Optical Measurement of Molar Absorption Coefficient of HbA1c: Comparison of Theoretical and Experimental Results
Abstract
1. Introduction
2. Methodology
2.1. Theoretical Method
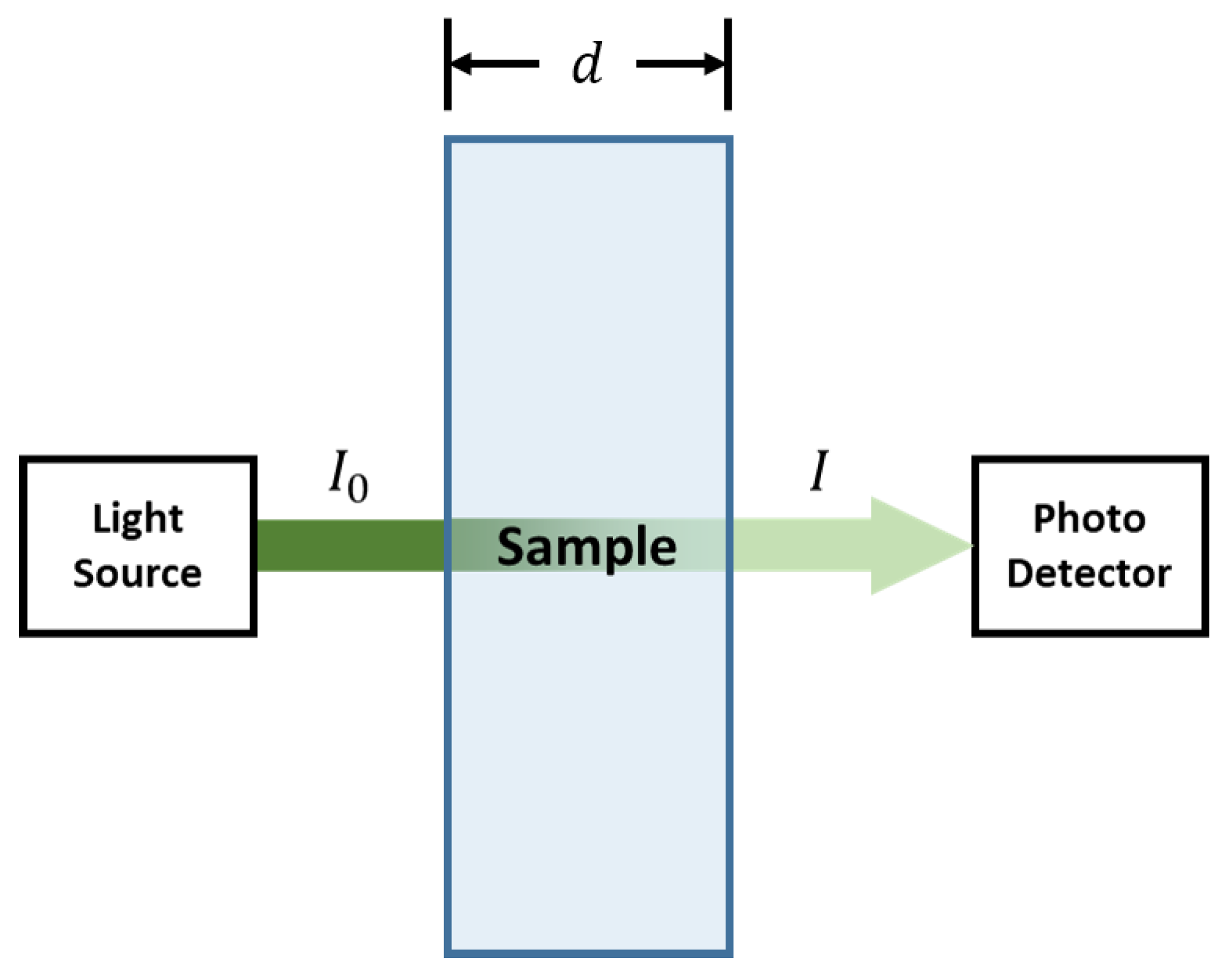
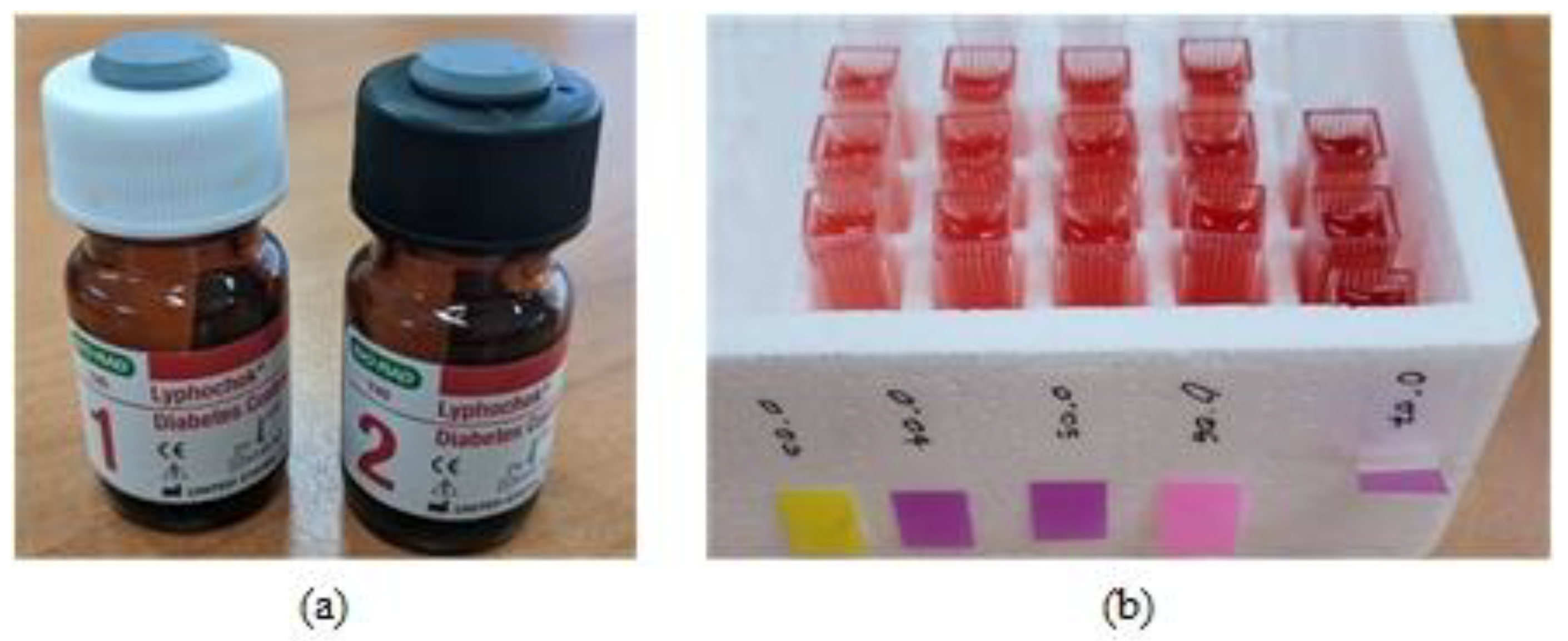
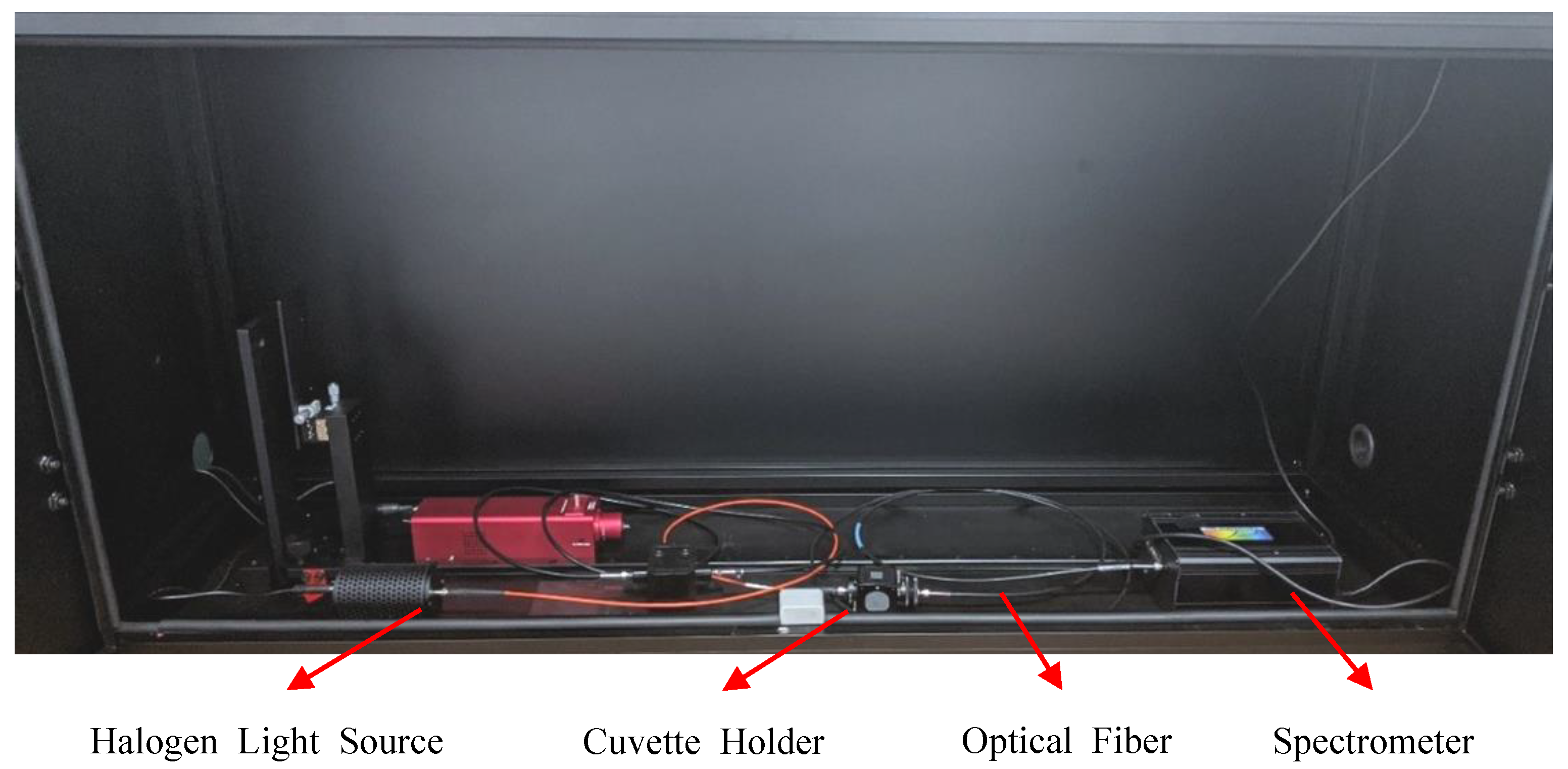
2.2. Proposed Experimental Method
3. Results and Discussion
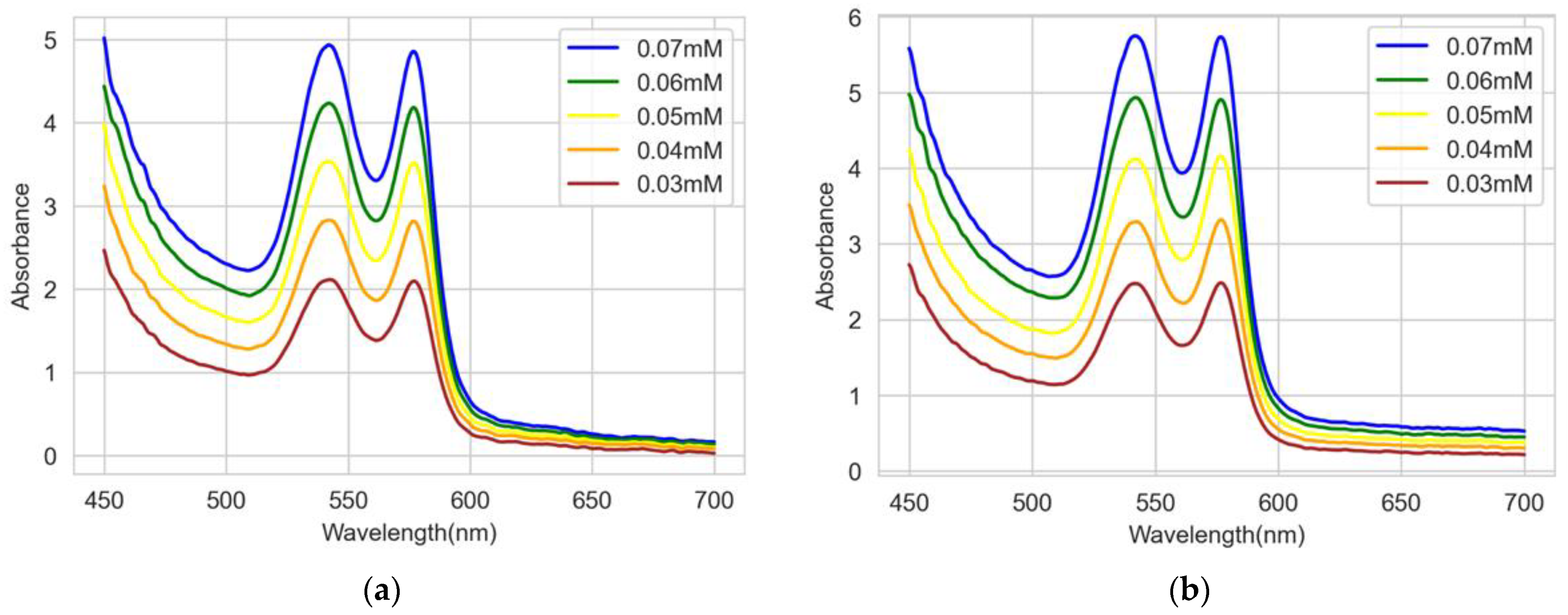
3.1. Experimental Results
3.2. Comparison and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Standards of Medical Care in Diabetes—2018 Abridged for Primary Care Providers. Clin. Diabetes 2018, 36, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E.G. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435. [Google Scholar] [CrossRef] [PubMed]
- Kagie, A.; Bishop, D.K.; Burdick, J.; La Belle, J.T.; Dymond, R.; Felder, R.; Wang, J. Flexible Rolled Thick-Film Miniaturized Flow-Cell for Minimally Invasive Amperometric Sensing. Electroanalysis 2008, 20, 1610–1614. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Mu, Z.; Zhang, Y.; Guo, L. Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sensors Actuators B Chem. 2014, 192, 261–268. [Google Scholar] [CrossRef]
- Mandal, S.; Marie, M.; Kuchuk, A.; Manasreh, M.O.; Benamara, M. Sensitivity enhancement in an in-vitro glucose sensor using gold nanoelectrode ensembles. J. Mater. Sci. Mater. Electron. 2017, 28, 5452–5459. [Google Scholar] [CrossRef]
- Little, R.R.; Roberts, W.L. A Review of Variant Hemoglobins Interfering with Hemoglobin A1c Measurement. J. Diabetes Sci. Technol. 2009, 3, 446–451. [Google Scholar] [CrossRef]
- Soboleva, A.; Mavropulo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.; Grishina, T.; Frolov, A. Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 2329. [Google Scholar] [CrossRef]
- Jung, D.G.; Jung, D.; Kong, S.H. A Lab-on-a-Chip-Based Non-Invasive Optical Sensor for Measuring Glucose in Saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Kwon, T.-H.; Hossain, S.; Kim, K.-D. Towards non-invasive blood glucose measurement using machine learning: An all-purpose PPG system design. Biomed. Signal Process. Control 2021, 68, 102706. [Google Scholar] [CrossRef]
- Arnold, M.A.; Small, G.W. Noninvasive glucose sensing. Anal. Chem. 2005, 77, 5429–5439. [Google Scholar] [CrossRef]
- Mandal, S.; Manasreh, M.O. An in-vitro optical sensor designed to estimate glycated hemoglobin levels. Sensors 2018, 18, 1084. [Google Scholar] [CrossRef]
- Saraoğlu, H.M.; Selvi, A.O. Determination of glucose and Hba1c values in blood from human breath by using Radial Basis Function Neural Network via electronic nose. In Proceedings of the 2014 18th National Biomedical Engineering Meeting, Istanbul, Turkey, 16–17 October 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Hossain, S.; Gupta, S.S.; Kwon, T.-H.; Kim, K.-D. Derivation and validation of gray-box models to estimate noninvasive in-vivo percentage glycated hemoglobin using digital volume pulse waveform. Sci. Rep. 2021, 11, 12169. [Google Scholar] [CrossRef]
- Hossain, S.; Kim, K.-D. Noninvasive Estimation of Glycated Hemoglobin In-Vivo Based on Photon Diffusion Theory and Genetic Symbolic Regression Models. IEEE Trans. Biomed. Eng. 2022, 69, 2053–2064. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Kim, K.-D. Machine-learning-based noninvasive in-vivo estimation of HbA1c using photoplethysmography signals. Sensors 2022, 22, 2963. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, L.; Sun, Y.; Huang, C.; Li, T. Optimal hemoglobin absorption coefficient data set for near-infrared spectroscopy. Biomed. Opt. Express 2017, 8, 5151–5159. [Google Scholar] [CrossRef]
- Mallya, M.; Shenoy, R.; Kodyalamoole, G.; Biswas, M.; Karumathil, J.; Kamath, S. Absorption spectroscopy for the estimation of glycated hemoglobin (HbA1c) for the diagnosis and management of diabetes mellitus: A pilot study. Photomed. Laser Surg. 2013, 31, 219–224. [Google Scholar] [CrossRef]
- Hossain, S.; Kwon, T.-H.; Kim, K.-D. Estimation of molar absorption coefficients of HbA1c in near UV-Vis-SW NIR light spectrum. JKICS 2021, 46, 1030–1039. [Google Scholar] [CrossRef]
- “Beer–Lambert Law,” Wikipedia. Available online: https://en.wikipedia.org/w/index.php?title=Beer%E2%80%93Lambert_law (accessed on 10 January 2021).
- The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Bio-rad Lyphocheck Diabetes Control Level1 (Lot 85821) and Level2 (Lot 85822) Datasheet. Available online: https://www.myeinserts.com/85820 (accessed on 30 April 2022).
- Prahl, S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water. Available online: https://omlc.org/spectra/hemoglobin/summary.html?fbclid=IwAR2F5VOmtzakCum3DpZYBps3ZC3qsAkSjnCVsRVKmac-gV6D4ha4lJY_1Iw (accessed on 10 June 2022).
- Clarke, W.L.; Cox, D.; Gonder-Frederick, L.A.; Carter, W.; Pohl, S.L. Evaluating Clinical Accuracy of Systems for Self-Monitoring of Blood Glucose. Diabetes Care 1987, 10, 622–628. [Google Scholar] [CrossRef] [PubMed]



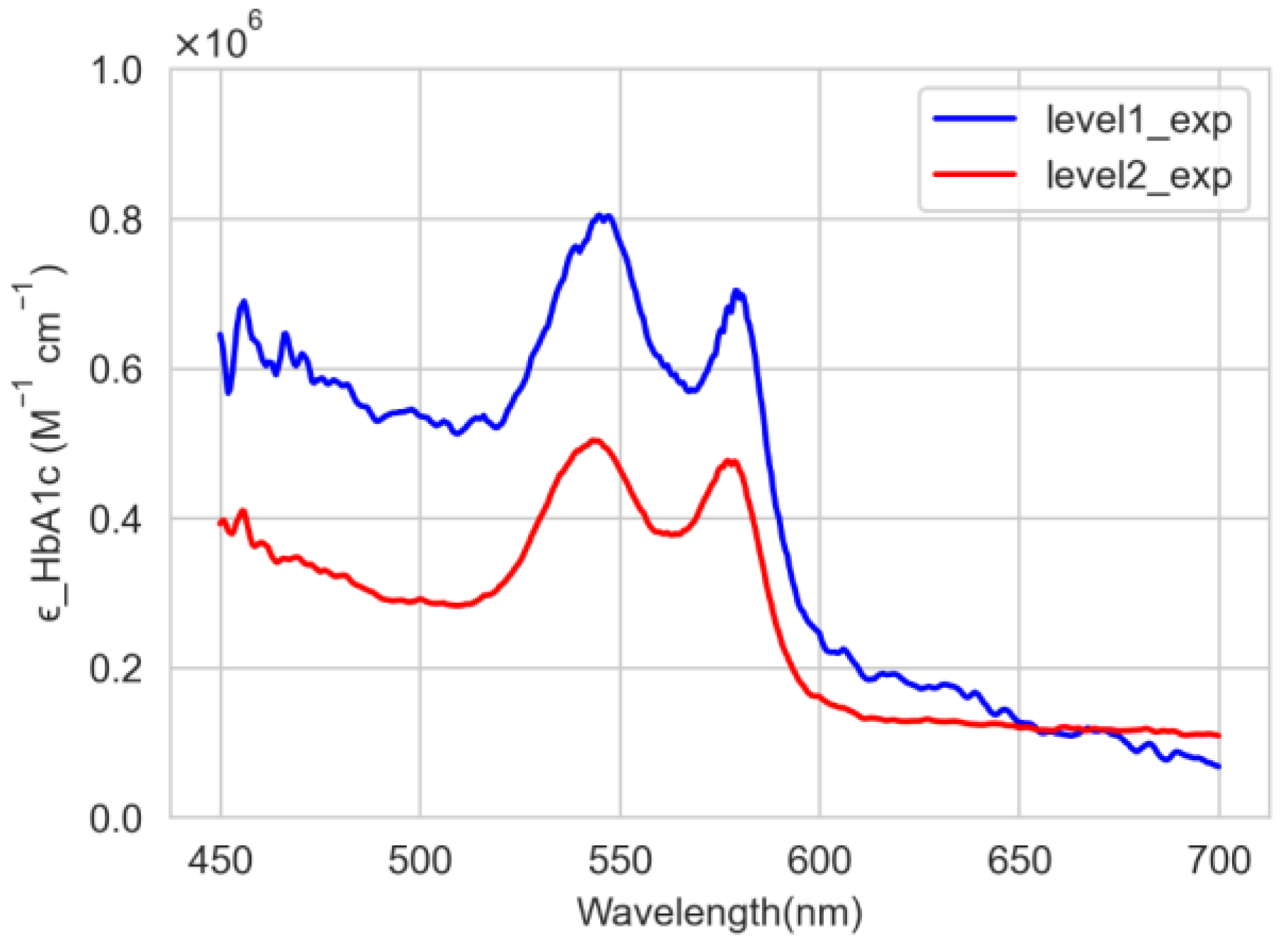
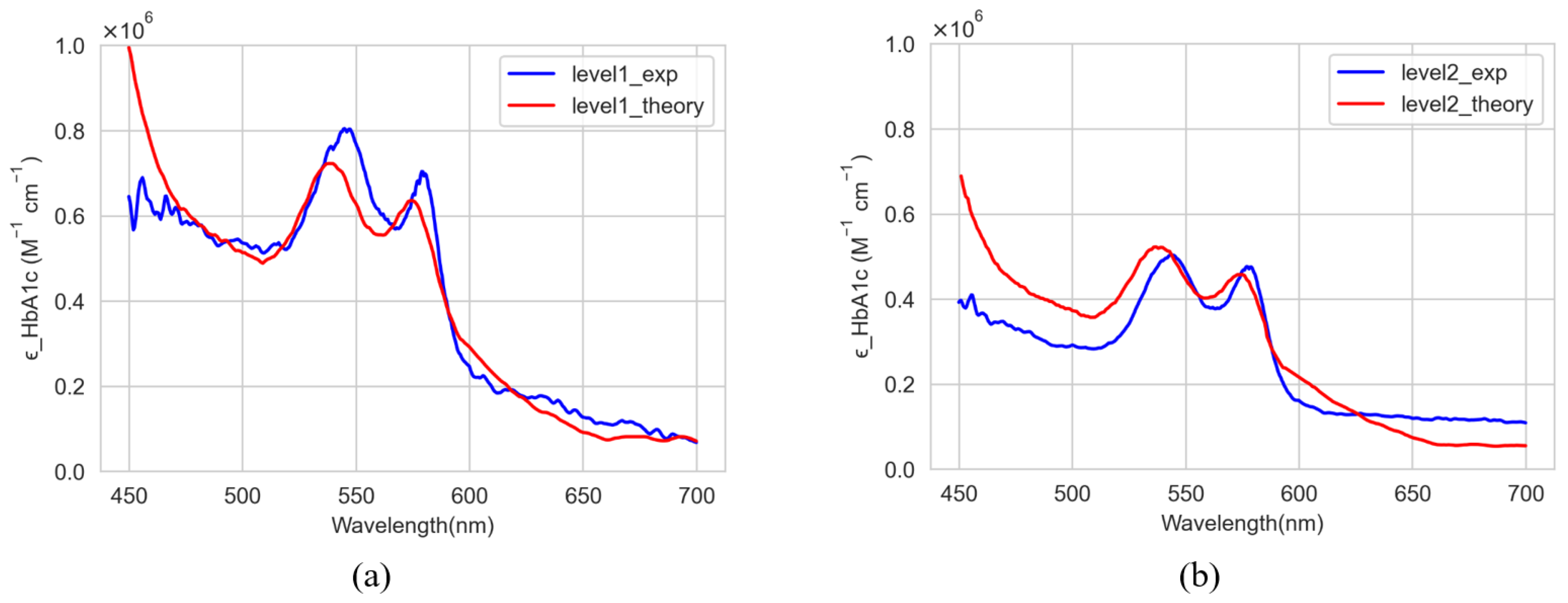
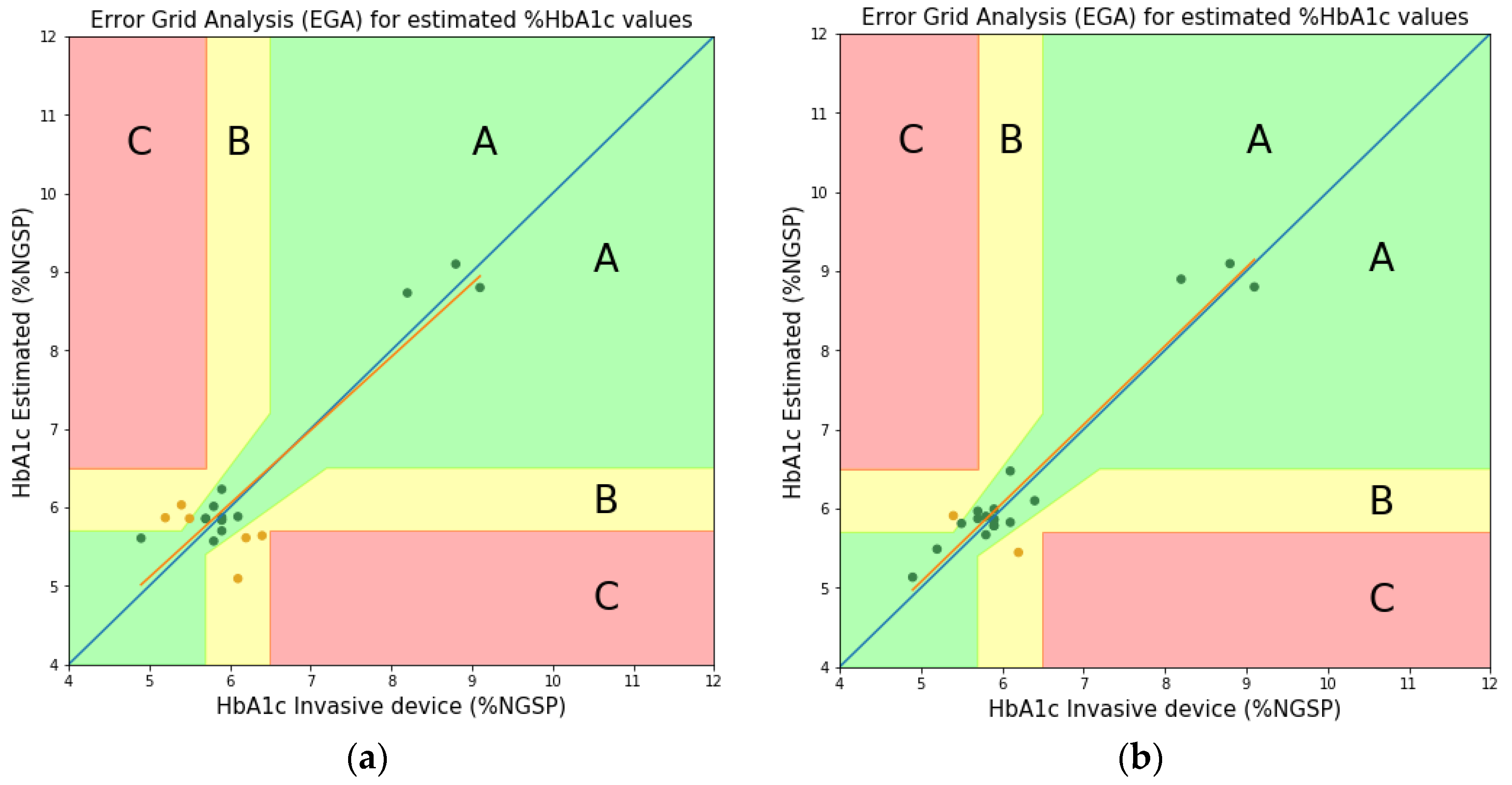
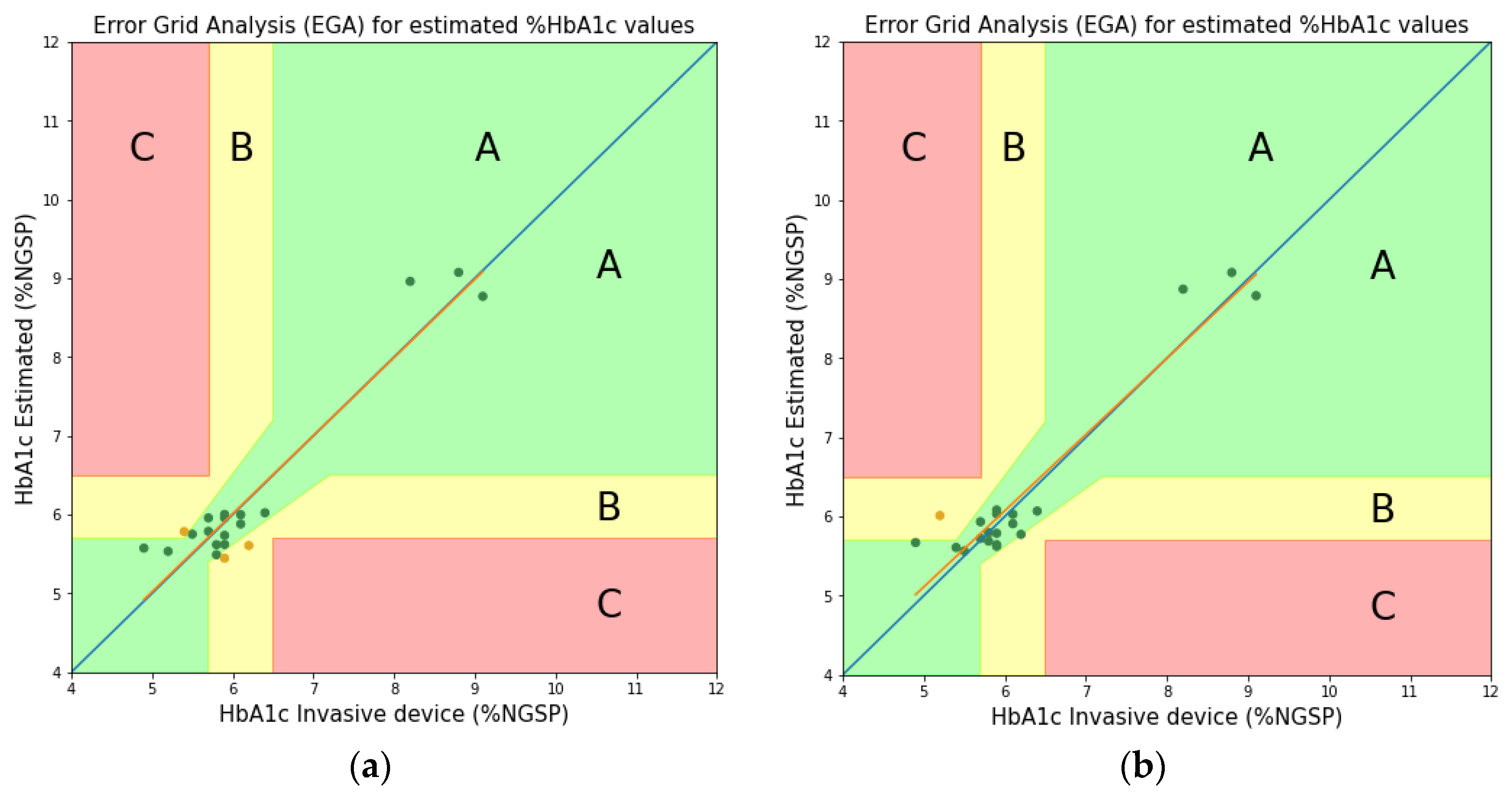
| Wavelengths at Two Peaks | |||
|---|---|---|---|
| Theoretical [21] | 540 nm | 722,205.0 | 520,757.0 |
| 576 nm | 632,437.9 | 455,116.5 | |
| Experimental (Proposed) | 545 (544) * nm | ||
| 579 (577) * nm | |||
| Wavelength (nm) | Molar Absorption Coefficients of HbA1c (M−1 cm−1) | |
|---|---|---|
| Theoretically [21] | Experimentally (Proposed) | |
| 465 | 549,025 | 342,907 |
| 525 | 455,140 | 341,034 |
| 615 | 170,555 | 131,187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, S.; Satter, S.; Kwon, T.-H.; Kim, K.-D. Optical Measurement of Molar Absorption Coefficient of HbA1c: Comparison of Theoretical and Experimental Results. Sensors 2022, 22, 8179. https://doi.org/10.3390/s22218179
Hossain S, Satter S, Kwon T-H, Kim K-D. Optical Measurement of Molar Absorption Coefficient of HbA1c: Comparison of Theoretical and Experimental Results. Sensors. 2022; 22(21):8179. https://doi.org/10.3390/s22218179
Chicago/Turabian StyleHossain, Shifat, Shama Satter, Tae-Ho Kwon, and Ki-Doo Kim. 2022. "Optical Measurement of Molar Absorption Coefficient of HbA1c: Comparison of Theoretical and Experimental Results" Sensors 22, no. 21: 8179. https://doi.org/10.3390/s22218179
APA StyleHossain, S., Satter, S., Kwon, T.-H., & Kim, K.-D. (2022). Optical Measurement of Molar Absorption Coefficient of HbA1c: Comparison of Theoretical and Experimental Results. Sensors, 22(21), 8179. https://doi.org/10.3390/s22218179








