Opto-Mechanical Eye Models, a Review on Human Vision Applications and Perspectives for Use in Industry
Abstract
1. Introduction
2. Physical Eye Models and Their Applications
3. Measurement Modalities of Eye Models
3.1. Double-Pass Measurements
3.2. Wavefront Measurements
3.3. Single-Pass Measurements
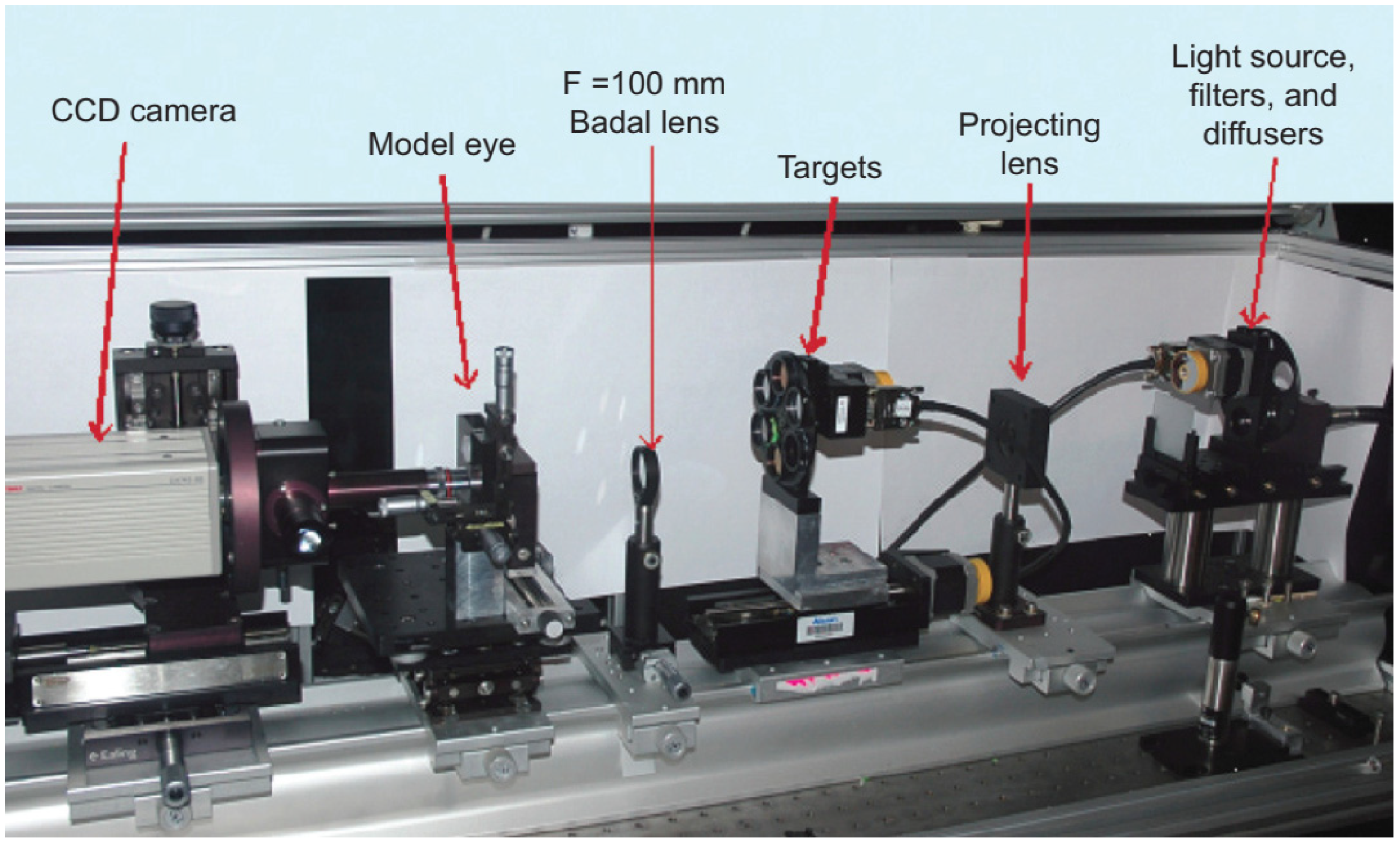
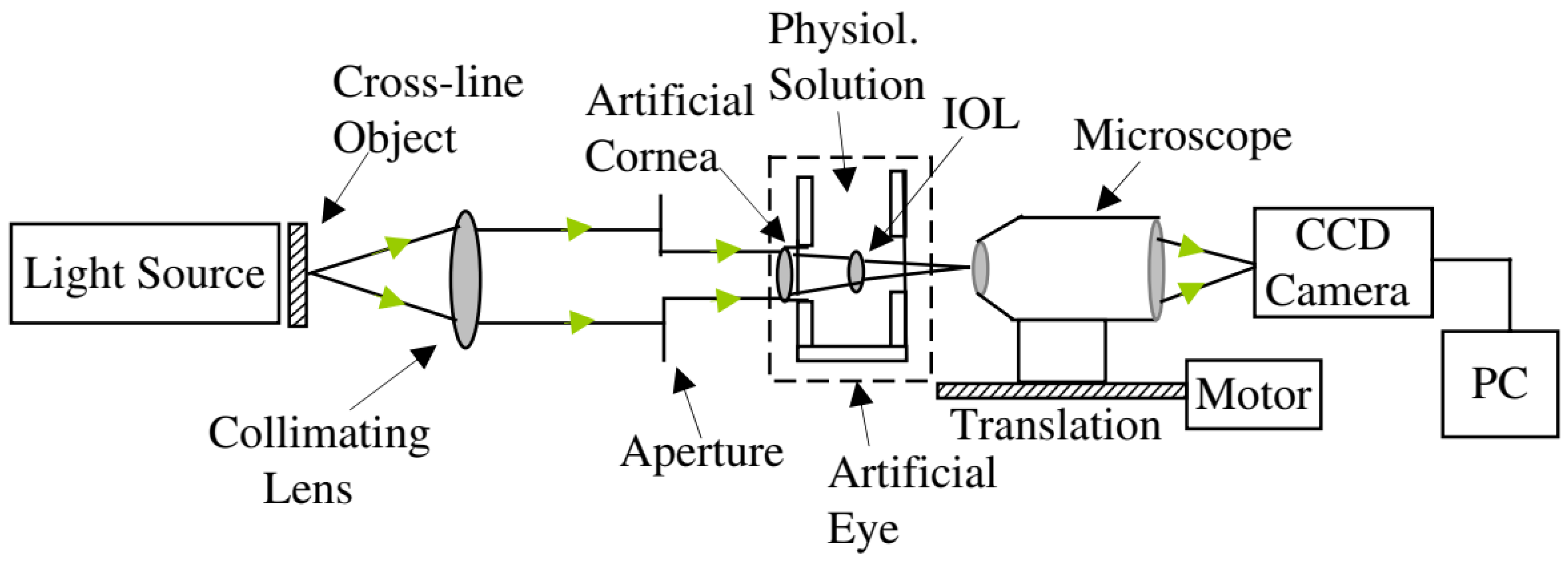
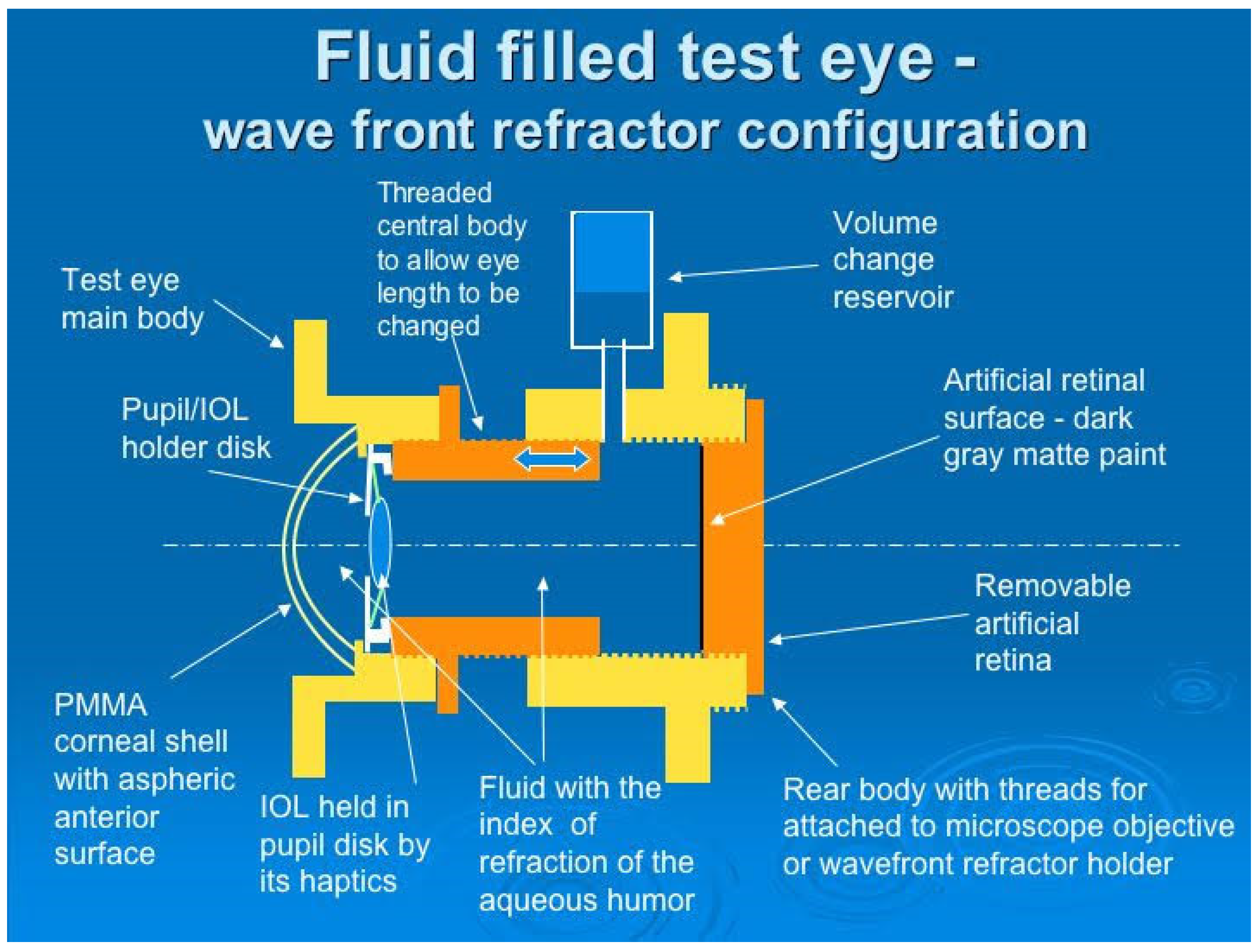
3.4. Fundus Imaging and Retinoscopy
3.5. Scheimpflug and Purkinje Imaging
3.6. Optical Biometry and OCT
3.7. Other Modalities
4. Eye Models for Human Vision
5. Components of a Physical Eye Model
5.1. Cornea
5.2. Crystalline Lens
5.3. Iris and Pupil
5.4. Retina
5.5. Chambers
5.6. Aqueous and Vitreous Fluids
6. Housing and Assembly
7. Eye Movements
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gullstrand, A. Appendices II and IV. In Helmholtz’s Handbuch der Physiologischen Optik; Butterworth-Heinemann: Oxford, UK, 1909; Volume 1, pp. 301–358, 382–415. [Google Scholar]
- Peng, J.; Jia, X.; Wang, J.; Hu, Y. A Simplifed Model Eye for Testing Fundus Imaging Device. Zhongguo Yi Liao Qi Xie Za Zhi 2019, 43, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Gliddon, G.H. An optical replica of the human eye for the study of the retinal image. Arch. Ophthalmol. 1929, 2, 138–163. [Google Scholar] [CrossRef]
- Arell, A.; Kolari, S. Experiments on a model eye. Am. J. Phys. 1978, 46, 613–614. [Google Scholar] [CrossRef]
- Heath, D.A.; McCormack, G.L.; Vaughan, W.H. Mapping of Ophthalmic Lens Distortions with a Pinhole Camera. Optom. Vis. Sci. 1987, 64, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Edgar, D.F.; Bennett, A.G. Construction of a model eye and its applications. Ophthalmic Physiol. Opt. 1992, 12, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Oshika, T.; Shiokawa, Y. Effect of folding on the optical quality of soft acrylic intraocular lenses. J. Cataract Refract. Surg. 1996, 22 (Suppl. S2), 1360–1364. [Google Scholar] [CrossRef]
- Pujol, J.; Arjona, M.; Arasa, J.; Badia, V. Influence of amount and changes in axis of astigmatism on retinal image quality. J. Opt. Soc. Am. A 1998, 15, 2514. [Google Scholar] [CrossRef]
- Moreno-Barriuso, E.; Navarro, R. Laser Ray Tracing versus Hartmann–Shack sensor for measuring optical aberrations in the human eye. J. Opt. Soc. Am. A 2000, 17, 974. [Google Scholar] [CrossRef]
- Trinavarat, A.; Atchaneeyasakul, L.; Udompunturak, S. Neodymium:YAG laser damage threshold of foldable intraocular lenses. J. Cataract Refract. Surg. 2001, 27, 775–780. [Google Scholar] [CrossRef]
- Dubbelman, M.; Van der Heijde, G.L. The shape of the aging human lens: Curvature, equivalent refractive index and the lens paradox. Vis. Res. 2001, 41, 1867–1877. [Google Scholar] [CrossRef]
- Barry, J.C.; Dunne, M.; Kirschkamp, T. Phakometric measurement of ocular surface radius of curvature and alignment: Evaluation of method with physical model eyes. Ophthalmic Physiol. Opt. 2001, 21, 450–460. [Google Scholar] [CrossRef]
- Pieh, S.; Marvan, P.; Lackner, B.; Hanselmayer, G.; Schmidinger, G.; Leitgeb, R.; Sticker, M.; Hitzenberger, C.K.; Fercher, A.F.; Skorpik, C. Quantitative performance of bifocal and multifocal intraocular lenses in a model eye: Point spread function in multifocal intraocular lenses. Arch. Ophthalmol. 2002, 120, 23–28. [Google Scholar] [CrossRef]
- Holladay, J.T.; Piers, P.A.; Koranyi, G.; Van der Mooren, M.; Norrby, N.E.S. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J. Refract. Surg. 2002, 18, 683–691. [Google Scholar] [CrossRef]
- Cheng, X.; Himebaugh, N.L.; Kollbaum, P.S.; Thibos, L.N.; Bradley, A. Validation of a clinical Shack-Hartmann aberrometer. Optom. Vis. Sci. 2003, 80, 587–595. [Google Scholar] [CrossRef]
- Barbero, S.; Marcos, S.; Jiménez-Alfaro, I. Optical aberrations of intraocular lenses measured in vivo and in vitro. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2003, 20, 1841–1851. [Google Scholar] [CrossRef]
- Letfullin, R.; Cherezova, T.; Belyakov, A.; Kudryashov, A. Human eye model based on bimorph flexible mirror. In Optics and Photonics, Proceedings of the Advanced Wavefront Control: Methods, Devices, and Applications III, San Diego, CA, USA, 31 July–4 August 2005; Gruneisen, M.T., Gonglewski, J.D., Giles, M.K., Eds.; SPIE: Bellingham, WA, USA, 2005; Volume 5894, p. 58940A. [Google Scholar]
- Rawer, R.; Stork, W.; Spraul, C.W.; Lingenfelder, C. Imaging quality of intraocular lenses. J. Cataract Refract. Surg. 2005, 31, 1618–1631. [Google Scholar] [CrossRef]
- Kawamorita, T.; Uozato, H. Modulation transfer function and pupil size in multifocal and monofocal intraocular lenses in vitro. J. Cataract Refract. Surg. 2005, 31, 2379–2385. [Google Scholar] [CrossRef]
- Gobbi, P.G.; Fasce, F.; Bozza, S.; Brancato, R. Optomechanical eye model with imaging capabilities for objective evaluation of intraocular lenses. J. Cataract Refract. Surg. 2006, 32, 643–651. [Google Scholar] [CrossRef]
- Galetskiĭ, S.O.; Cherezova, T.Y.; Belyakov, A.I.; Kudryashov, A.V. Creating a model of the human eye by the methods of adaptive optics. J. Opt. Technol. 2006, 73, 491. [Google Scholar] [CrossRef]
- De Castro, A.; Rosales, P.; Marcos, S. Tilt and decentration of intraocular lenses in vivo from Purkinje and Scheimpflug imaging. Validation study. J. Cataract Refract. Surg. 2007, 33, 418–429. [Google Scholar] [CrossRef]
- Norrby, S.; Piers, P.; Campbell, C.; van der Mooren, M. Model eyes for evaluation of intraocular lenses. Appl. Opt. 2007, 46, 6595–6605. [Google Scholar] [CrossRef]
- Fernández, E.J.; Artal, P. Dynamic eye model for adaptive optics testing. Appl. Opt. 2007, 46, 6971. [Google Scholar] [CrossRef]
- Artigas, J.M.; Menezo, J.L.; Peris, C.; Felipe, A.; Díaz-Llopis, M. Image quality with multifocal intraocular lenses and the effect of pupil size: Comparison of refractive and hybrid refractive-diffractive designs. J. Cataract Refract. Surg. 2007, 33, 2111–2117. [Google Scholar] [CrossRef]
- Choi, J.; Schwiegerling, J. Optical performance measurement and night driving simulation of ReSTOR, ReZoom, and Tecnis multifocal intraocular lenses in a model eye. J. Refract. Surg. 2008, 24, 218–222. [Google Scholar] [CrossRef]
- Terwee, T.; Weeber, H.; van der Mooren, M.; Piers, P. Visualization of the retinal image in an eye model with spherical and aspheric, diffractive, and refractive multifocal intraocular lenses. J. Refract. Surg. 2008, 24, 223–232. [Google Scholar] [CrossRef]
- Campbell, C.E. Wavefront measurements of diffractive and refractive multifocal intraocular lenses in an artificial eye. J. Refract. Surg. 2008, 24, 308–311. [Google Scholar] [CrossRef]
- Eppig, T.; Scholz, K.; Langenbucher, A. Assessing the optical performance of multifocal (diffractive) intraocular lenses. Ophthalmic Physiol. Opt. 2008, 28, 467–474. [Google Scholar] [CrossRef]
- Barcik, A.; Nowak, J.; Siedlecki, D.; Zając, M.; Zarówny, J. Physical model of human eye with implantable intraocular lenses. In Proceedings of the 16th Polish-Slovak-Czech Optical Conference on Wave and Quantum Aspects of Contemporary Optics, Polanica Zdroj, Poland, 8–12 September 2008; Popiolek-Masajada, A., Jankowska, E., Urbanczyk, W., Eds.; SPIE: Bellingham, WA, USA, 2008; Volume 7141, p. 71411A. [Google Scholar]
- Maxwell, W.A.; Lane, S.S.; Zhou, F. Performance of presbyopia-correcting intraocular lenses in distance optical bench tests. J. Cataract Refract. Surg. 2009, 35, 166–171. [Google Scholar] [CrossRef]
- McKelvie, J.; Ku, J.Y.; McArdle, B.; McGhee, C. Wavefront aberrometry: Comparing and profiling higher-order aberrations produced by intraocular lenses in vitro using a physical model eye system and Hartman-Shack aberrometry. J. Cataract Refract. Surg. 2009, 35, 547–555. [Google Scholar] [CrossRef]
- Pieh, S.; Fiala, W.; Malz, A.; Stork, W. In vitro strehl ratios with spherical, aberration-free, average, and customized spherical aberration-correcting intraocular lenses. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1264–1270. [Google Scholar] [CrossRef]
- Shen, J.; Thibos, L.N. Measuring ocular aberrations and image quality in peripheral vision with a clinical wavefront aberrometer. Clin. Exp. Optom. 2009, 92, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, A.V.; Lerat, B.; Nowakowski, M.; Dainty, C. Inverse optical design: Building and testing an artificial eye. In SPIE Europe Optical Metrology, Proceedings of the Modeling Aspects in Optical Metrology II, Munich, Germany, 14–18 June 2009; Bosse, H., Bodermann, B., Silver, R.M., Eds.; SPIE: Bellingham, WA, USA, 2009; Volume 7390, p. 739005. [Google Scholar]
- Eppig, T.; Scholz, K.; Löffler, A.; Meßner, A.; Langenbucher, A. Effect of decentration and tilt on the image quality of aspheric intraocular lens designs in a model eye. J. Cataract Refract. Surg. 2009, 35, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bakaraju, R.C.; Ehrmann, K.; Falk, D.; Ho, A.; Papas, E. Physical human model eye and methods of its use to analyse optical performance of soft contact lenses. Opt. Express 2010, 18, 16868–16882. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Noda, T.; Mihashi, T.; Ohnuma, K.; Bissen-Miyajima, H.; Hirakata, A. Quality of Image of Grating Target Placed in Model of Human Eye with Corneal Aberrations as Observed Through Multifocal Intraocular Lenses. Am. J. Ophthalmol. 2011, 151, 644–652.e1. [Google Scholar] [CrossRef]
- Birkner, S.; Einighammer, J.; Oltrup, T.; Bende, T.; Jean, B. Biometric measurements inside the model eye using a two wavelengths Fourier domain low coherence interferometer. Biomed. Tech. 2011, 56, 65–71. [Google Scholar] [CrossRef]
- Ohnuma, K.; Kayanuma, H.; Lawu, T.; Negishi, K.; Yamaguchi, T.; Noda, T. Retinal image contrast obtained by a model eye with combined correction of chromatic and spherical aberrations. Biomed. Opt. Express 2011, 2, 1443. [Google Scholar] [CrossRef]
- Kim, M.J.; Zheleznyak, L.; Macrae, S.; Tchah, H.; Yoon, G. Objective evaluation of through-focus optical performance of presbyopia-correcting intraocular lenses using an optical bench system. J. Cataract Refract. Surg. 2011, 37, 1305–1312. [Google Scholar] [CrossRef]
- Petelczyc, K.; Bará, S.; Lopez, A.C.; Jaroszewicz, Z.; Kakarenko, K.; Kolodziejczyk, A.; Sypek, M. Imaging properties of the light sword optical element used as a contact lens in a presbyopic eye model. Opt. Express 2011, 19, 25602–25616. [Google Scholar] [CrossRef]
- Pepose, J.S.; Wang, D.; Altmann, G.E. Comparison of Through-Focus Image Sharpness Across Five Presbyopia-Correcting Intraocular Lenses. Am. J. Ophthalmol. 2012, 154, 20–28.e1. [Google Scholar] [CrossRef]
- Montés-Micó, R.; López-Gil, N.; Pérez-Vives, C.; Bonaque, S.; Ferrer-Blasco, T. In vitro optical performance of nonrotational symmetric and refractive-diffractive aspheric multifocal intraocular lenses: Impact of tilt and decentration. J. Cataract Refract. Surg. 2012, 38, 1657–1663. [Google Scholar] [CrossRef]
- Ackermann, R.; Kammel, R.; Merker, M.; Kamm, A.; Tünnermann, A.; Nolte, S. Optical side-effects of fs-laser treatment in refractive surgery investigated by means of a model eye. Biomed. Opt. Express 2013, 4, 220. [Google Scholar] [CrossRef]
- Arianpour, A.; Tremblay, E.J.; Stamenov, I.; Ford, J.E.; Schanzlin, D.J.; Lo, Y. An optomechanical model eye for ophthalmological refractive studies. J. Refract. Surg. 2013, 29, 126–132. [Google Scholar] [CrossRef]
- Gatinel, D.; Houbrechts, Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. J. Cataract Refract. Surg. 2013, 39, 1093–1099. [Google Scholar] [CrossRef]
- Drauschke, A.; Rank, E.; Traxler, L.; Lux, K.; Krutzler, C. Mechanical Eye Model for Comparison of Optical and Physiological Imaging Properties. IFAC Proc. Vol. 2013, 46, 1–12. [Google Scholar] [CrossRef]
- Ruiz-Alcocer, J.; Madrid-Costa, D.; García-Lázaro, S.; Ferrer-Blasco, T.; Montés-Micó, R. Optical performance of two new trifocal intraocular lenses: Through-focus modulation transfer function and influence of pupil size. Clin. Exp. Ophthalmol. 2014, 42, 271–276. [Google Scholar] [CrossRef]
- Hill, W.; Carson, D.; Hong, X.; Karakelle, M. Optical bench performance of Acrysof® IQ resTOr®, AT LISA® tri, and FineVision® intraocular lenses. Clin. Ophthalmol. 2014, 249, 2105. [Google Scholar] [CrossRef][Green Version]
- Liang, D.; Xiang, K.; Du, J.-W.; Yang, J.-N.; Wang, X.-Y. Biomimetic optical system using polymer lenses with tunable focus. Opt. Eng. 2014, 53, 105101. [Google Scholar] [CrossRef]
- Xie, P.; Hu, Z.; Zhang, X.; Li, X.; Gao, Z.; Yuan, D.; Liu, Q. Application of 3-dimensional printing technology to construct an eye model for fundus viewing study. PLoS ONE 2014, 9, e109373. [Google Scholar] [CrossRef]
- Fung, T.H.M.; Yusuf, I.H.; Xue, K.; Smith, L.M.; Patel, C.K. Heidelberg Spectralis ultra-widefield fundus fluorescein angiography in infants. Am. J. Ophthalmol. 2015, 159-1, 78–84.e2. [Google Scholar] [CrossRef]
- Förster, E.; Stürmer, M.; Wallrabe, U.; Korvink, J.; Brunner, R. Bio-inspired variable imaging system simplified to the essentials: Modelling accommodation and gaze movement. Opt. Express 2015, 23, 929. [Google Scholar] [CrossRef]
- Santiago-Alvarado, A.; Cruz-Félix, A.; Hernández Méndez, A.; Pérez-Maldonado, Y.; DomÍnguez-Osante, C. Design and characterization of a tunable opto-mechatronic system to mimic the focusing and the regulation of illumination in the formation of images made by the human eye. In SPIE Defense + Security, Proceedings of the Micro- and Nanotechnology Sensors, Systems, and Applications VII, Baltimore, MD, USA, 20–24 April 2015; George, T., Dutta, A.K., Islam, M.S., Eds.; SPIE: Bellingham, WA, USA, 2015; Volume 9467, p. 94671Y. [Google Scholar]
- Vega, F.; Alba-Bueno, F.; Millán, M.S.; Varón, C.; Gil, M.A.; Buil, J.A. Halo and Through-Focus Performance of Four Diffractive Multifocal Intraocular Lenses. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3967–3975. [Google Scholar] [CrossRef]
- Esteve-Taboada, J.J.; Del Águila-Carrasco, A.J.; Marín-Franch, I.; Bernal-Molina, P.; Montés-Micó, R.; López-Gil, N. Opto-mechanical artificial eye with accommodative ability. Opt. Express 2015, 23, 19396. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Fung, T.H.M.; Patel, C.K. Ultra-widefield retinal imaging through a black intraocular lens. J. Cataract Refract. Surg. 2015, 41, 1926–1933. [Google Scholar] [CrossRef]
- García-Guerra, C.E.; Aldaba, M.; Arjona, M.; Pujol, J. Binocular open-view system to perform estimations of aberrations and scattering in the human eye. Appl. Opt. 2015, 54, 9504–9508. [Google Scholar] [CrossRef]
- Domínguez-Vicent, A.; Esteve-Taboada, J.J.; Del Águila-Carrasco, A.J.; Ferrer-Blasco, T.; Montés-Micó, R. In vitro optical quality comparison between the Mini WELL Ready progressive multifocal and the TECNIS Symfony. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1387–1397. [Google Scholar] [CrossRef]
- Mao, X.; Banta, J.T.; Ke, B.; Jiang, H.; He, J.; Liu, C.; Wang, J. Wavefront Derived Refraction and Full Eye Biometry in Pseudophakic Eyes. PLoS ONE 2016, 11, e0152293. [Google Scholar] [CrossRef]
- Petsch, S.; Schuhladen, S.; Dreesen, L.; Zappe, H. The engineered eyeball, a tunable imaging system using soft-matter micro-optics. Light Sci. Appl. 2016, 5, e16068. [Google Scholar] [CrossRef]
- Winter, S.; Sabesan, R.; Tiruveedhula, P.; Privitera, C.; Unsbo, P.; Lundström, L.; Roorda, A. Transverse chromatic aberration across the visual field of the human eye. J. Vis. 2016, 16, 9. [Google Scholar] [CrossRef]
- Coughlan, M.F.; Mihashi, T.; Goncharov, A.V. Opto-mechanical design of a dispersive artificial eye. Appl. Opt. 2017, 56, 4338–4346. [Google Scholar] [CrossRef]
- Son, H.S.; Tandogan, T.; Liebing, S.; Merz, P.; Choi, C.Y.; Khoramnia, R.; Auffarth, G.U. In Vitro optical quality measurements of three intraocular lens models having identical platform. BMC Ophthalmol. 2017, 17, 108. [Google Scholar] [CrossRef]
- Alba-Bueno, F.; Garzón, N.; Vega, F.; Poyales, F.; Millán, M.S. Patient-Perceived and Laboratory-Measured Halos Associated with Diffractive Bifocal and Trifocal Intraocular Lenses. Curr. Eye Res. 2018, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohamedi, H.; Kelly-Pérez, I.; Prinz, A.; Oltrup, T.; Leitritz, M.; Cayless, A.; Bende, T. A systematic comparison and evaluation of three different Swept-Source interferometers for eye lengths biometry. Z. Med. Phys. 2019, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Petelczyc, K.; Kolodziejczyk, A.; Błocki, N.; Byszewska, A.; Jaroszewicz, Z.; Kakarenko, K.; Kołacz, K.; Miler, M.; Mira-Agudelo, A.; Torres-Sepúlveda, W.; et al. Model of the light sword intraocular lens: In-Vitro comparative studies. Biomed. Opt. Express 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Poddar, S.; Lin, Y.; Long, Z.; Zhang, D.; Zhang, Q.; Shu, L.; Qiu, X.; Kam, M.; Javey, A.; et al. A biomimetic eye with a hemispherical perovskite nanowire array retina. Nature 2020, 581, 278–282. [Google Scholar] [CrossRef]
- Regal, S.; Troughton, J.; Delattre, R.; Djenizian, T.; Ramuz, M. Changes in temperature inside an optomechanical model of the human eye during emulated transscleral cyclophotocoagulation. Biomed. Opt. Express 2020, 11, 4548. [Google Scholar] [CrossRef]
- Chae, S.H.; Son, H.S.; Khoramnia, R.; Lee, K.H.; Choi, C.Y. Laboratory evaluation of the optical properties of two extended-depth-of-focus intraocular lenses. BMC Ophthalmol. 2020, 20, 53. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Hu, Z.; Li, X.; Li, F.; Duan, L. Model eye tool for retinal optical coherence tomography instrument calibration. J. Innov. Opt. Health Sci. 2021, 14, 2150010. [Google Scholar] [CrossRef]
- Schneider, R.T.; Keates, R.H. Apparatus and Methods for Evaluating Vision through an Intraocular Lens. U.S. Patent 5,532,770, 2 July 1996. [Google Scholar]
- Schneider, R.T.; Keates, R.H. Vision Simulating Apparatus and Method. U.S. Patent 5,652,640, 29 July 1997. [Google Scholar]
- Ohnuma, K.; Qi, H.; Minato, A. Ocular Optical System Simulation ApparatuS. U.S. Patent 5,875,017, 23 February 1999. [Google Scholar]
- Sheehy, J.B.; Gish, K.W.; Sprenger, J.J. Artificial Human Eye and Test Apparatus. U.S. Patent 6,485,142 B1, 26 November 2002. [Google Scholar]
- Altmann, G.E. Lens-Eye Model and Method for Prfdicting In-Vivo Lens Performance. U.S. Patent 6,626,535 B2, 30 September 2003. [Google Scholar]
- Yamaguchi, T.; Nakazaw, N.; Toshifumi, M.; Hirohara, Y. Model Eye for Eye Characteristic Measuring Device and Calibration Method for The Same. U.S. Patent 7,036,933 B2, 2 May 2006. [Google Scholar]
- Niven, G.D. Eye Model for Measurement. U.S. Patent 7,066,598 B2, 27 June 2006. [Google Scholar]
- Ehrmann, K.; Bakaraju, R.C.; Falk, D. Physical Model Eye Systems and Methods. U.S. Patent 8,814,356 B2, 26 August 2014. [Google Scholar]
- Bennett, A.G.; Rabbetts, R.B. Clinical Visual Optics, 2nd ed.; Butterworths: London, UK, 1989. [Google Scholar]
- Portney, V. Optical testing and inspection methodology for modern intraocular lenses. J. Cataract Refract. Surg. 1992, 18, 607–613. [Google Scholar] [CrossRef]
- ISO 11979–2:1999; Ophthalmic Implants—Intraocular Lenses—Part 2: Optical Properties and Test Methods. International Organization for Standardization, ISO: Geneva, Switzerland, 1999.
- Bajrovic, S.; Zimmermann, D. ISO-compliant IOL inspection Implementing the ISO-compliant Intraocular Lens (IOL) inspection in your production. GlobalCONTACT 2018, 20–23. [Google Scholar]
- ISO 11979–2:2014; Ophthalmic Implants—Intraocular Lenses—Part 2: Optical Properties and Test Methods. International Organization for Standardization, ISO: Geneva, Switzerland, 2014.
- Swift, A.; Gooi, P. Artificial eye models: An opportunity to increase surgical training exposure in ophthalmology during and beyond the COVID-19 pandemic. J. Clin. Ophthalmol. 2020, 4, 15–17. [Google Scholar]
- Swift, A.; Waldner, D.; Gorner, A.; Chung, H.; Ahmed, Y.; Docherty, G.; Gooi, P. Face and content validity of an artificial eye model for Ab-Interno Goniotomy. Eur. J. Ophthalmol. 2021, 31, 2418–2423. [Google Scholar] [CrossRef]
- Wang, D.; Mulvey, F.B.; Pelz, J.B.; Holmqvist, K. A study of artificial eyes for the measurement of precision in eye-trackers. Behav. Res. Methods 2017, 49, 947–959. [Google Scholar] [CrossRef]
- Gu, J.J.; Meng, M.; Cook, A.; Liu, P.X. Design, sensing and control of a robotic prosthetic eye for natural eye movement. Appl. Bionics Biomech. 2006, 3, 29–41. [Google Scholar] [CrossRef]
- Model Eye for Ultrasonic Biometry. 3B Scientific. Item No. 1012869 [U10018]. Available online: https://www.3bscientific.com/us/model-eye-for-ultrasonic-biometry-1012869-u10018-3b-scientific,p_838_18625.html (accessed on 3 May 2022).
- Díaz-Doutón, F.; Benito, A.; Pujol, J.; Arjona, M.; Güell, J.L.; Artal, P. Comparison of the retinal image quality with a Hartmann-Shack wavefront sensor and a double-pass instrument. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1710–1716. [Google Scholar] [CrossRef]
- Escudero-Sanz, I.; Navarro, R. Off-axis aberrations of a wide-angle schematic eye model. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1999, 16, 1881–1891. [Google Scholar] [CrossRef]
- Liou, H.L.; Brennan, N.A. Anatomically accurate, finite model eye for optical modeling. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 1684–1695. [Google Scholar] [CrossRef]
- Le Grand, Y.; Hage, S.G. El Physiological Optics; Springer: Berlin, Gernamy, 1980; pp. 65–66. [Google Scholar]
- Kirschkamp, T.; Jöckel, M.; Wählisch, G.; Barry, J.C. Konstruktion eines Modellauges zur Simulation von Purkinje-Spiegelbildern für die Bestimmung der Krümmungsradien und der Lage der Augenlinse-Construction of a Model Eye to Simulate Purkinje Reflections for the Determination of the Radii of Curvature and of the Position of the Crystalline Lens of the Eye. Biomed. Tech. Eng. 1998, 43, 318–325. [Google Scholar] [CrossRef]
- Navarro, R.; Santamaría, J.; Bescós, J. Accommodation-dependent model of the human eye with aspherics. J. Opt. Soc. Am. A 1985, 2, 1273–1281. [Google Scholar] [CrossRef]
- Gobbi, P.G.; Fasce, F.; Bozza, S.; Brancato, R. Experimental characterization of the imaging properties of multifocal intraocular lenses. In Biomedical Optics, Proceedings of the Ophthalmic Technologies XIII, San Jose, CA, USA, 25–31 January 2003; Manns, F., Söderberg, P.G., Ho, A., Eds.; SPIE: Bellingham, WA, USA, 2003; Volume 4951, p. 104. [Google Scholar]
- Panos, J.G.; Ho, A.; Ehrmann, K.; Bakaraju, R.C. An Optically Equivalent Physical Eye Model for In-Vitro Assessment of Intraocular Lenses. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3701. [Google Scholar]
- Arianpour, A.; Tremblay, E.; Ford, J.; Lo, Y. Optomechanical Fluid-filled Model of the Human Eye. In Proceedings of the Optics in the Life Sciences; OSA: Washington, DC, USA, 2011; p. BTuA4. [Google Scholar]
- Xiong, Y.; Li, J.; Wang, N.; Liu, X.; Wang, Z.; Tsai, F.F.; Wan, X. The analysis of corneal asphericity (Q value) and its related factors of 1,683 Chinese eyes older than 30 years. PLoS ONE 2017, 12, e0176913. [Google Scholar] [CrossRef]
- Dubbelman, M.; Sicam, V.A.D.P.; Van der Heijde, G.L. The shape of the anterior and posterior surface of the aging human cornea. Vis. Res. 2006, 46, 993–1001. [Google Scholar] [CrossRef]
- Dubbelman, M.; Weeber, H.A.; van der Heijde, R.G.L.; Völker-Dieben, H.J. Radius and asphericity of the posterior corneal surface determined by corrected Scheimpflug photography. Acta Ophthalmol. Scand. 2002, 80, 379–383. [Google Scholar] [CrossRef]
- Haigis, W.; Lege, B.; Miller, N.; Schneider, B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 765–773. [Google Scholar] [CrossRef]
- Atchison, D.A.; Markwell, E.L.; Kasthurirangan, S.; Pope, J.M.; Smith, G.; Swann, P.G. Age-related changes in optical and biometric characteristics of emmetropic eyes. J. Vis. 2008, 8, 29. [Google Scholar] [CrossRef]
- Richdale, K.; Bullimore, M.A.; Zadnik, K. Lens thickness with age and accommodation by optical coherence tomography. Ophthalmic Physiol. Opt. 2008, 28, 441–447. [Google Scholar] [CrossRef]
- Praveen, M.R.; Vasavada, A.R.; Shah, S.K.; Shah, C.B.; Patel, U.P.; Dixit, N.V.; Rawal, S. Lens thickness of Indian eyes: Impact of isolated lens opacity, age, axial length, and influence on anterior chamber depth. Eye 2009, 23, 1542–1548. [Google Scholar] [CrossRef]
- Manns, F.; Fernandez, V.; Zipper, S.; Sandadi, S.; Hamaoui, M.; Ho, A.; Parel, J.M. Radius of curvature and asphericity of the anterior and posterior surface of human cadaver crystalline lenses. Exp. Eye Res. 2004, 78, 39–51. [Google Scholar] [CrossRef]
- Ji, S.; Ponting, M.; Lepkowicz, R.S.; Rosenberg, A.; Flynn, R.; Beadie, G.; Baer, E. A bio-inspired polymeric gradient refractive index (GRIN) human eye lens. Opt. Express 2012, 20, 26746–26754. [Google Scholar] [CrossRef]
- Martinez, E.J.F.; Soriano, P.A. Variable-Power Accommodative Intraocular Lens and Assembly of Variablepower Accommodative Intraocuilar Lens and Capsular Ring. U.S. Patent 10,130,461 B2, 20 November 2018. [Google Scholar]
- Carpi, F.; Frediani, G.; Turco, S.; De Rossi, D. Bioinspired Tunable Lens with Muscle-Like Electroactive Elastomers. Adv. Funct. Mater. 2011, 21, 4152–4158. [Google Scholar] [CrossRef]
- Leung, C.K.; Palmiero, P.-M.; Weinreb, R.N.; Li, H.; Sbeity, Z.; Dorairaj, S.; Leung, D.; Liu, S.; Liebmann, J.M.; Congdon, N.; et al. Comparisons of anterior segment biometry between Chinese and Caucasians using anterior segment optical coherence tomography. Br. J. Ophthalmol. 2010, 94, 1184–1189. [Google Scholar] [CrossRef]
- Li, Q.; Zong, Y.; Wen, H.; Yu, J.; Zhou, C.; Jiang, C.; Liu, G.; Sun, X. Measurement of Iris Thickness at Different Regions in Healthy Chinese Adults. J. Ophthalmol. 2021, 2021, 2653564. [Google Scholar] [CrossRef] [PubMed]
- Schuhladen, S.; Preller, F.; Rix, R.; Petsch, S.; Zentel, R.; Zappe, H. Iris-like tunable aperture employing liquid-crystal elastomers. Adv. Mater. 2014, 26, 7247–7251. [Google Scholar] [CrossRef] [PubMed]
- Kimmle, C.; Schmittat, U.; Doering, C.; Fouckhardt, H. Compact dynamic microfluidic iris for active optics. Microelectron. Eng. 2011, 88, 1772–1774. [Google Scholar] [CrossRef]
- Wässle, H.; Boycott, B.B. Functional architecture of the mammalian retina. Physiol. Rev. 1991, 71, 447–480. [Google Scholar] [CrossRef]
- Polyak, S.L. The Retina: The Anatomy and the Histology of the Retina in Man, Ape, and Monkey, Including the Consideration of Visual Functions, the History of Physiological Optics, and the Histological Laboratory Technique; University of Chicago Press: Chicago, IL, USA, 1941. [Google Scholar]
- Guenter, B.; Joshi, N.; Stoakley, R.; Keefe, A.; Geary, K.; Freeman, R.; Hundley, J.; Patterson, P.; Hammon, D.; Herrera, G.; et al. Highly curved image sensors: A practical approach for improved optical performance. Opt. Express 2017, 25, 13010. [Google Scholar] [CrossRef]
- Jung, I.; Xiao, J.; Malyarchuk, V.; Lu, C.; Li, M.; Liu, Z.; Yoon, J.; Huang, Y.; Rogers, J.A. Dynamically tunable hemispherical electronic eye camera system with adjustable zoom capability. Proc. Natl. Acad. Sci. USA 2011, 108, 1788–1793. [Google Scholar] [CrossRef]
- Lee, K.E.; Klein, B.E.K.; Klein, R.; Quandt, Z.; Wong, T.Y. Association of age, stature, and education with ocular dimensions in an older white population. Arch. Ophthalmol. 2009, 127, 88–93. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, L.; Bian, A.; Zhou, Q. Iris lens distance to predict the risk of intraocular pressure elevation after dark room provocative test. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2723–2728. [Google Scholar] [CrossRef]
- Nover, A.; Grote, W. On the determination of the length of the axis of the human eye with ultrasound in the living person. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1965, 168, 405–418. [Google Scholar] [CrossRef]
- Rieger, C.J.; Carpenter, F.G. Light scattering by commercial sugar solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1959, 63A, 205. [Google Scholar] [CrossRef]
- Wagholikar, N.K.; Jagtap, S.B. Optical Characterization of Sucrose (C12H21O11) at Different Temperatures. IJIRSET 2017, 6, 2625–2630. [Google Scholar] [CrossRef]
- Thibos, L.N.; Ye, M.; Zhang, X.; Bradley, A. The chromatic eye: A new reduced-eye model of ocular chromatic aberration in humans. Appl. Opt. 1992, 31, 3594–3600. [Google Scholar] [CrossRef]
- Schwiegerling, J. Field Guide to Visual and Ophthalmic Optics; SPIE: Bellingham, Washington, DC, USA, 2004; ISBN 9780819478191. [Google Scholar]
- Wang, K.; Venetsanos, D.T.; Hoshino, M.; Uesugi, K.; Yagi, N.; Pierscionek, B.K. A Modeling Approach for Investigating Opto-Mechanical Relationships in the Human Eye Lens. IEEE Trans. Biomed. Eng. 2020, 67, 999–1006. [Google Scholar] [CrossRef]
- Atchison, D.A.; Thibos, L.N. Optical models of the human eye. Clin. Exp. Optom. 2016, 99, 99–106. [Google Scholar] [CrossRef]
- Lang, A.J.; Lakshminarayanan, V.; Portney, V. Phenomenological model for interpreting the clinical significance of the in vitro optical transfer function. J. Opt. Soc. Am. A 1993, 10, 1600–1610. [Google Scholar] [CrossRef]
- Piers, P.A.; Norrby, N.E.S.; Mester, U. Eye models for the prediction of contrast vision in patients with new intraocular lens designs. Opt. Lett. 2004, 29, 733–735. [Google Scholar] [CrossRef]
- Li, G.; Zwick, H.; Stuck, B.; Lund, D.J. On the use of schematic eye models to estimate retinal image quality. J. Biomed. Opt. 2000, 5, 307–314. [Google Scholar] [CrossRef][Green Version]
- Dalimier, E.; Dainty, C. Use of a customized vision model to analyze the effects of higher-order ocular aberrations and neural filtering on contrast threshold performance. J. Opt. Soc. Am. A 2008, 25, 2078. [Google Scholar] [CrossRef]
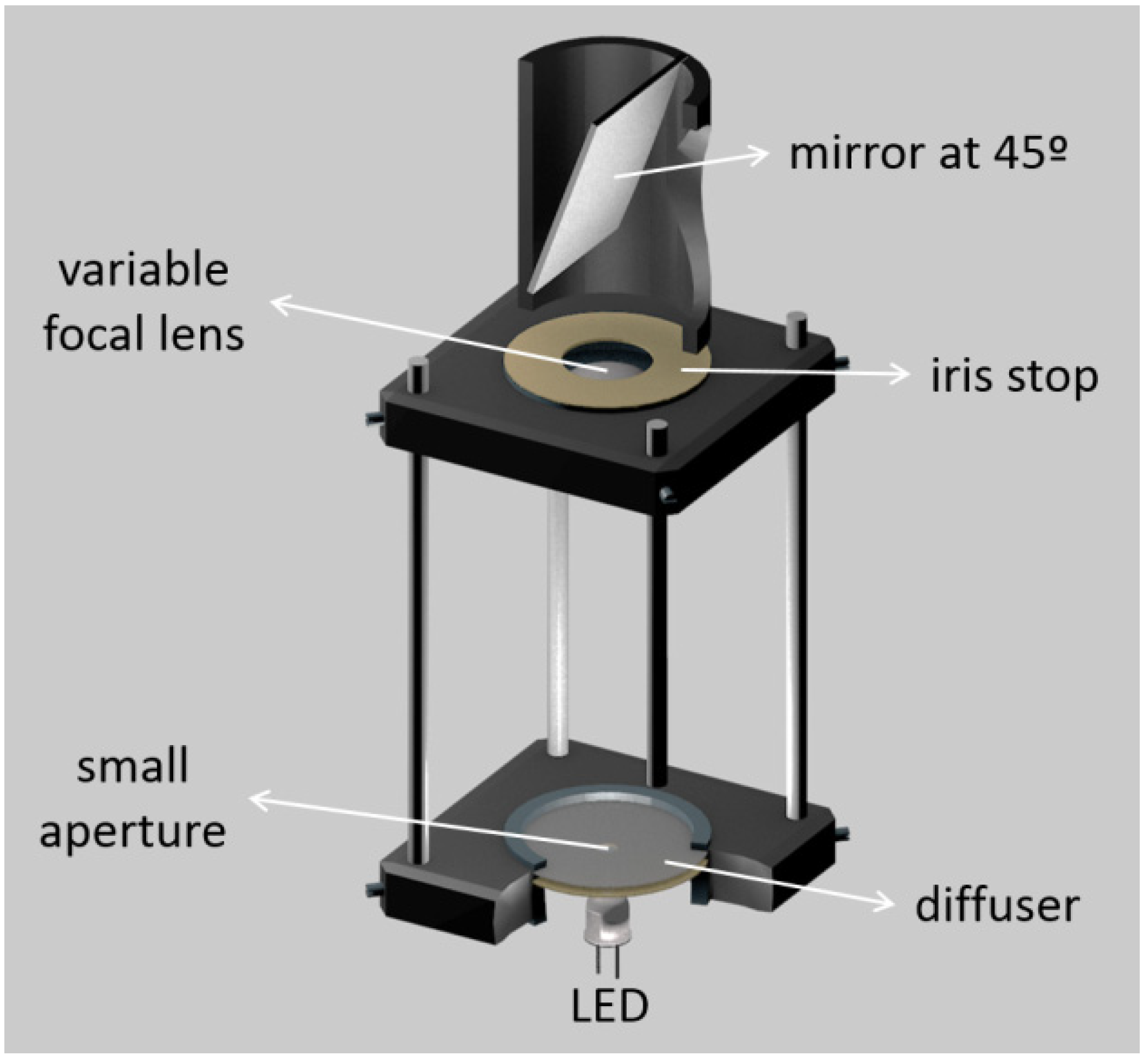
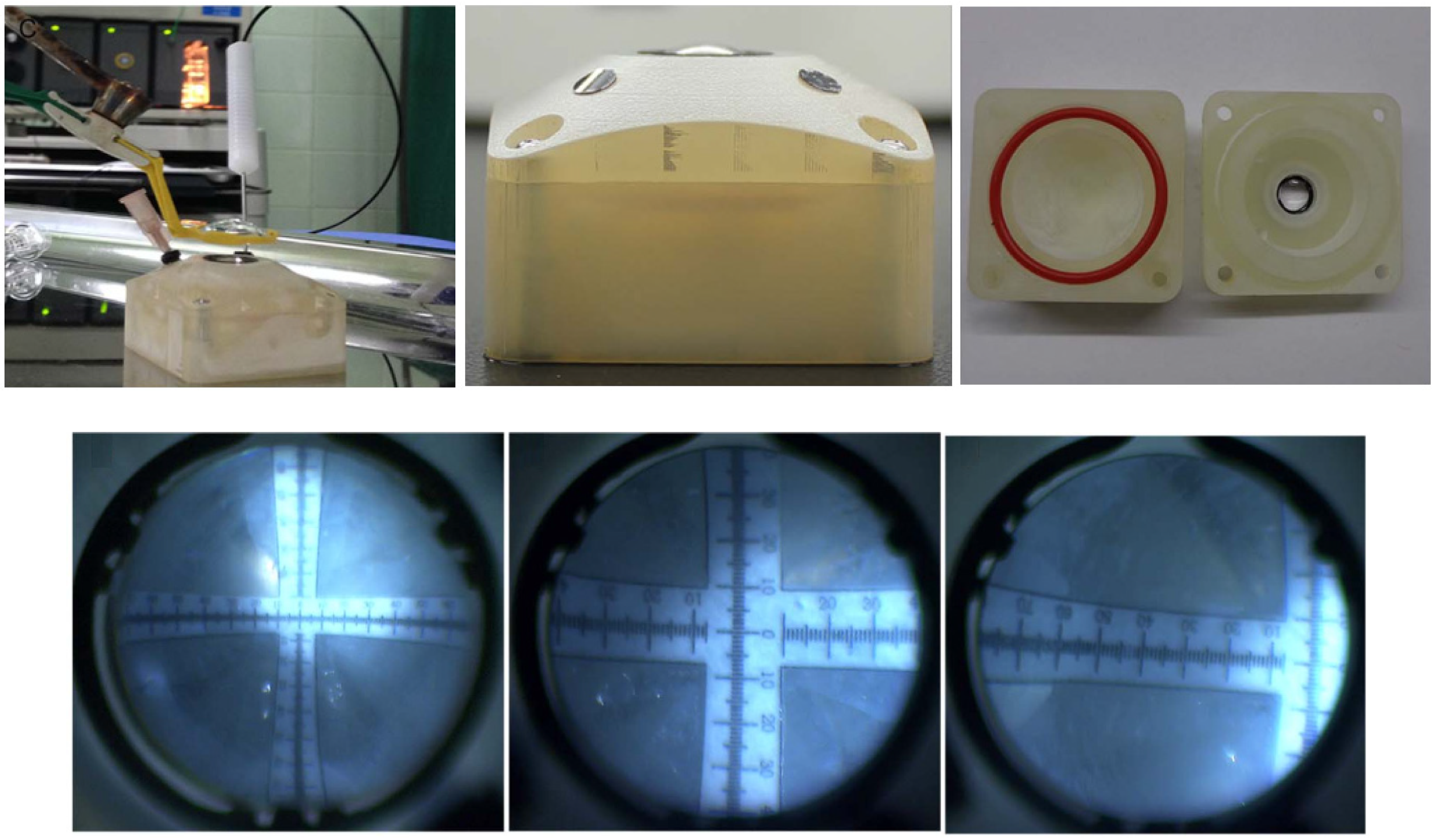
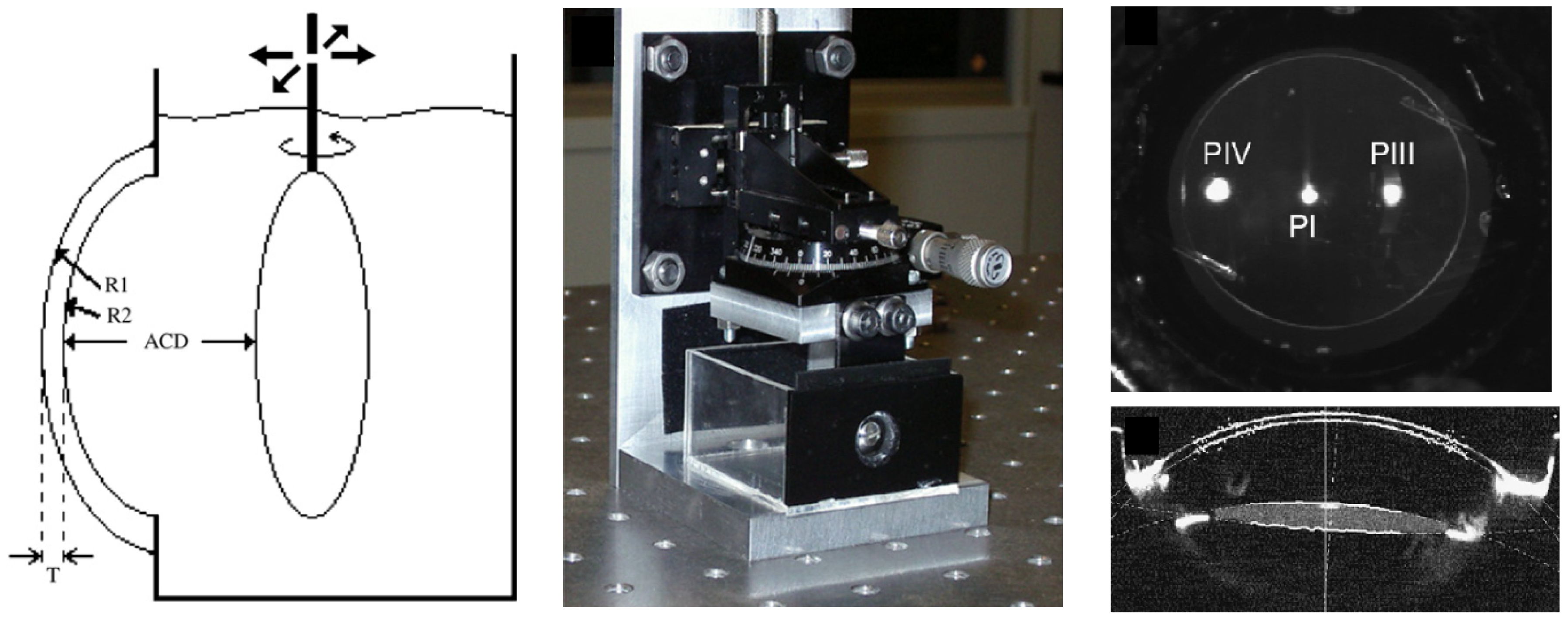
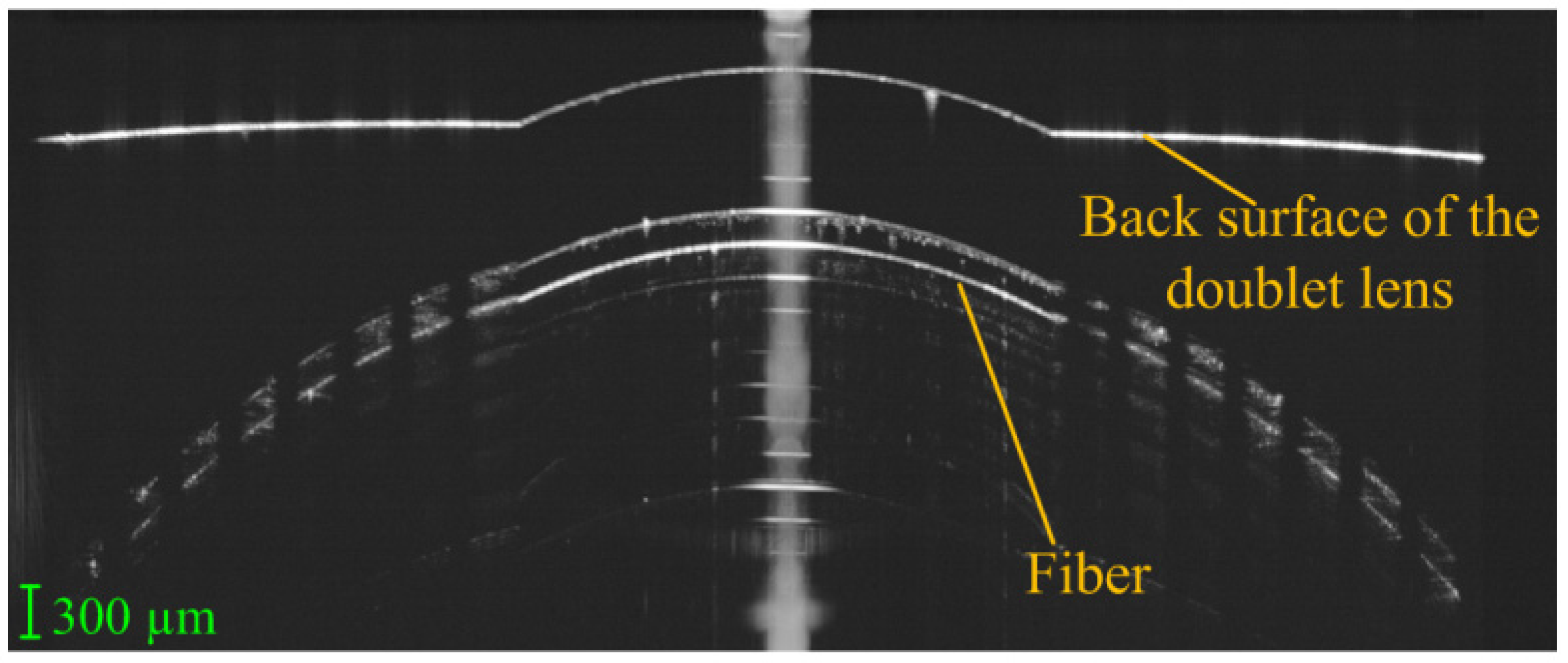
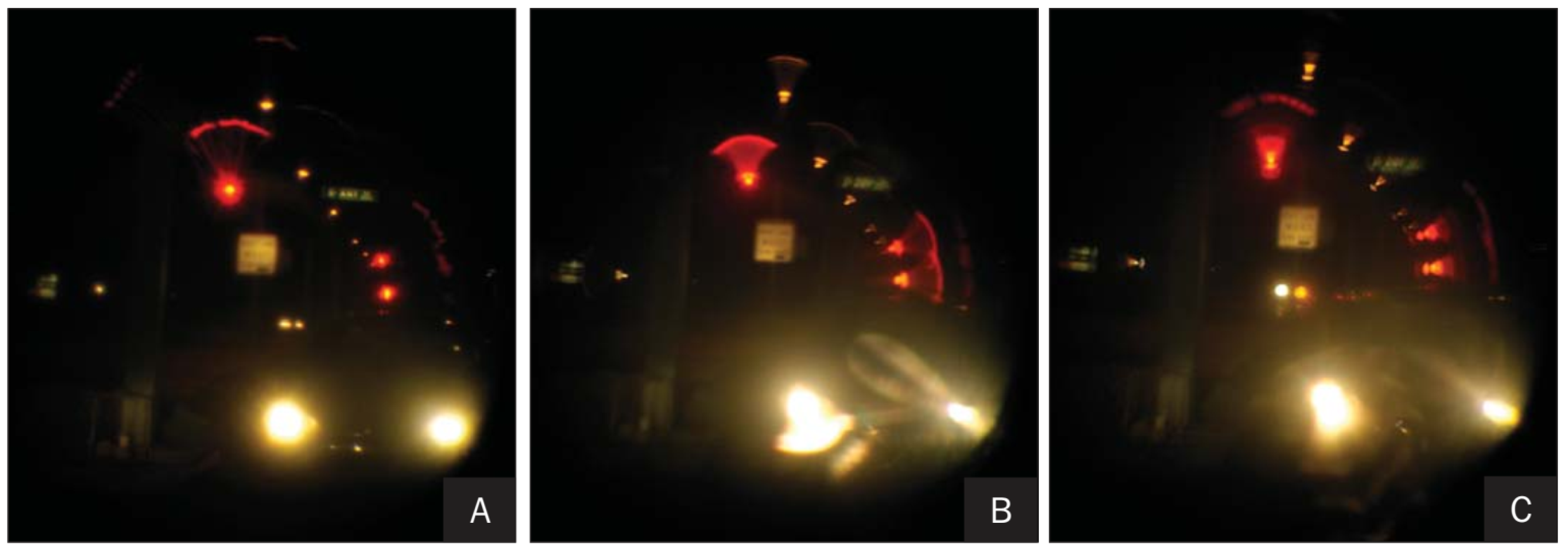
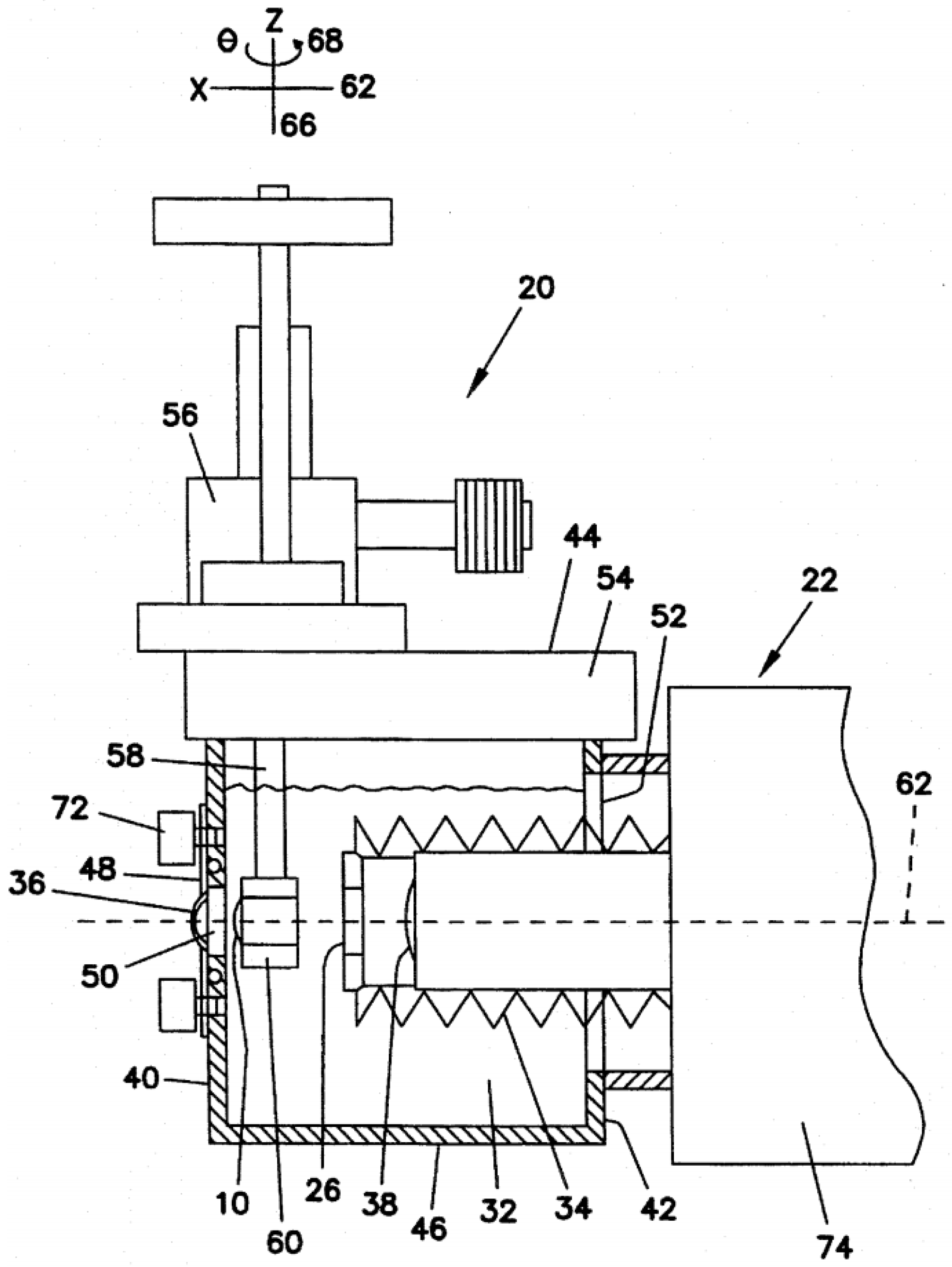
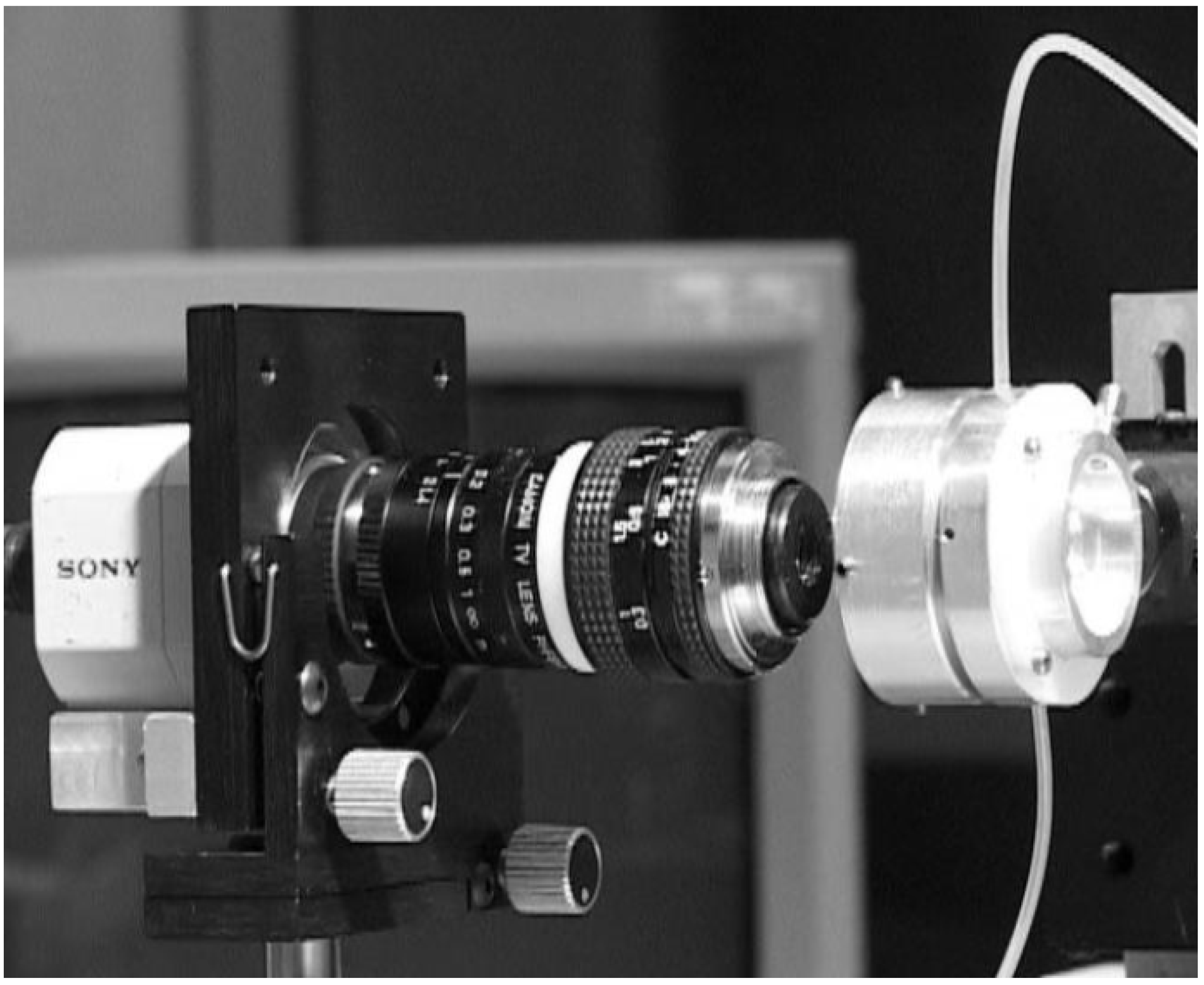
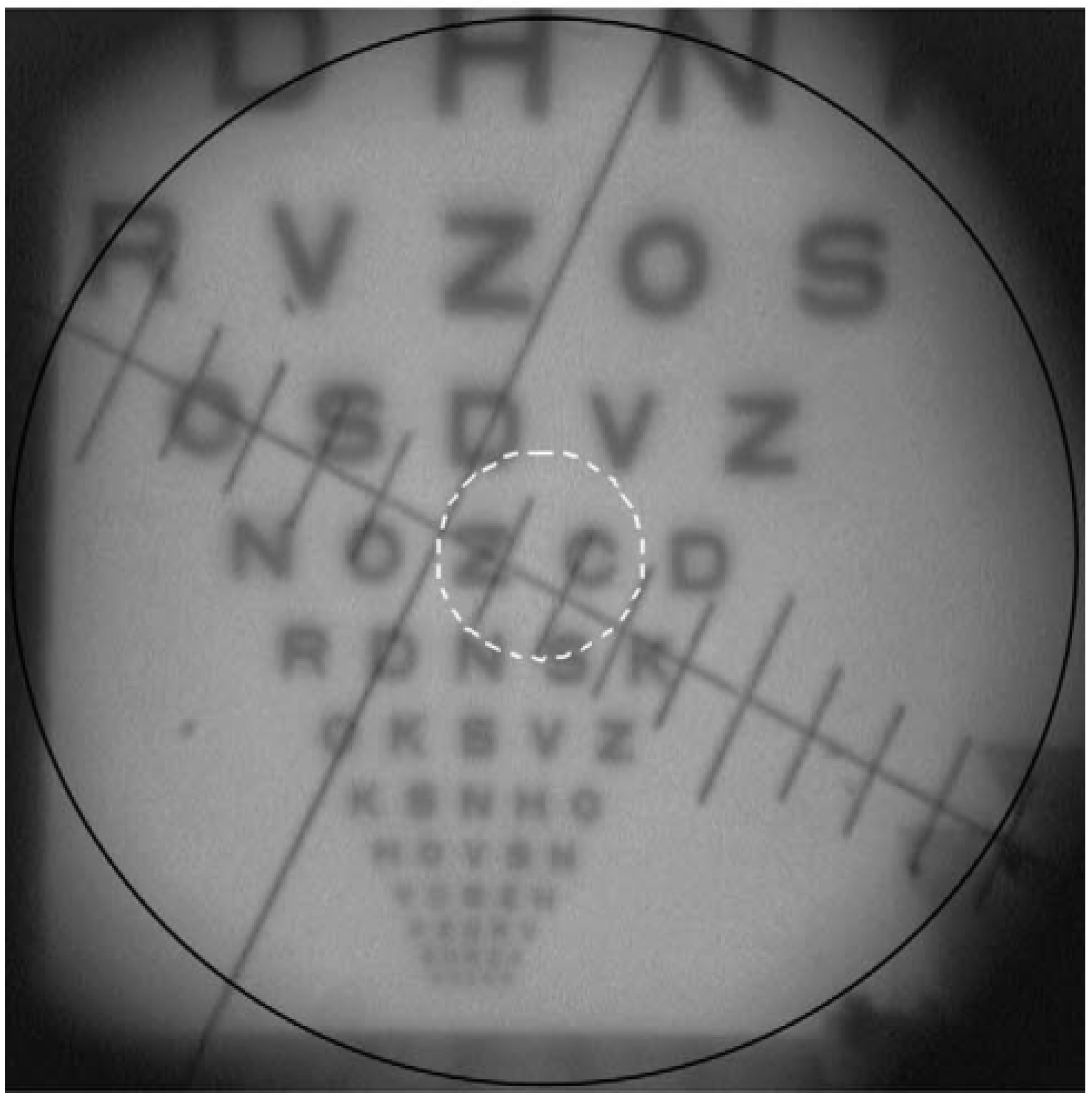
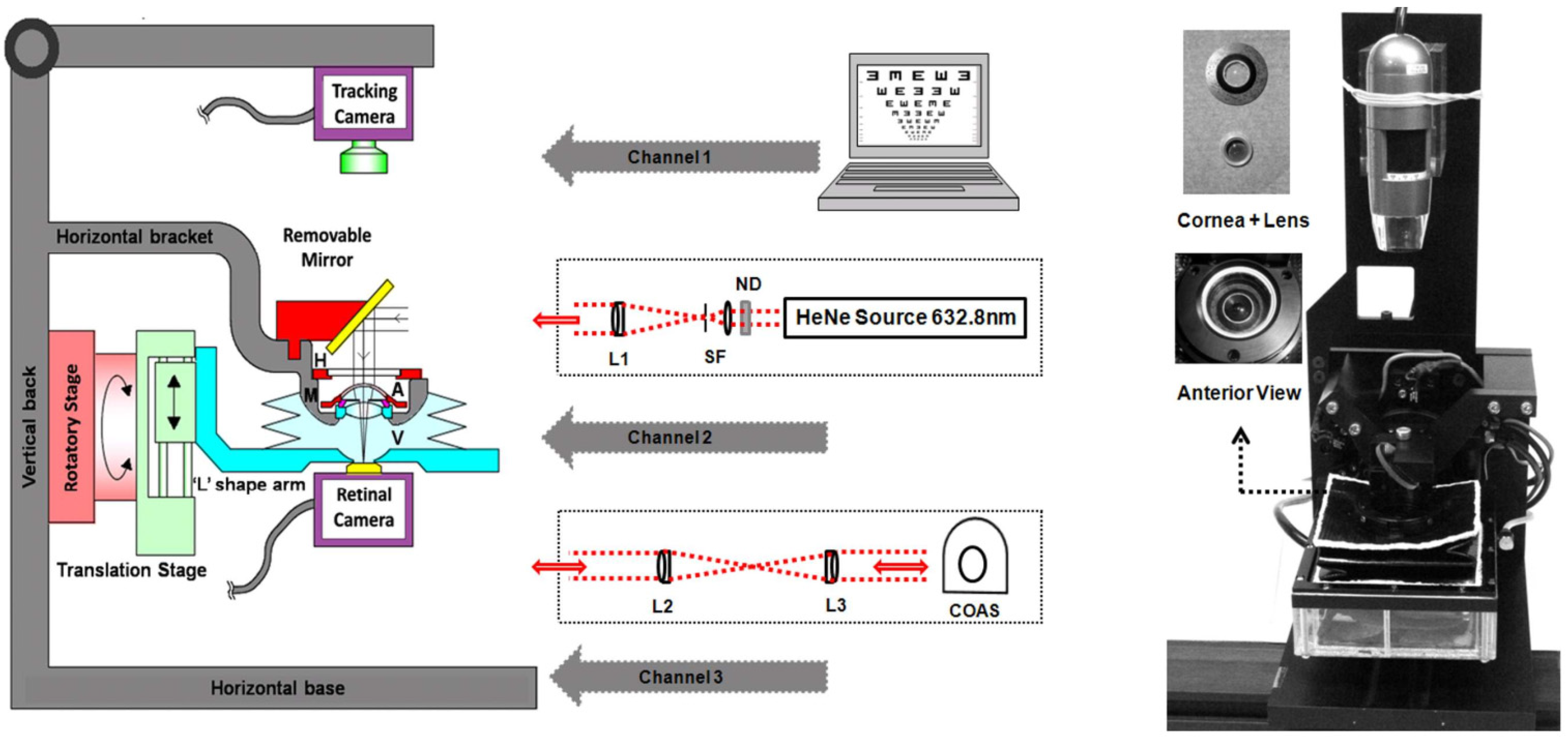

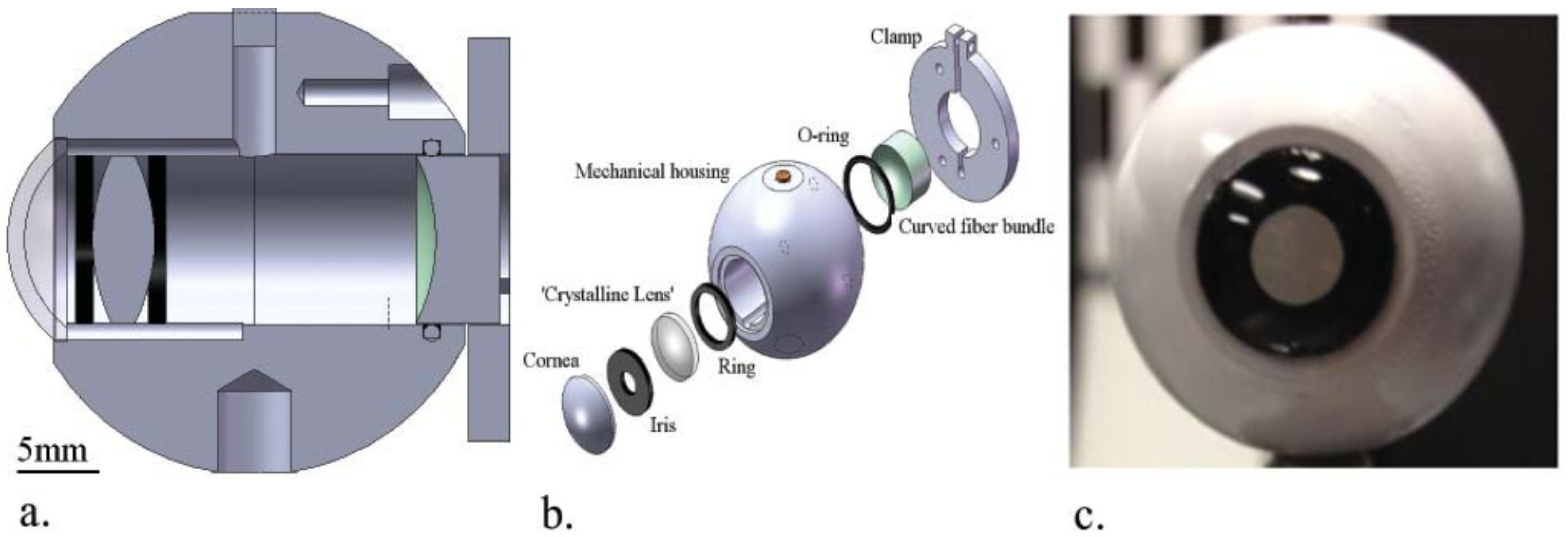
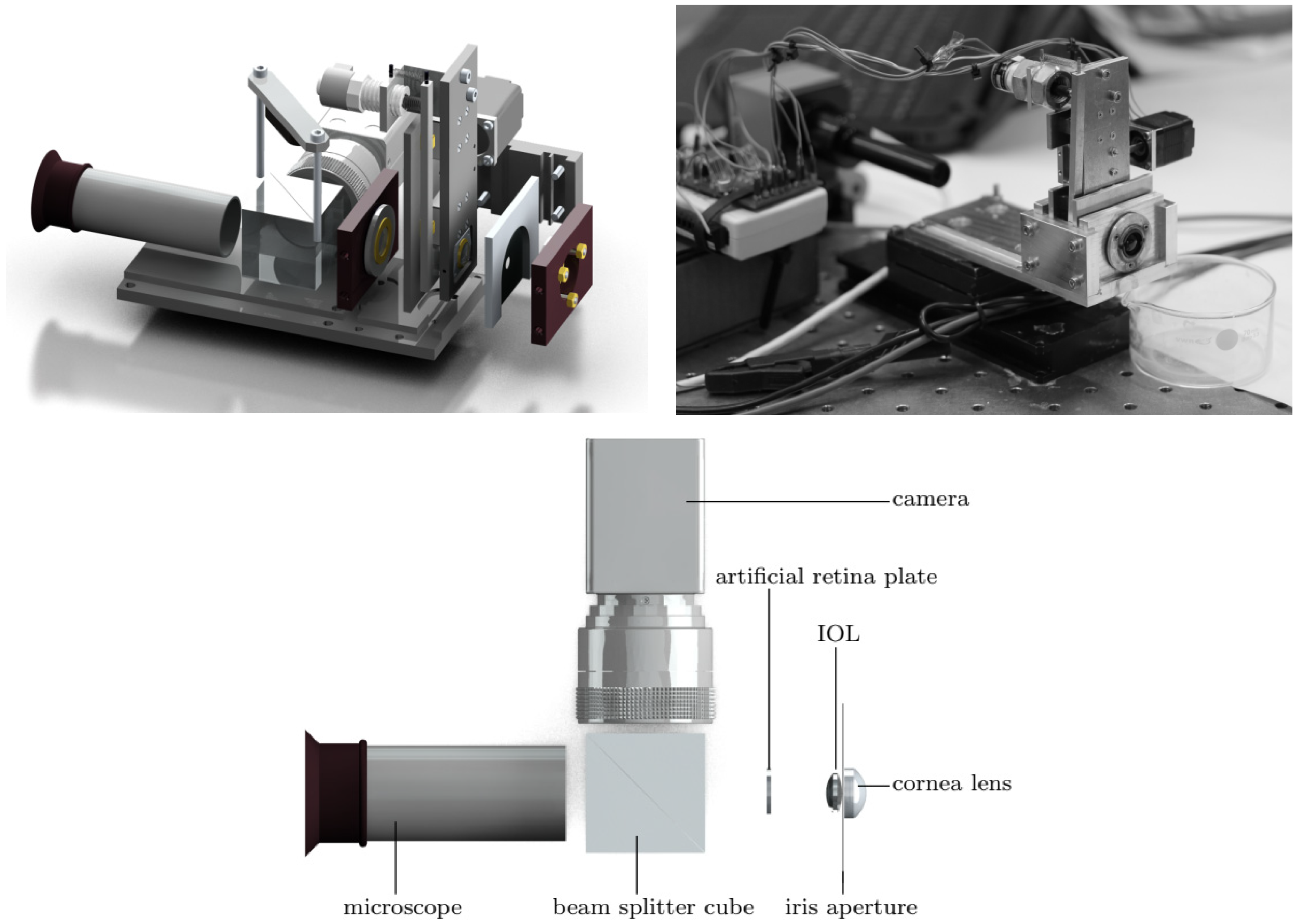
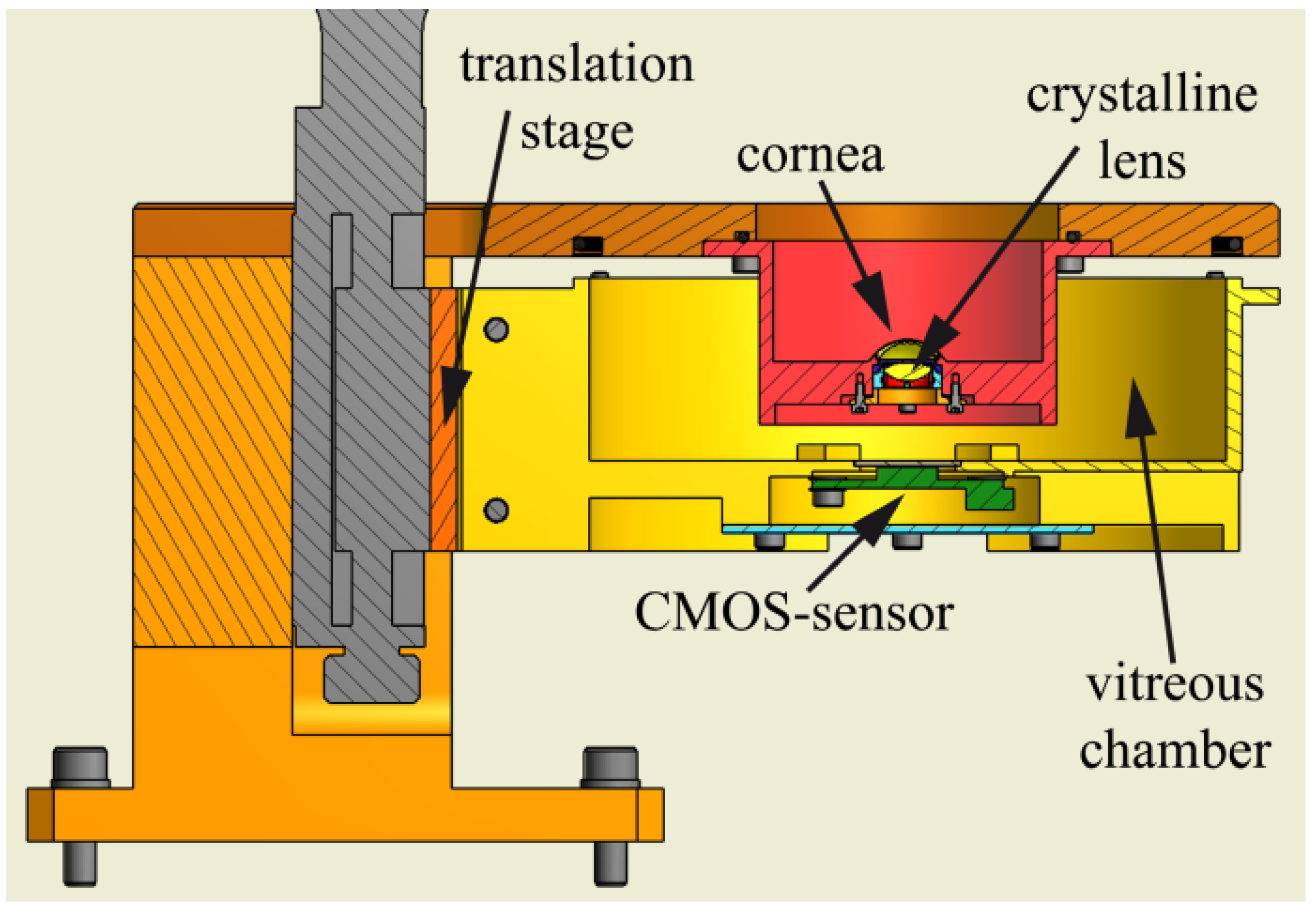

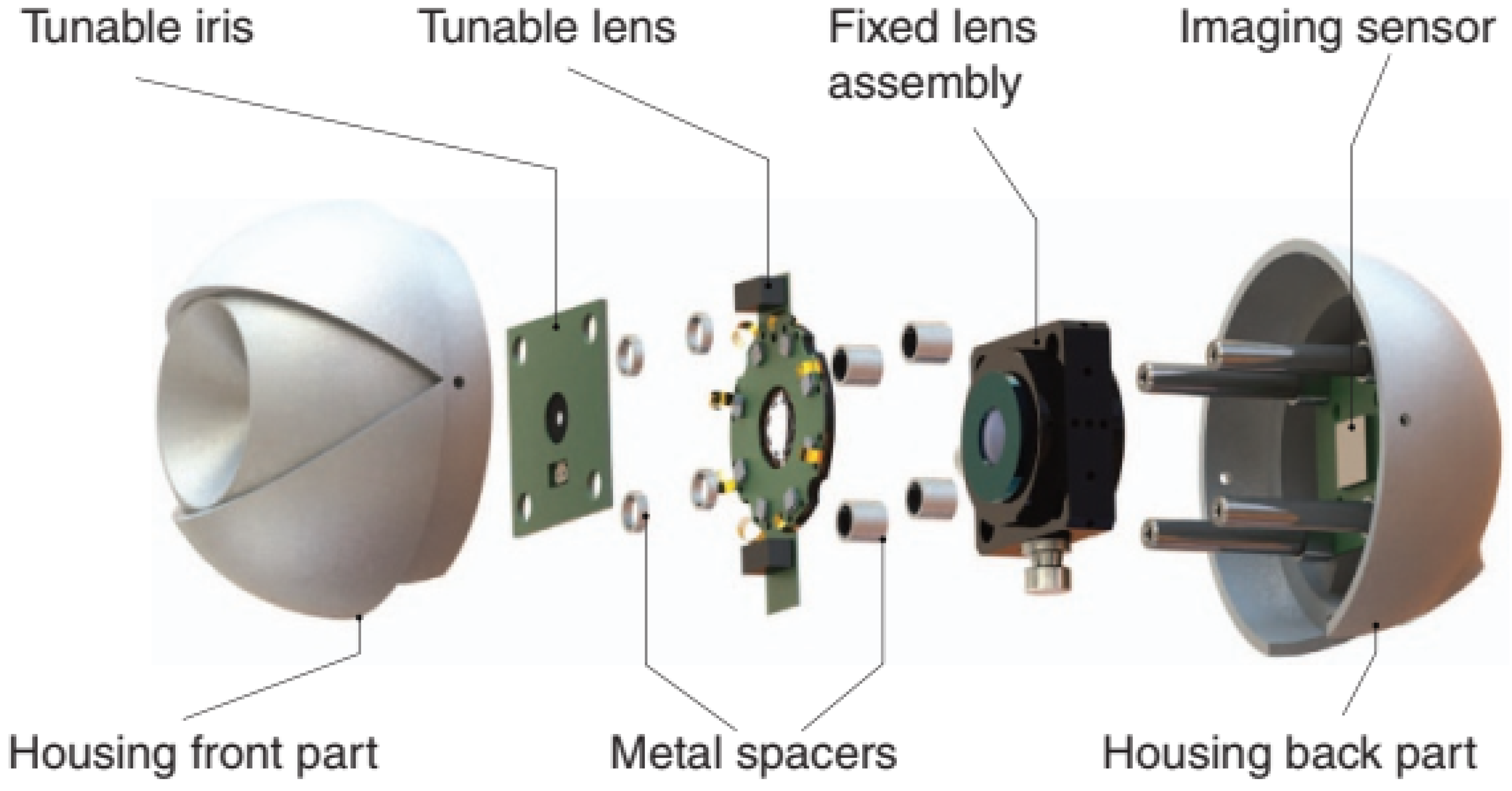
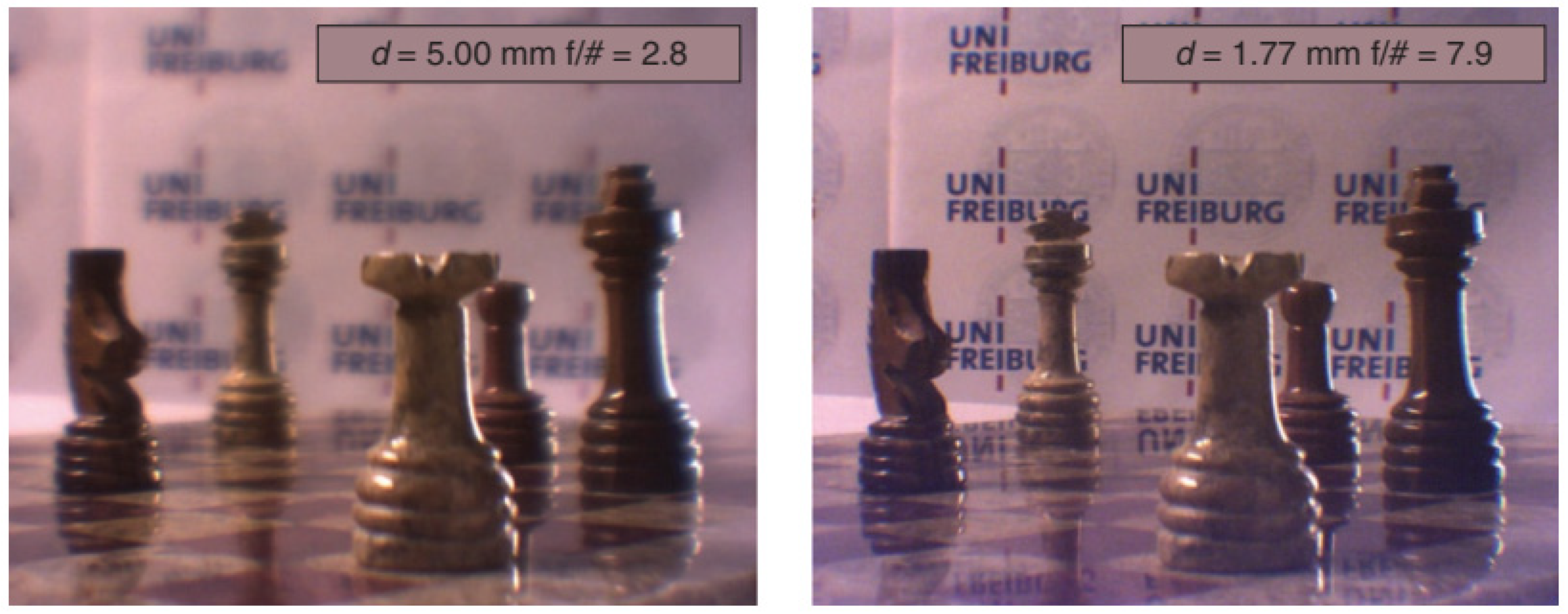

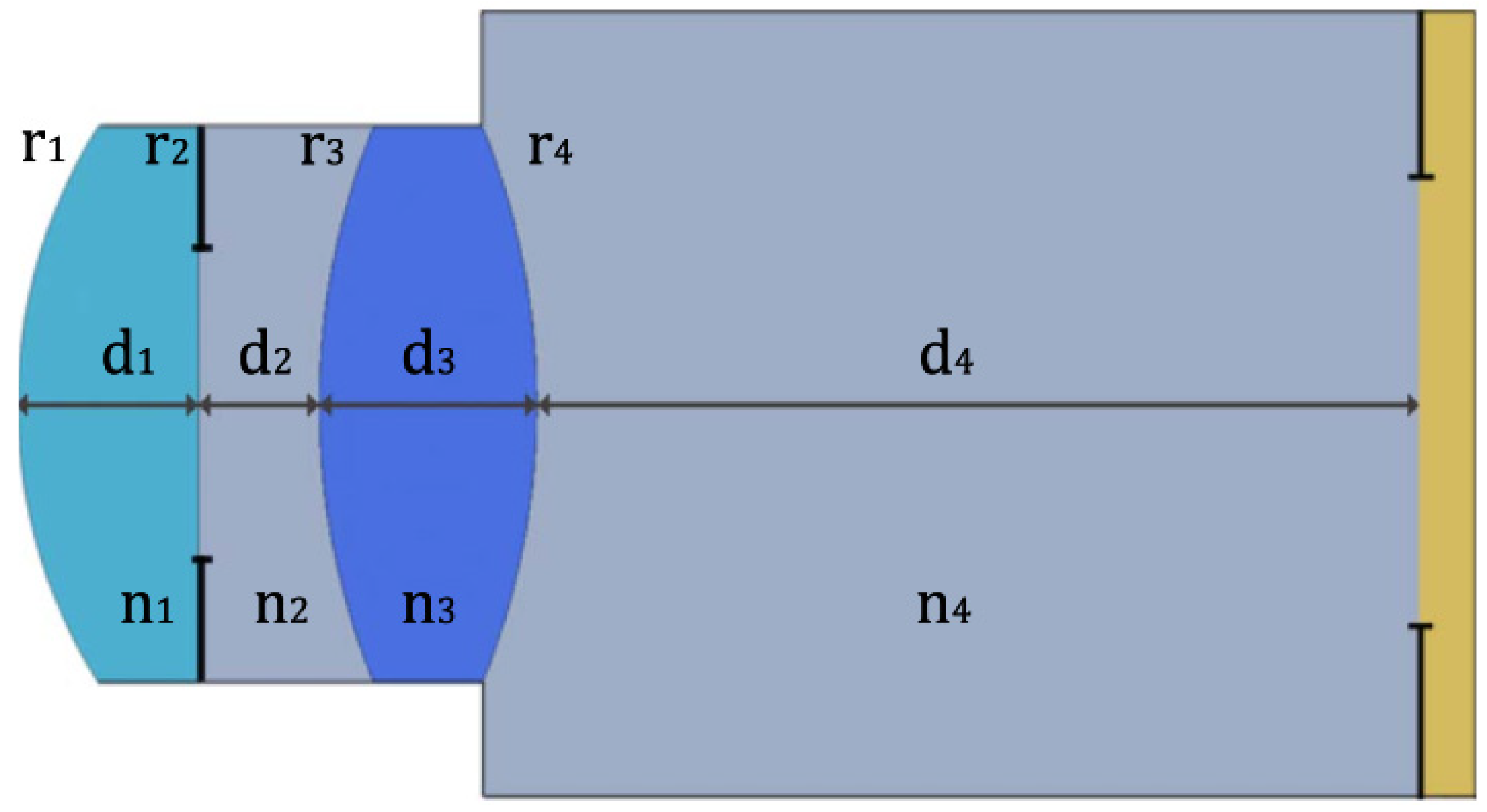
| Publication (Year) | Reference |
|---|---|
| Gliddon (1929) | [3] |
| Arell (1978) | [4] |
| Heath (1987) | [5] |
| Rudnicka (1992) | [6] |
| Oshika (1996) | [7] |
| Pujol (1998) | [8] |
| Moreno-Barriuso (2000) | [9] |
| Trinavarat (2001) | [10] |
| Dubbelman (2001) | [11] |
| Barry (2001) | [12] |
| Pieh (2002) | [13] |
| Holladay (2002) | [14] |
| Cheng (2003) | [15] |
| Barbero (2003) | [16] |
| Letfullin (2005) | [17] |
| Rawer (2005) | [18] |
| Kawamorita (2005) | [19] |
| Gobbi (2006) | [20] |
| Galetskiĭ (2006) | [21] |
| De Castro (2007) | [22] |
| Norrbi (2007) | [23] |
| Fernández (2007) | [24] |
| Artigas (2007) | [25] |
| Choi (2008) | [26] |
| Terwee (2008) | [27] |
| Campbell (2008) | [28] |
| Eppig (2008) | [29] |
| Barcik (2008) | [30] |
| Maxwell (2009) | [31] |
| McKelvie (2009) | [32] |
| Pieh (2009) | [33] |
| Shen (2009) | [34] |
| Goncharov (2009) | [35] |
| Eppig (2009) | [36] |
| Bakaraju (2010) | [37] |
| Inoue (2011) | [38] |
| Birkner (2011) | [39] |
| Ohnuma (2011) | [40] |
| Kim (2011) | [41] |
| Petelczyc (2011) | [42] |
| Pepose (2012) | [43] |
| Montés-Micó (2012) | [44] |
| Ackermann (2013) | [45] |
| Arianpour (2013) | [46] |
| Gatinel (2013) | [47] |
| Drauschke (2013) | [48] |
| Ruiz-Alcocer (2014) | [49] |
| Carson (2014) | [50] |
| Liang (2014) | [51] |
| Xie (2014) | [52] |
| Fung (2015) | [53] |
| Förster (2015) | [54] |
| Santiago-Alvarado (2015) | [55] |
| Vega (2015) | [56] |
| Esteve-Taboada (2015) | [57] |
| Yusuf (2015) | [58] |
| Guerra (2015) | [59] |
| Domínguez-Vicent (2015) | [60] |
| Mao (2016) | [61] |
| Petsch (2016) | [62] |
| Winter (2016) | [63] |
| Coughlan (2017) | [64] |
| Son (2017) | [65] |
| Alba-Bueno (2017) | [66] |
| Al-Mohamedi (2018) | [67] |
| Petelczy (2019) | [68] |
| Gu (2020) | [69] |
| Regal (2020) | [70] |
| Chae (2020) | [71] |
| Wang (2021) | [72] |
| Schneider (1996) * | [73] |
| Schneider (1997) * | [74] |
| Ohnuma (1999) * | [75] |
| Sheehy (2002) * | [76] |
| Altmann (2003) * | [77] |
| Yamaguchi (2006) * | [78] |
| Niven (2006) * | [79] |
| Ehrmann (2014) * | [80] |
| Application | Literature References |
|---|---|
| Evaluate intraocular lenses: | [7,10,13,14,16,17,18,19,20,22,23,25,26,27,28,29,30,31,32,33,38,41,43,44,47,48,49,50,56,60,65,66,68,71,73,74,75,77,80] |
| Evaluate spectacles/contact lenses: | [5,17,37,38,42,74,75,76,77,80] |
| Evaluate ophtalmic equipment: | [6,15,17,24,34,35,53,59,64,67,78,79,80] |
| Evaluate measurement methods: | [9,11,12,22,39,52,58,59,61,72,80] |
| Evaluate treatments: | [45,70] |
| Modeling optical conditions: | [3,6,8,17,21,30,37,40,46,48,64,80] |
| Simulate human visual conditions: | [26,45,54,66] |
| Modeling accomodation: | [24,51,54,55,57,62,63] |
| Modeling other eye behaviour | [17,21,54,55,69] |
| Research/optical design: | [3,6,35,46,51,64] |
| Teaching purposes: | [4] |
| Optical Principles Used | Literature References |
|---|---|
| Wavefront measurements: | [9,15,17,21,23,24,28,32,34,35,37,41,44,48,59,64] |
| Double-pass measurements: * | [6,8,9,35,42,57,59,72] |
| Single-pass measurements: | [3,5,7,9,13,14,16,18,19,20,25,26,27,28,29,30,31,33,37,40,41,42,43,45,46,47,48,49,50,51,54,55,56,60,62,65,66,68,69,70,71] |
| Fundus imaging and retinoscopy | [4,6,38,52,53,58,63] |
| Scheimpflug/Purkinje imaging: | [11,12,22] |
| Optical biometry and OCT | [39,52,61,67,72] |
| Other: | [10,52,70,76] |
| Ref. | Strengths | Limitations |
|---|---|---|
| [37,80] |
|
|
| [45] |
|
|
| [46] |
|
|
| [64] |
|
|
| [48] |
|
|
| [62] |
|
|
| [54] |
|
|
| [30] |
|
|
| [20] |
|
|
| [23] |
|
|
| [28] |
| No dynamic componentsNot focused on single-pass measurementsRequires relay optics (single-pass) |
| [73,74] |
|
|
| [77] |
|
|
| [55] |
|
|
| [51] |
|
|
| [40] |
|
|
| [42] |
|
|
| [26] |
|
|
| [27] |
|
|
| [75] |
|
|
| [76] |
|
|
| [36] |
|
|
| [66] |
|
|
| [13] |
|
|
| Other * |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, A.R.; Bret, B.; González-Méijome, J.M. Opto-Mechanical Eye Models, a Review on Human Vision Applications and Perspectives for Use in Industry. Sensors 2022, 22, 7686. https://doi.org/10.3390/s22197686
Amorim AR, Bret B, González-Méijome JM. Opto-Mechanical Eye Models, a Review on Human Vision Applications and Perspectives for Use in Industry. Sensors. 2022; 22(19):7686. https://doi.org/10.3390/s22197686
Chicago/Turabian StyleAmorim, André Rino, Boris Bret, and José M. González-Méijome. 2022. "Opto-Mechanical Eye Models, a Review on Human Vision Applications and Perspectives for Use in Industry" Sensors 22, no. 19: 7686. https://doi.org/10.3390/s22197686
APA StyleAmorim, A. R., Bret, B., & González-Méijome, J. M. (2022). Opto-Mechanical Eye Models, a Review on Human Vision Applications and Perspectives for Use in Industry. Sensors, 22(19), 7686. https://doi.org/10.3390/s22197686






