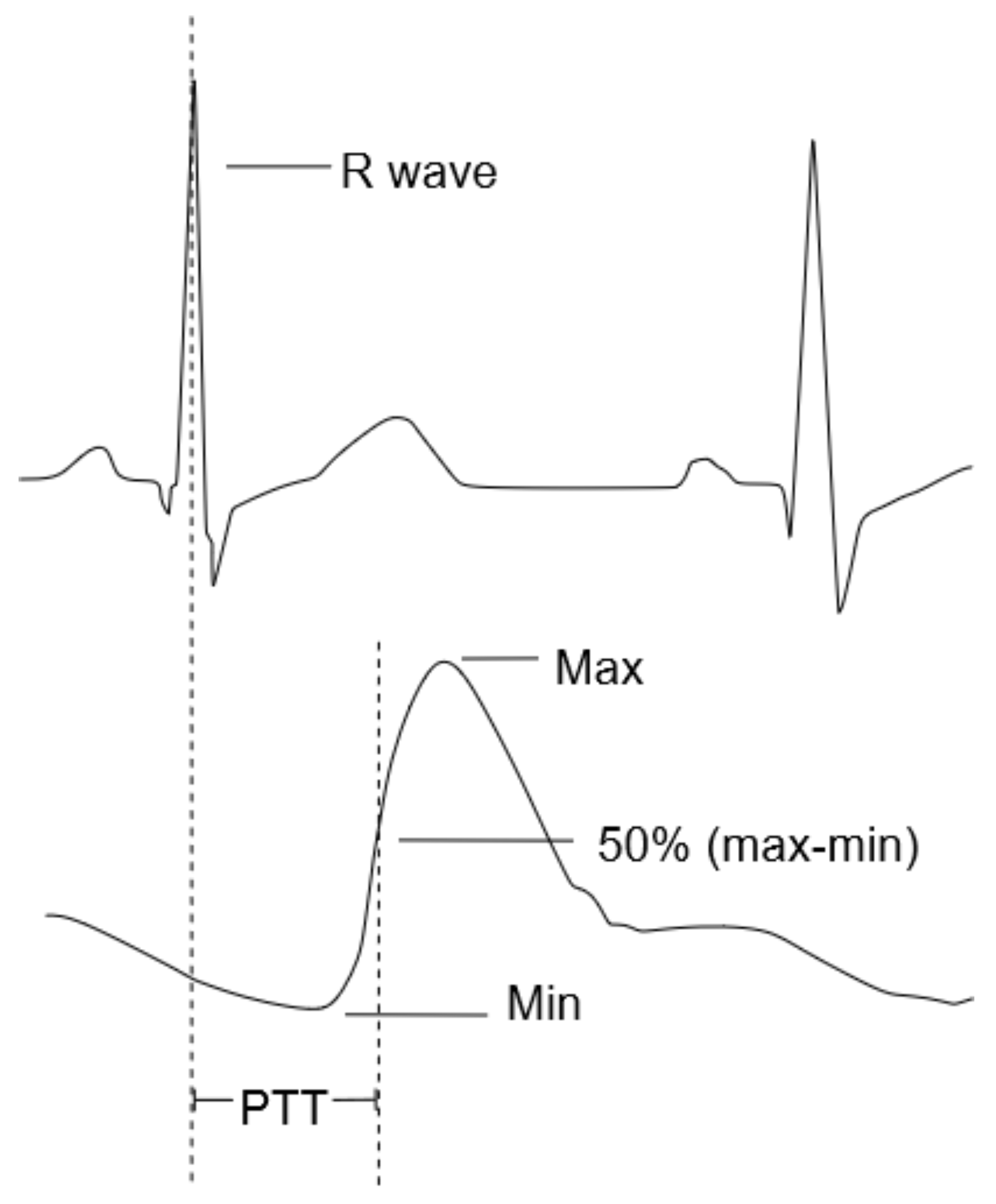
Figure 2 graphically illustrates the PTT as the time difference between the R-peak of the ECG signal and the inflection point of the PPG signal.
2.1. Sensor Selection
The correct evaluation of the PTT requires synchronized recordings of ECG and PPG and a data acquisition rate of approximately 1 . SPECTRE 3.0 is equipped with a Texas Instruments four-channel AFE4900 analog-front-end (AFE) that has the ability to synchronously acquire several different biosignals, i.e., one ECG and up to three PPG signals. The sensor therefore allows the recording of PPG and Peripheral Oxygen Saturation (SpO2 ) data. PPG data acquisition is achieved by measuring changes in green LED light absorbance via Photodetectors (PDs). To allow parallel measurement of SpO2, another two LEDs are needed—one red and one infrared (IR) LED. For this purpose, with selected OSRAM’s SFH7072, a multichip package that provides two green (526 nm) emitters, one red (660 nm) emitter, one IR (950 nm) emitter, one broadband PD, and one IR PD. It is also optimized for PPG signal acquisition in wearable devices. The AFE4900 utilizes different time slots for each of the three LEDs during which one LED is turned on and the resulting PD signal is recorded. One full sample thus contains four datasets consisting of one PPG-signal for each LED and one ECG-signal.
The pressure chamber can induce claustrophobia and is generally, at least during the first few exposures, considered stressful for patients. While high-speed datatransfer of PTT data out of the chamber was rejected, the metal pressure chamber acts as a Faraday cage, low-speed data transfer is possible. Therefore, an additional sensor is added to continuously monitor the patient’s heart rate during HBOT and transmit this information to the chamber operator via the control station outside the chamber. This allows medical staff to notice early signs of distress and to intervene in a timely manner. The MAX30003 by Maxim Integrated is an integrated biopotential AFE for wearable applications that is capable of R-to-R detection in an ECG-signal and thus quickly computes the momentary heart rate.
2.2. Hardware Design
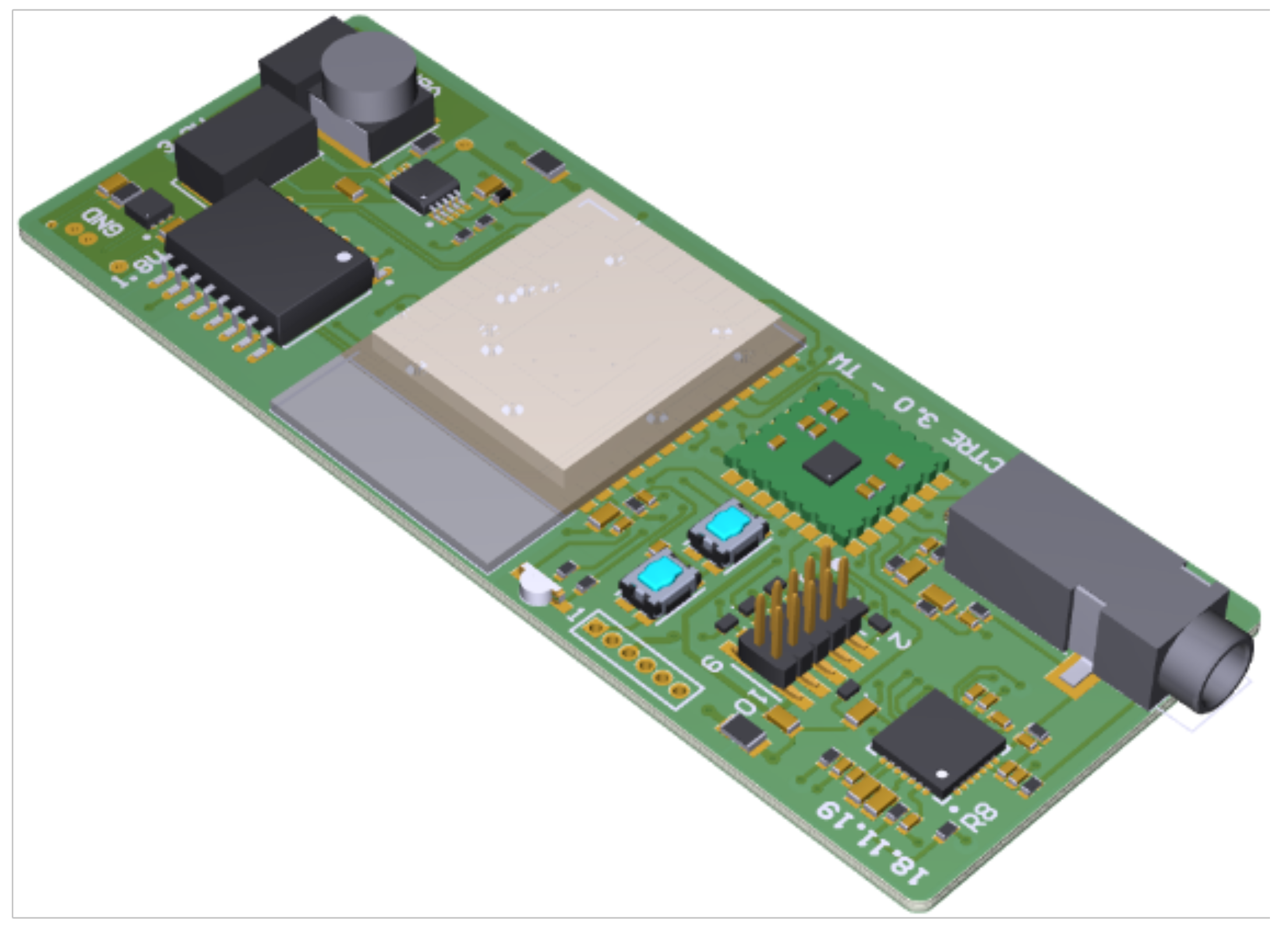
A 28 × 73
printed circuit board (PCB) is designed that hosts the bulk of the circuitry (shown in
Figure 3), namely, a microcontroller unit (MCU) for controlling the system, a programming interface, flash-memory for recorded data, power-supply via batteries, electrode and sensor connectors, the AFE4900, and the MAX30003. The system’s integrated flash memory, Cypress Semiconductor Corporation’s S70FL01GS, provides a sufficient amount of memory (1 Gbit in total) to store the entirety of the data generated by the AFE4900 during a two-hour therapy session. Another smaller PCB with a diameter of 15
hosts the PPG sensor LEDs and PDs, can be mounted on the finger, and is connected to the main board with a suitable ribbon cable and pin header connector.
The AFE4900 and MAX30003 receive the ECG signal from a three-lead ECG electrode audio jack connector. The ECG signal is collected from the human body, with the help of adhesive electrodes in an Einthoven configuration on the left and right forearm of the patient, and transferred to the connector on the main PCB via two cable electrodes. The AFE4900 has an additional Right Leg Drive (RLD) amplifier output, which is connected to a third ECG cable electrode and used to bias the patient to a fixed direct current (DC) operating point to ensure a defined common mode of the ECG signal suitable for the AFE’s operating conditions. Before entering the AFE4900, the ECG-signal is filtered through a passive analog first-order RC low-pass filter (LPF) to reduce high-frequency noise on the ECG-signal such as potentially coupled 50 power line noise.
SPECTRE 3.0’s power is supplied by two AAA alkaline batteries connected in series on a battery holder on the bottom side of the main PCB. This integrated battery holder facilitates quick battery (re-)placement without cumbersome wire connections. A piece of circuitry is added that allows SPECTRE 3.0 to be switched off by using a detachable magnet. Once a magnet is placed near the PCB’s reed sensor, SPECTRE 3.0 is powered off by disconnecting the device from the battery power. Withdrawing the magnet restores power supply and SPECTRE 3.0 is powered on. This approach allows the use of SPECTRE 3.0 even if no magnet is available. In this case, SPECTRE 3.0 can always be powered off by removing the batteries from the battery holder. The two batteries with 1300 mAh last for approximately 7 h of recording time and pose no danger of fire or other unwanted reactions to elevated ambient pressures.
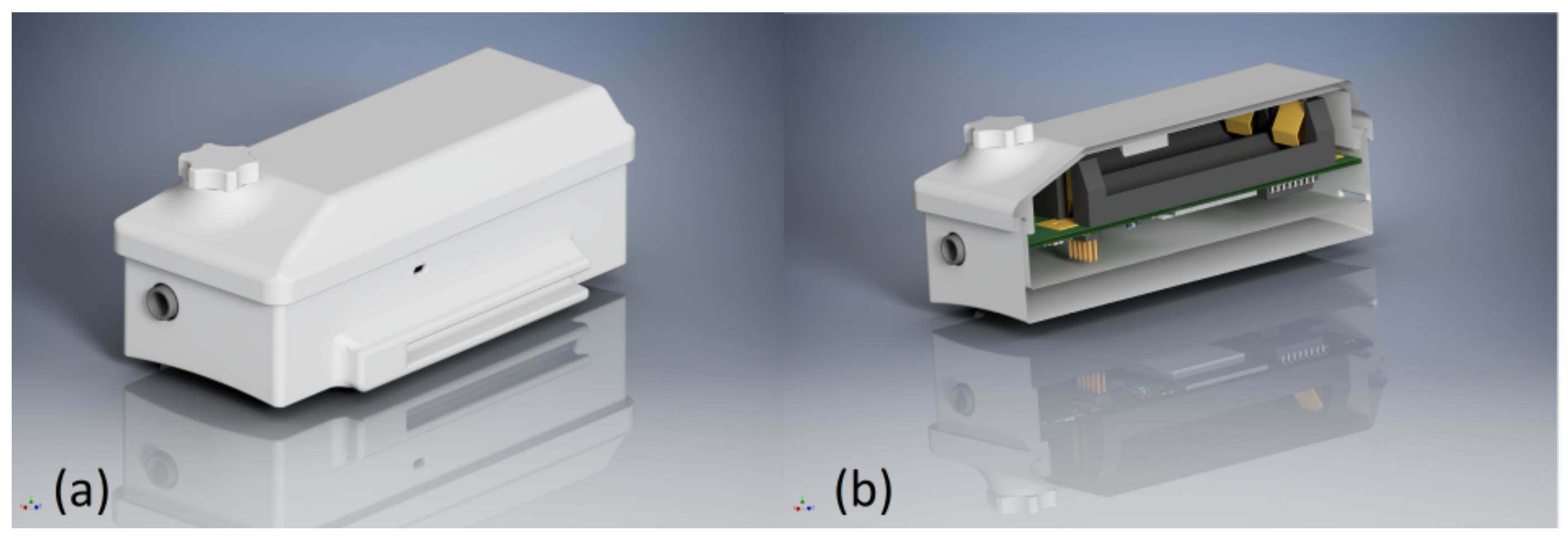
SPECTRE 3.0 is placed in a 3D-printed case that has a detachable lid to facilitate battery exchange, and it is shown in
Figure 4. The housing incorporates recesses for connectors, cable routing, and indicator LEDs, as well as a fixture for attaching a wristband for easy application and a mount for the detachable magnet on top.
Figure 5 shows pictures of the assembled device including the PPG sensor and ECG electrodes.
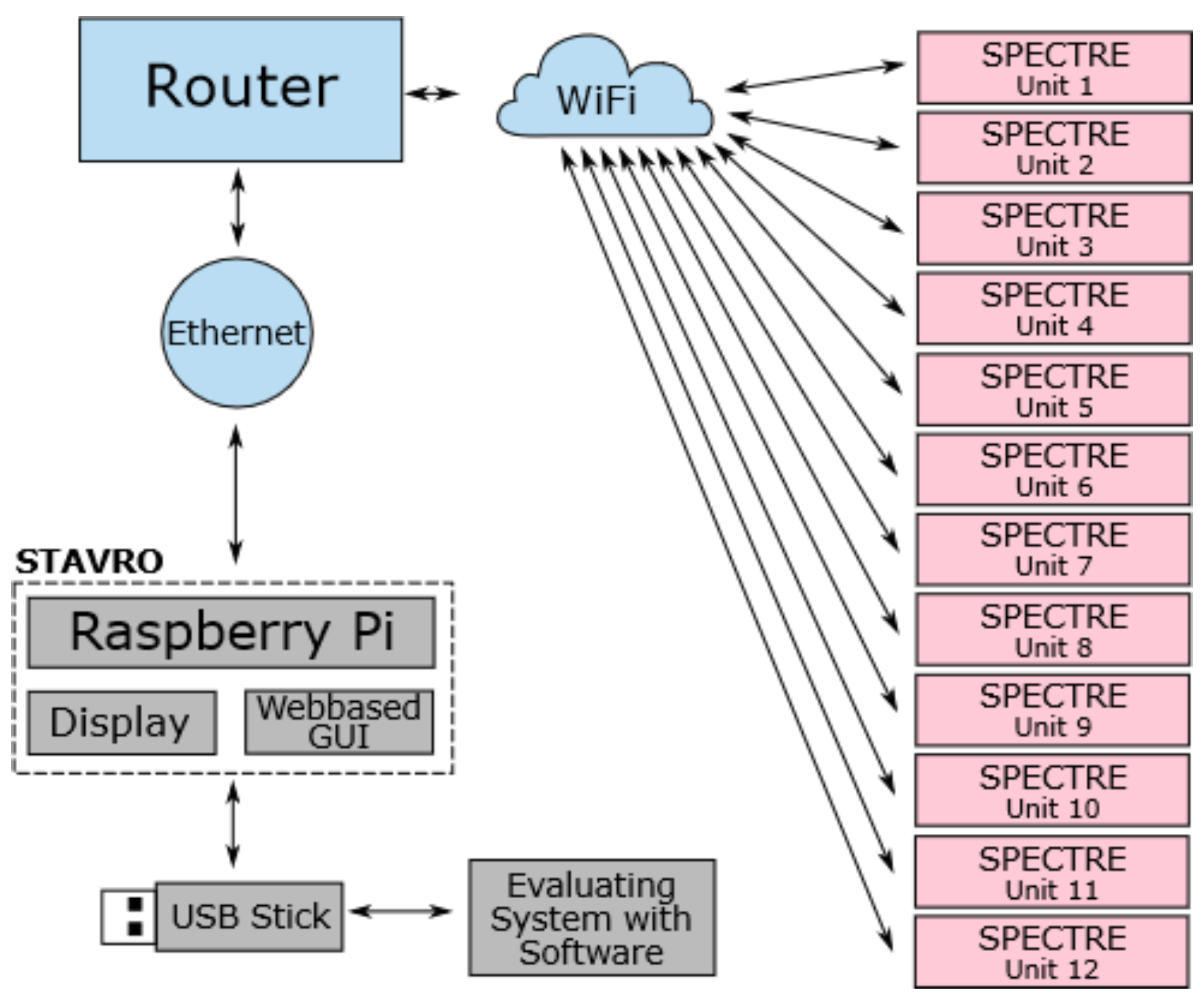
The overall topology of the system is designed to allow data acquisition during HBOT and the subsequent evaluation of that data. The system consists of one control unit and up to twelve SPECTRE 3.0 measuring devices that are connected to a Wireless Local Area Network (WLAN) created by an access point, e.g., a router.
Figure 6 illustrates this overall system topology with arrows demonstrating the directions of data transfer between the components. The control unit is a single board computer (Raspberry Pi 3 Model B+) with a touch display and a Graphical User Interface (GUI).
Figure 7 shows a photograph of the complete setup consisting of the control unit, a router and a measuring device. The measuring units can be worn by patients inside the hyperbaric chamber during HBOT. The control unit remains outside of the chamber and is to be operated by the chamber personnel. Subsequent to HBOT monitoring, the raw data are first transferred from the individual measuring units to the control unit via WiFi. Afterwards, the data are transferred to an evaluating system, e.g., a personal computer (PC) running an evaluation software, via wireless connection or external storage devices.
2.3. Software Design
The measuring unit’s central MCU controls the system, initially processes and stores the data provided by the biosensors and communicates with the control unit. The communication relies on the MQTT protocol for transmitting requests and status information. SPECTRE 3.0 can receive
record data,
send data, and
delete data requests from the control unit and it can send status and heart rate information to be displayed in the GUI. The heart rate information is calculated by using the MAX30003’s data on R-to-R intervals and transmitted to the control unit once per second. The requests
send data and
delete data are only available when SPECTRE 3.0 is not recording data. The delete flash command erases the entire flash memory storage. The send data command reads out the flash memory storage until the last entry and transmits the resulting data packages to the host via the Transmission Control Protocol (TCP). The data are sent in binary format and saved as a binary file for subsequent processing using the evaluation software. Furthermore, the chamber personnel can transmit the specific onset of oxygen administration to the measuring unit via the control unit by pressing a button in the device’s GUI. This allows SPECTRE 3.0 to add the actual time of oxygen onset to the data set to improve the accuracy of the results. In order to provide minimal and unobtrusive visual feedback for fast and reliable user-updates on the basic operational state of SPECTRE 3.0, the main PCB is equipped with a multi-colour LED indicator, whose state can be observed through an opening on the side of the housing. Information encoding is described in
Table 1.
Most errors can be corrected by restarting the device, checking the WLAN network, or changing the batteries. Potential errors include:
Battery power is too low for WLAN connection;
Cannot connect to WLAN network;
Cannot connect to MQTT broker (automatically attempts reconnect);
Error while initialising any hardware component;
Error while erasing the flash memory.
2.4. Data Processing and Evaluation
The recorded data are processed and evaluated using an appropriate evaluation software running on Windows Systems. The software is based on MATLAB® algorithms for digitally processing and filtering ECG and PPG data. A subsequent analysis detects the ECG signal’s R-peaks and computes the time difference to the next PPG signal’s inflection point, thus computing the PTT’s progression during the measuring interval. Furthermore, the software evaluates the R-to-R intervals of the ECG signals, thereby computing heart rate progression as well as heart rate variability.
2.5. Detecting Changes in Pulse Transit Time during Nicotine Administration
To test the measuring method, the effects of nicotine intake on the cardiovascular system were measured using SPECTRE 3.0. Nicotine is known to have a vasoconstrictive effect and to increase the PWV [
20,
21,
22,
23]. We therefore expect a visible decrease in PTT during nicotine intake. The trial was performed on one of the authors as follows:
Volunteer rests for 5 ;
Volunteer smokes 3 cigarettes in quick succession (approx. 5 );
Volunteer rests for another 10 .
The data acquired with SPECTRE 3.0 during the trial were afterwards processed with the evaluating software to compute the PTT’s and heart rate’s progression over time.
3. Results
3.1. Validation of Measuring Methods
The basic functionality of the device was validated by using a phantom 320 ECG simulator by the company Müller und Sebastiani Elektronik GmbH. The ECG was measured both with an oscilloscope and SPECTRE 3.0. The comparison is shown in
Figure 8. The excellent agreement between SPECTRE 3.0’s data and the oscilloscope’s reference data confirms that SPECTRE 3.0 is able to obtain valid ECG data from human subjects.
Additional tests are performed to test the ECG signal quality. The upper graph in
Figure 9 shows the raw (unfiltered) ECG signal obtained with SPECTRE 3.0. from one of the authors. The signal shows a drift resulting from minor motion artifacts. Additional digital filtering using the evaluation software further enhances the signal by reducing noise, normalizing the signal, and eliminating trends and motion artifacts, as seen in
Figure 9 in the graph below. The result is a signal with clearly visible R-peaks that allows subsequent PTT calculation.
The heart rate calculated with the MAX30003 data is validated by comparing the displayed value to other methods of heart rate evaluation. First, the ECG-simulator phantom 320 ECG simulator by Müller und Sebastiani Elektronik GmbH is connected to SPECTRE 3.0. The phantom 320 can output various ECG signals with different frequencies. SPECTRE 3.0’s displayed heart rate values match the configured phantom 320 ECG output frequencies. The value is consistent at lower frequencies (i.e., 60 beats per minute (BPM) and lower), whereas a small deviation (up to ±5%) can be observed in higher frequencies (i.e., 75 BPM +). Further tests on the authors are performed and the obtained heart rates are compared to the computed values of two other devices: the Apple Watch Series 5 and the Sanitas SPO25 finger pulse oximeter. These two devices were chosen as they were available in the laboratory and allowed for easy comparison of simultaneously recorded heart rate measurements on human subjects. In addition, we expect the Apple Watch Series 5 heart rate data to be reliable since its predecessor is the FDA-approved Apple Watch Series 4, which has been shown to be able to accurately detect heart rate and rhythm [
24]. SPECTRE 3.0’s calculated heart rate values match the displayed values of the other two devices with deviations of up to ±10%. Motion artifacts are observed. The findings indicate that the system can reliably monitor the heart rate of patients while recording if the movement of the patient is limited.
The PPG signal recorded with SPECTRE 3.0 is compared to the characteristics of a pulse wave described in the work of Lax et al. [
25] according to which a characteristic pulse wave is comprised of an initial wave and a lower dicrotic wave. The initial wave reaches its peak “within 0.1 to 0.15 s of the start of the wave” [
25]. The dicrotic wave generally appears “from 0.35 to 0.45 s after the start of the initial wave” [
25]. Similar intervals for the specified time periods can be observed in the PPG signals recorded with SPECTRE 3.0 shown in
Figure 10. SPECTRE 3.0’s PPG signals are therefore considered reliable for determining the PPG’s inflection point and consequently calculating the PTT.
3.2. Nicotine Administration
The PTT progression over time during nicotine administration shown in
Figure 11 displays a distinct drop at the onset of nicotine intake at 300
and recovers shortly after discontinuing substance administration at approx. 600
. As a by-product of this measurement, we determined the pharmacokinetic profile of inhaled nicotine with regard to the PTT to be in the range of
250
and
400
. The relaxation time constant of the heart rate (R-to-R intervals) is in excellent agreement with the decline of the mean plasma nicotine concentration after cigarette consumption [
26], whereas the relaxation of the PTT is slightly faster. The vasoconstrictive effects of nicotine administration are clearly visible in the recorded data set. We therefore conclude that SPECTRE 3.0 is suitable for detecting changes in the course of the PTT over time.
3.3. Recording during HBOT
The system was tested under hyperbaric conditions during a standard HBOT at the University Hospital Duesseldorf. Two of the authors were equipped with SPECTRE 3.0 during the therapy session and recordings were managed by the control unit outside the hyperbaric chamber. Excellent data could be obtained from the devices, both during the therapy session (live heart rate monitoring) and after subsequently processing the data using the evaluation software. SPECTRE 3.0 has therefore proven to be operational under hyperbaric conditions and during HBOT. Multiple SPECTRE 3.0 units are currently involved in a clinical trial at University Clinic Duesseldorf, Center for Hyperbaric Medicine, Clinic for Orthopedics and Trauma Surgery. Several units have undergone dozens of therapy session, sometimes to pressures of up to 6 ATA with minimal failure rates. Replacement of defect units is simple and affordable due to the low cost of each individual unit. Study results are to be published at a later time.
4. Discussion
The aim of this project was to develop an advanced measuring unit that facilitates the acquisition of data on the changes in pulse transit time during HBOT and thereby enable researches to further examine the effects of hyperbaric oxygen on the vascular system. We should note, however, that the accuracy of the PTT evaluation results is highly affected by the quality of the captured ECG and PPG signals. Testing phases in the device’s developmental state showed that signal strength varies strongly between individuals. Subsequent processing and filtering of the recorded data is thus essential for obtaining reliable results.
The duration during which recordings can be obtained is limited by the devices battery capacity and the memory size. Although two AAA batteries with 1300 mAh are sufficient for ensuring a measuring period of up to 7 h, the memory size shortens this period significantly. With a storage capacity of 1 Gbit, the memory lasts only for approximately 2 h of recording time and is therefore sufficient for measurements during the aforementioned TS 240-90 therapy scheme. Currently, SPECTRE 3.0’s storage size might not provide full coverage for other therapy schemes requiring longer periods of HBO exposure. Future device enhancements could therefore include optimized data processing and compression directly on the microcontroller to better take advantage of the memory storage and expand the available recording period. Additionally, the data are not deleted automatically once a new recording is started. On the one hand, this prevents accidental deletion of data, but, in turn, it requires the device operator to actively clear the memory space in advance of a new measurement. In general, the device requires some attention from the chamber personnel when recording data as the onset of oxygen administration has to be marked actively using the control unit. In turn, the device offers additional monitoring of the patients by displaying the individuals’ momentary heart rate. However, this feature is still in need of improvement as a patient’s movement greatly affects the accuracy of the measurements.
Furthermore, even though the device allows recording of red and IR LED light absorption, the evaluation of these data with regard to monitoring SpO2 is currently not implemented due to lack of relevance. The SpO2 of all but the most severe cases of lung dysfunction in humans during HBOT is stable around 100%. The main objective of measuring changes in pulse transit time could be reached and demonstrated during trials using nicotine to create vascular responses.
5. Conclusions
The 28 × 73 small measuring unit, SPECTRE 3.0 is operational under hyperbaric conditions and records ECG and PPG data synchronously with a sample frequency of 1 kHz. These data can be subsequently used to calculate the current PTT and heart rate with great precision. Additional data can be acquired that allow the evaluation of blood oxygen saturation (SpO2). Moreover, SPECTRE 3.0 reliably computes the subject’s heart rate and, every second, transmits these data to the control unit outside the hyperbaric chamber. There, the heart rate is displayed in a GUI and thus allows live monitoring of the patients by the chamber personnel.
Author Contributions
Conceptualization, T.W., D.P.H. and D.B.; methodology, T.W.; software, T.W. and D.P.H.; validation, T.W.; formal analysis, T.W.; investigation, T.W.; resources, D.B.; writing—original draft preparation, T.W.; writing—review and editing, D.P.H. and D.B.; visualization, T.W.; supervision, D.B.; project administration, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The supporting firmware of the measuring unit and control unit is available at
https://github.com/BerbenLabs/SPECTRE. The evaluation software is available from the corresponding author upon reasonable request.
Acknowledgments
This work was motivated by our cooperation with Sven Dreyer, Hartmut Strelow, and Karsten Krispin; Center for Hyperbaric Medicine Duesseldorf.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HBO | Hyperbaric Oxygen |
| HBOT | Hyperbaric Oxygen Therapy |
| PTT | Pulse Transit Time |
| PWV | Pulse Wave Velocity |
| ECG | Electrogardiogram |
| PPG | Photoplethysmogram |
| LPF | Low-Pass Filter |
| ATA | Atmosphere Absolute |
| SpO2 | Peripheral Oxygen Saturation |
| LED | Light Emitting Diode |
| PD | Photodetector |
| IR | Infrared |
| AFE | Analog Front-End |
| PCB | Printed Circuit Board |
| MCU | Microcontroller Unit |
| DC | Direct Current |
| WLAN | Wireless Local Area Network |
| GUI | Graphical User Interface |
| PC | Personal Computer |
| BPM | Beats per minute |
| MQTT | Message Queuing Telemetry Transport |
| TCP | Transmission Control Protocol |
References
- Clauss, W.; Clauss, C. Humanbiologie Kompakt, 2nd ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2018; pp. 278,298. [Google Scholar]
- Jain, K. Textbook of Hyperbaric Medicine, 6th ed.; Springer International Publishing: Basel, Switzerland, 2017; p. 39 et seq. [Google Scholar]
- Strelow, H. Therapie in einer Druckkammer: Hyperbare Sauerstofftherapie (HBO)—Grundlagen, Indikationen und Forschung. Prakt. Arbeitsmedizin 2009, 16, 38–41. [Google Scholar]
- GTÜM HBO-Therapie. Available online: https://www.gtuem.org/hbo-therapie/sh-25.html (accessed on 20 September 2022).
- Gesell, L.B. (Ed.) Hyperbaric Oxygen Therapy Indications, 12th ed.; Undersea and Hyperbaric Medical Society: Durham, NC, USA, 2008. [Google Scholar]
- Brem, H.; Sheehan, P.; Boulton, A.J.M. Protocol for treatment of diabetic foot ulcers. Am. J. Surg. 2004, 187 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef]
- Zamboni, W.; Wong, H.; Stephenson, L. Evaluation of hyperbaric oxygen for diabetic wounds: A Prospective Study. Undersea Hyperb. Med. 1997, 24, 175–179. [Google Scholar] [PubMed]
- Shaha, J. Hyperbaric Oxygen Therapy. J. Am. Col. Certif. Wound Spec. 2010, 2, 9–13. [Google Scholar]
- Goyal, A.; Chonis, T.; Cooper, J.S. Hyperbaric Cardiovascular Effects; StatPearls: Treasure Island, FL, USA, 2020; pp. 32–58. [Google Scholar]
- Mehm, D. Noninvasive assessment of the vasoconstrictive effects of hyperoxigenation. JHM 1990, 4, 177. [Google Scholar]
- Watson, N.A.; Beards, S.C.; Altaf, N.; Kassner, A.; Jackson, A. The effect of hyperoxia on cerebral blood flow: A study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur. J. Anaesthesiol. 2000, 17, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Floyd, T.F.; Clark, J.M.; Gelf, R.; Detre, J.A.; Ratcliffe, S.; Guvakov, D.; Lambertsen, C.J.; Eckenhoff, R.G. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J. Appl. Physiol. 2003, 95, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.I. Over de Voortplantingssnelheid van den Pols [On the Speed of Propagation of the Pulse]; SC Van Doesburgh: Leiden, The Netherlands, 1877. [Google Scholar]
- Moens, A.I. Die Pulskurve; E.J. Brill: Leiden, The Netherlands, 1878; p. 86. [Google Scholar]
- Korteweg, D.J. Ueber die Fortpflanzungsgeschwindigkeit des Schalles in elastischen Röhren. Ann. Der Phys. Chem. 1878, 241, 525–542. [Google Scholar] [CrossRef]
- Pai, R. Relationship between the pulse wave and the flow velocity wave and their propagation velocities in the arterial system: Implications for the assessment of regional physical properties of the arterial beds. Int. J. Angiol. 1999, 8, 127–130. [Google Scholar] [CrossRef]
- Smith, R.P.; Argod, J.; Pépin, J.L.; Lévy, P.A. Pulse transit time: An appraisal of potential clinical applications. Thorax 1999, 54, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Hennig, A.; Patzak, A. Continuous blood pressure measurement using pulse transit time. Somnologie Schlafforschung Schlafmed. 2013, 17, 104–110. [Google Scholar] [CrossRef]
- Geddes, L.A.; Voelz, M.H.; Babbs, C.F.; Bourl, J.D.; Tacker, W.A. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology 1981, 18, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular Toxicity of Nicotine: Implications for Electronic Cigarette Use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Smoking-induced coronary vasoconstriction: Implications for therapetic use of nicotine. J. Am. Coll. Cardiol. 1993, 22, 648–649. [Google Scholar] [CrossRef]
- Moreyra, A.E.; Lacy, C.R.; Wilson, A.C.; Kumar, A.; Kostis, B.J. Arterial blood nicotine concentration and coronary vasoconstrictive effect of low-nicotine cigarette smoking. Am. Heart J. 1992, 124, 392–397. [Google Scholar] [CrossRef]
- Zhu, B.; Parmley, W.W. Hemodynamic and vascular effects of active and passive smoking. Am. Heart J. 1995, 130, 1270–1275. [Google Scholar] [CrossRef]
- Lee, C.; Feinberg, A.W.; Cohen, B.M. Comparison of Apple Watch Series 4 vs. KardiaMobile: A Tale of Two Devices. CJC Open 2022, in press. [Google Scholar] [CrossRef]
- Lax, H.; Feinberg, A.W.; Cohen, B.M. Studies of the arterial pulse wave. J. Chronic Dis. 1956, 3, 618–631. [Google Scholar] [CrossRef]
- Yuki, D.; Sakaguchi, C.; Kikuchi, A.; Futamura, Y. Pharmacokinetics of nicotine following the controlled use of a prototype novel tobacco vapor product. Regul. Toxicol. Pharmacol. RTP 2017, 87, 30–35. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).