Preliminary Evidence of EEG Connectivity Changes during Self-Objectification of Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Objectifying Task and Self-Objectification Evaluation
2.2.2. EEG Recording and Analysis
2.2.3. ERS Analysis
2.3. PDC Analysis
3. Results
3.1. Self-Objectification Evaluation
3.2. ERS/ERD Analysis
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EEG | Electroencepalography |
| ERD | Event-related desynchronization |
| ERS | Event-related synchronization |
| ERP | Event-related potential |
| NSOs | Non-self-objectified subjects |
| PSD | Power spectral density |
| PDC | Partial directed coherence |
| SOI | Self-objectification index |
| SOs | Self-objectified subjects |
References
- Baldissarri, C.; Andrighetto, L.; Volpato, C. Feeling like an object: A field study on working self-objectification and belief in personal free will. TPM Test. Psychom. Methodol. Appl. Psychol. 2019, 26, 185–197. [Google Scholar] [CrossRef]
- Auzoult, A.L. Can self-consciousness and team reflexivity guard against the consequences of objectification? Pol. Psychol. Bull. 2021, 52, 67–75. [Google Scholar] [CrossRef]
- Baldissarri, C.; Andrighetto, L.; Gabbiadini, A.; Volpato, C. Work and freedom? Working self-objectification and belief in personal free will. Br. J. Soc. Psychol. 2017, 56, 250–269. [Google Scholar] [CrossRef]
- Baldissarri, C.; Andrighetto, L.; Volpato, C. The longstanding view of workers as objects: Antecedents and consequences of working objectification. Eur. Rev. Soc. Psychol. 2022, 33, 81–130. [Google Scholar] [CrossRef]
- Baldissarri, C.; Andrighetto, L.; Volpato, C. When work does not ennoble man: Psychological consequences of working objectification. TPM Test. Psychom. Methodol. Appl. Psychol. 2014, 21, 327–339. [Google Scholar] [CrossRef]
- Venegas, M.A.; Ramírez, R.; Mateo, E.A. The moderating role of organizational dehumanization in the relationship between authentic leadership and organizational citizenship behaviors. Ucjc Bus. Soc. Rev. 2021, 18, 90–127. [Google Scholar] [CrossRef]
- Auzoult, L. Can meaning at work guard against the consequences of objectification? Psychol. Rep. 2020, 123, 872–884. [Google Scholar] [CrossRef]
- Andrighetto, L.; Baldissarri, C.; Gabbiadini, A.; Sacino, A.; Valtorta, R.R.; Volpato, C. Objectified conformity: Working self-objectification increases conforming behavior. Soc. Influ. 2018, 13, 78–90. [Google Scholar] [CrossRef]
- Auzoult, L.; Personnaz, B. The role of organizational culture and self-consciousness in self-objectification in the workplace. TPM Test. Psychom. Methodol. Appl. Psychol. 2016, 23, 271–284. [Google Scholar] [CrossRef]
- Vaes, J.; Cristoforetti, G.; Ruzzante, D.; Cogoni, C.; Mazza, V. Assessing neural responses towards objectified human targets and objects to identify processes of sexual objectification that go beyond the metaphor. Sci. Rep. 2019, 9, 6699. [Google Scholar] [CrossRef]
- Bernard, P.; Hanoteau, F.; Gervais, S.; Servais, L.; Bertolone, I.; Deltenre, P.; Colin, C. Revealing clothing does not make the object: ERP evidences that cognitive objectification is driven by posture suggestiveness, not by revealing clothing. Personal. Soc. Psychol. Bull. 2019, 45, 16–36. [Google Scholar] [CrossRef]
- Jayarathna, S.; Jayawardana, Y.; Jaime, M.; Thapaliya, S. Electroencephalogram (EEG) for delineating objective measure of autism spectrum disorder. In Computational Models for Biomedical Reasoning and Problem Solving; Chen, C., Cheung, S.S., Eds.; IGI Global: Hershey, PA, USA, 2019; pp. 34–65. [Google Scholar] [CrossRef]
- Wang, S.; Gwizdka, J.; Chaovalitwongse, W.A. Using wireless EEG signals to assess memory workload in the n-back task. IEEE Trans. Hum. Mach. Syst. 2015, 46, 424–435. [Google Scholar] [CrossRef]
- Hankins, T.C.; Wilson, G.F. A comparison of heart rate, eye activity, EEG and subjective measures of pilot mental workload during flight. Aviat. Space Environ. Med. 1998, 69, 360–367. [Google Scholar]
- Al-Ezzi, A.; Kamel, N.; Faye, I.; Gunaseli, E. Review of EEG, ERP, and brain connectivity estimators as predictive biomarkers of social anxiety disorder. Front. Psychol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Bridwell, D.A.; Cavanagh, J.F.; Collins, A.G.; Nunez, M.D.; Srinivasan, R.; Stober, S.; Calhoun, V.D. Moving beyond ERP components: A selective review of approaches to integrate EEG and behavior. Front. Hum. Neurosci. 2018, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Grandchamp, R.; Delorme, A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef] [PubMed]
- Baccalá, L.A.; Sameshima, K. Partial directed coherence: A new concept in neural structure determination. Biol. Cybern. 2001, 84, 463–474. [Google Scholar] [CrossRef]
- Baldissarri, C.; Gabbiadini, A.; Andrighetto, L.; Volpato, C. The ACME shop: A paradigm to investigate working (self-) objectification. J. Soc. Psychol. 2021, 161, 526–542. [Google Scholar] [CrossRef]
- Chengaiyan, S.; Retnapandian, A.S.; Anandan, K. Identification of vowels in consonant–vowel–consonant words from speech imagery based EEG signals. Cogn. Neurodynamics 2020, 14, 1–19. [Google Scholar] [CrossRef]
- Gliem, J.A.; Gliem, R.R. Calculating, interpreting, and reporting Cronbach’s alpha reliability coefficient for Likert-type scales. In Proceedings of the Midwest Research to Practice Conference in Adult, Continuing, and Community Education, Columbus, OH, USA, 8–10 October 2003; pp. 82–88. [Google Scholar]
- Zou, Y.; Dehzangi, O.; Nathan, V.; Jafari, R. Automatic removal of EEG artifacts using electrode-scalp impedance. In Proceedings of the 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; pp. 2054–2058. [Google Scholar]
- Somers, B.; Francart, T.; Bertrand, A. A generic EEG artifact removal algorithm based on the multi-channel Wiener filter. J. Neural Eng. 2018, 15, 036007. [Google Scholar] [CrossRef]
- Trujillo-Ortiz, A.; Hernandez-Walls, R. Welch’s ANOVA: Welch ANOVA Test for Unequal Variances. 2012. Available online: http://www.mathworks.com/matlabcentral/fileexchange/37121-welchanova (accessed on 17 October 2017).
- Trujillo-Ortiz, A.; Hernandez-Walls, R. GHtest: Games-Howell’s Approximate Test of Equality of Means from Normal Population When Variances Are Heterogeneous; The MathWorks, Inc.: Natick, MA, USA, 2003. [Google Scholar]
- Gaxiola-Tirado, J.A.; Salazar-Varas, R.; Gutiérrez, D. Using the partial directed coherence to assess functional connectivity in electroencephalography data for brain–computer interfaces. IEEE Trans. Cogn. Dev. Syst. 2017, 10, 776–783. [Google Scholar] [CrossRef]
- Sameshima, K.; Baccala, L.A. Methods in Brain Connectivity Inference through Multivariate Time Series Analysis; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Angulo-Sherman, I.N.; Gutiérrez, D. A link between the increase in electroencephalographic coherence and performance improvement in operating a brain-computer interface. Comput. Intell. Neurosci. 2015, 2015, 64. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Salinsky, M.C.; Elsas, S. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clin. Neurophysiol. 2006, 117, 1885–1901. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Brzezicka, A.; Gola, M.; Wróbel, A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Braboszcz, C.; Delorme, A. Lost in thoughts: Neural markers of low alertness during mind wandering. Neuroimage 2011, 54, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, Y.G.; Kotchoubey, B. EEG correlates of working memory performance in females. BMC Neurosci. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Scharinger, C.; Soutschek, A.; Schubert, T.; Gerjets, P. Comparison of the working memory load in n-back and working memory span tasks by means of EEG frequency band power and P300 amplitude. Front. Hum. Neurosci. 2017, 11, 6. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Kreiter, A.; Bertrand, O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis. Neurosci. 1999, 16, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O.; Hénaff, M.A.; Isnard, J.; Fischer, C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb. Cortex 2005, 15, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Müller, M.M. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb. Cortex 2005, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lei, X. Wandering minds with wandering brain networks. Neurosci. Bull. 2018, 34, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, J.; Schooler, J.W. The restless mind. Psychol. Bull. 2006, 132, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Grubb, E.A. Assembly line boredom and individual differences in recreation participation. J. Leis. Res. 1975, 7, 256–269. [Google Scholar] [CrossRef]
- Cummings, M.L.; Gao, F.; Thornburg, K.M. Boredom in the workplace: A new look at an old problem. Hum. Factors 2016, 58, 279–300. [Google Scholar] [CrossRef]
- Katayama, T.; Natsume, K. The change in EEG when we are bored. J. Signal Process. 2012, 16, 637–641. [Google Scholar] [CrossRef]
- Thackray, R.I.; Bailey, J.P.; Touchstone, R.M. Physiological, subjective, and performance correlates of reported boredom and monotony while performing a simulated radar control task. In Vigilance; Mackie, R.R., Ed.; NATO Conference Series; Springer: Boston, MA, USA, 1977; Volume 3, pp. 203–215. [Google Scholar] [CrossRef]
- Frankenhaeuser, M.; Nordheden, B.; Myrsten, A.L.; Post, B. Psychophysiological reactions to understimulation and overstimulation. Acta Psychol. 1971, 35, 298–308. [Google Scholar] [CrossRef]
- Khan, D.M.; Kamel, N.; Muzaimi, M.; Hill, T. Effective connectivity for default mode network analysis of alcoholism. Brain Connect. 2021, 11, 12–29. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Danckert, J.; Merrifield, C. Boredom, sustained attention and the default mode network. Exp. Brain Res. 2018, 236, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C. Using partial directed coherence to study alpha-band effective brain networks during a visuospatial attention task. Behav. Neurol. 2019, 2019, 1410425. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y. EEG effective connectivity networks for an attentive task requiring vigilance based on dynamic partial directed coherence. J. Integr. Neurosci. 2020, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Yanagihara, T.K.; Zhang, X.; Meitzler, T.; Hirsch, J. Frontal–occipital connectivity during visual search. Brain Connect. 2012, 2, 164–175. [Google Scholar] [CrossRef]
- Peelen, M.V.; Kastner, S. A neural basis for real-world visual search in human occipitotemporal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 12125–12130. [Google Scholar] [CrossRef] [PubMed]
- Paneri, S.; Gregoriou, G.G. Top-down control of visual attention by the prefrontal cortex. Functional specialization and long-range interactions. Front. Neurosci. 2017, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; van der Leij, A.R.; Sligte, I.G.; Lamme, V.A.; Scholte, H.S. Bottom-up and top-down attention are independent. J. Vis. 2013, 13, 1–14. [Google Scholar] [CrossRef]
- Katsuki, F.; Constantinidis, C. Bottom-up and top-down attention: Different processes and overlapping neural systems. Neuroscientist 2014, 20, 509–521. [Google Scholar] [CrossRef]
- Long, J.; Xie, Q.; Ma, Q.; Urbin, M.; Liu, L.; Weng, L.; Huang, X.; Yu, R.; Li, Y.; Huang, R. Distinct interactions between fronto-parietal and default mode networks in impaired consciousness. Sci. Rep. 2016, 6, 38866. [Google Scholar] [CrossRef]
- Pascucci, D.; Hervais-Adelman, A.; Plomp, G. Gating by induced A–Γ asynchrony in selective attention. Hum. Brain Mapp. 2018, 39, 3854–3870. [Google Scholar] [CrossRef]
- Niki, C.; Kumada, T.; Maruyama, T.; Tamura, M.; Muragaki, Y. Role of frontal functions in executing routine sequential tasks. Front. Psychol. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, P.K.; Sarraf, J. Brain Computer Interface issues on hand movement. J. King Saud-Univ.-Comput. Inf. Sci. 2018, 30, 18–24. [Google Scholar] [CrossRef]
- Wellbrink, J.C.; Buss, A.H. Vigilance performance modeled as a complex adaptive system with listener event graph objects (LEGOS). In Proceedings of the 2004 Winter Simulation Conference, Washington, DC, USA, 5–8 December 2004; Volume 1, pp. 755–759. [Google Scholar] [CrossRef]
- Teasdale, J.D.; Dritschel, B.H.; Taylor, M.J.; Proctor, L.; Lloyd, C.A.; Nimmo-Smith, I.; Baddeley, A.D. Stimulus-independent thought depends on central executive resources. Mem. Cogn. 1995, 23, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, J.D.; Proctor, L.; Lloyd, C.A.; Baddeley, A.D. Working memory and stimulus-independent thought: Effects of memory load and presentation rate. Eur. J. Cogn. Psychol. 1993, 5, 417–433. [Google Scholar] [CrossRef]
- Fredrickson, B.L.; Harrison, K. Throwing like a girl: Self-objectification predicts adolescent girls’ motor performance. J. Sport Soc. Issues 2005, 29, 79–101. [Google Scholar] [CrossRef]

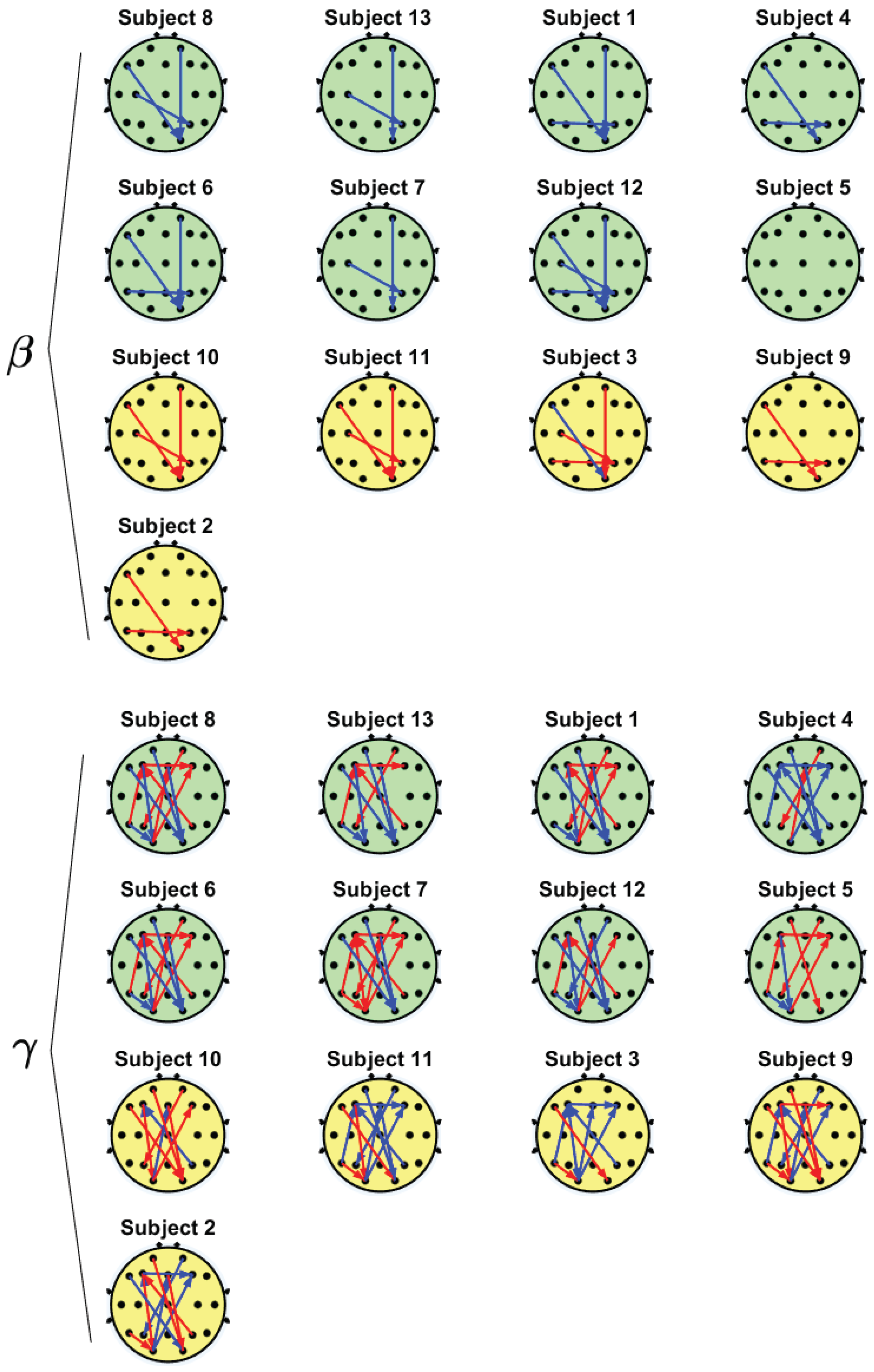
| Subject | Self-Objectification Index |
|---|---|
| 8 | −4.2 |
| 13 | −3.4 |
| 1 | −2.6 |
| 4 | −2 |
| 6 | −2 |
| 7 | −1.6 |
| 12 | −1.4 |
| 5 | −0.4 |
| 10 | 1.6 |
| 11 | 3 |
| 3 | 3.6 |
| 9 | 4 |
| 2 | 6 |
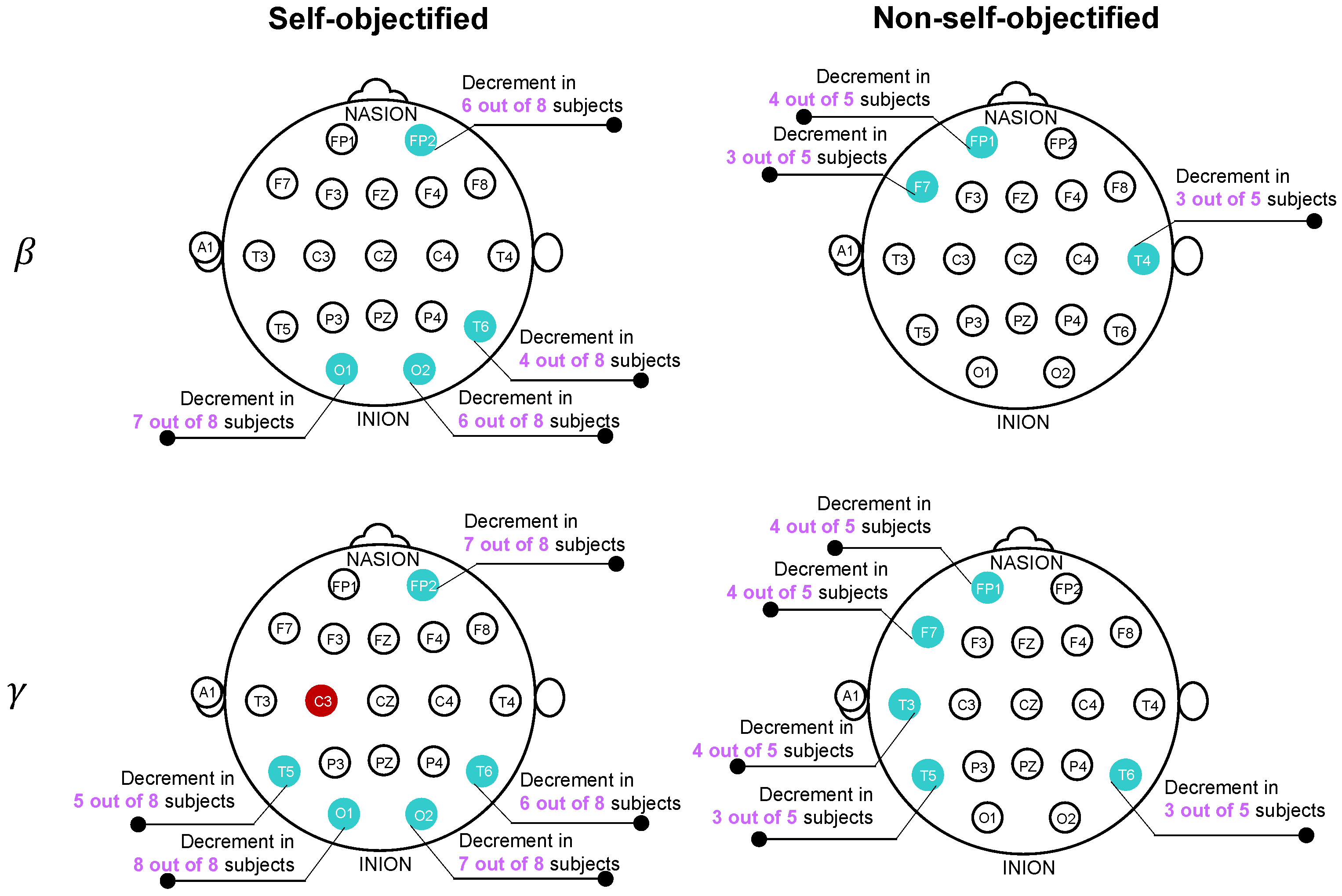
| Sensor | Group | Trend | Subjects (S) | p | |
|---|---|---|---|---|---|
| Beta | |||||
| Fp1 | NSOs | ERD | 2, 3, 9, 10 | <0.001 | 0.53, 0.24, 0.24, 0.33 |
| F7 | NSOs | ERD | 3, 9, 10 | <0.001 | 0.17, 0.08, 0.56 |
| T4 | NSOs | ERD | 3, 10, 11 | <0.001 | 0.25, 0.52, 0.27 |
| Fp2 | SOs | ERD | 1, 4, 7, 8, 12, 13 | <0.001 | 0.09, 0.45, 0.15, 0.29, 0.27, 0.33 |
| O1 | SOs | ERD | 1, 5–8, 12, 13 | S13: 0.001 | 0.15, 0.16, 0.64, 0.26, 0.10, 0.33, 0.09 |
| Other: <0.001 | |||||
| O2 | SOs | ERD | 1, 4, 6–8, 13 | <0.001 | 0.51, 0.34, 0.59, 0.30, 0.21, 0.12 |
| T6 | SOs | ERD | 4, 6, 7, 13 | <0.001 | 0.28, 0.17, 0.27, 0.06 |
| Gamma | |||||
| Fp1 | NSOs | ERD | 2, 3, 9, 10 | <0.001 | 0.53, 0.21, 0.18, 0.57 |
| Fp2 | SOs | ERD | 1, 4, 5, 7, 8, 12, 13 | <0.001 | 0.14, 0.67, 0.25, 0.16, 0.24, 0.23, 0.37 |
| F7 | NSOs | ERD | 2, 3, 10, 11 | <0.001 | 0.14, 0.29, 0.74, 0.06 |
| T3 | NSOs | ERD | 3, 9, 10, 11 | <0.001 | 0.24, 0.35, 0.82, 0.07 |
| T5 | SOs | ERD | 1, 6, 7, 12, 13 | <0.001 | 0.20, 0.45, 0.44, 0.49, 0.43 |
| T5 | NSOs | ERD | 3, 9, 10 | <0.001 | 0.38, 0.57, 0.69 |
| T6 | SOs | ERD | 4, 6–8, 12, 13 | <0.001 | 0.51, 0.44, 0.32, 0.08, 0.22, 0.13 |
| T6 | NSOs | ERD | 3, 9, 10 | <0.001 | 0.32, 0.41, 0.38 |
| O1 | SOs | ERD | 1, 4–8, 12, 13 | <0.001 | 0.61, 0.09, 0.25, 0.76, 0.32, 0.12, 0.57, 0.14 |
| O2 | SOs | ERD | 1, 4, 6–8, 12, 13 | <0.001 | 0.83, 0.63, 0.72, 0.64, 0.24, 0.46, 0.32 |
| C3 | SOs | ERS | 4–8, 12, 13 | <0.001 | 0.12, 0.08, 0.05, 0.10, 0.12, 0.17, 0.48 |
| Interaction | r | p-Value | PDC Tendency in SOs | PDC Tendency in NSOs |
|---|---|---|---|---|
| F7 → O2 | 0.65 | 0.016 | Decremental | Incremental |
| Fp2 → O2 | 0.66 | 0.014 | Decremental | Incremental |
| T5 → P4 | 0.63 | 0.020 | Decremental | Incremental |
| C3 → P4 | 0.57 | 0.041 | Decremental | Incremental |
| Interaction | r | p-Value | PDC Tendency in SOs | PDC Tendency in NSOs |
|---|---|---|---|---|
| F3 → O1 | 0.58 | 0.036 | Decremental | Incremental |
| O1 → Fz | −0.76 | 0.003 | Incremental | Decremental |
| O1 → F4 | −0.60 | 0.029 | Incremental | Decremental |
| F7 → O2 | 0.63 | 0.021 | Decremental | Incremental |
| Fp1 → O2 | 0.62 | 0.024 | Decremental | Incremental |
| Fz → O2 | 0.63 | 0.022 | Decremental | Incremental |
| F3 → F4 | −0.77 | 0.002 | Incremental | Decremental |
| P4 → F3 | −0.62 | 0.024 | Incremental | Decremental |
| Fp2 → P3 | −0.62 | 0.024 | Incremental | Decremental |
| T5 → O1 | 0.62 | 0.025 | Decremental | Incremental |
| T5 → F3 | −0.68 | 0.011 | Incremental | Decremental |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo-Sherman, I.N.; Saavedra-Hernández, A.; Urbina-Arias, N.E.; Hernández-Granados, Z.; Sainz, M. Preliminary Evidence of EEG Connectivity Changes during Self-Objectification of Workers. Sensors 2022, 22, 7906. https://doi.org/10.3390/s22207906
Angulo-Sherman IN, Saavedra-Hernández A, Urbina-Arias NE, Hernández-Granados Z, Sainz M. Preliminary Evidence of EEG Connectivity Changes during Self-Objectification of Workers. Sensors. 2022; 22(20):7906. https://doi.org/10.3390/s22207906
Chicago/Turabian StyleAngulo-Sherman, Irma N., Annel Saavedra-Hernández, Natalia E. Urbina-Arias, Zahamara Hernández-Granados, and Mario Sainz. 2022. "Preliminary Evidence of EEG Connectivity Changes during Self-Objectification of Workers" Sensors 22, no. 20: 7906. https://doi.org/10.3390/s22207906
APA StyleAngulo-Sherman, I. N., Saavedra-Hernández, A., Urbina-Arias, N. E., Hernández-Granados, Z., & Sainz, M. (2022). Preliminary Evidence of EEG Connectivity Changes during Self-Objectification of Workers. Sensors, 22(20), 7906. https://doi.org/10.3390/s22207906









