EEG-Validated Photobiomodulation Treatment of Dementia—Case Study
Abstract
1. Introduction
1.1. Causes and Symptoms
1.2. Diagnostics
1.3. Treatment of AD
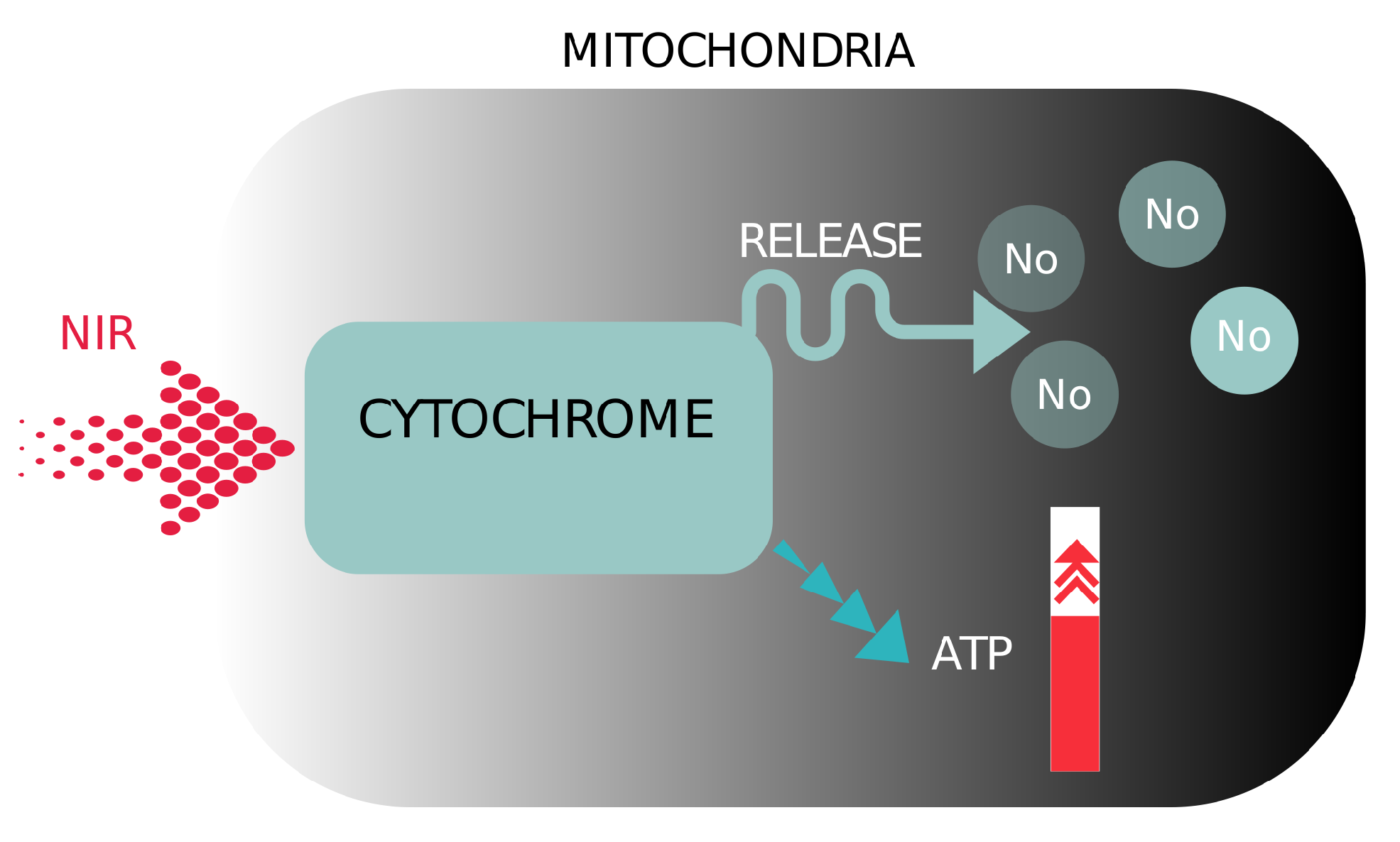
1.4. Photobiomodulation (PBM)
2. Materials and Methods
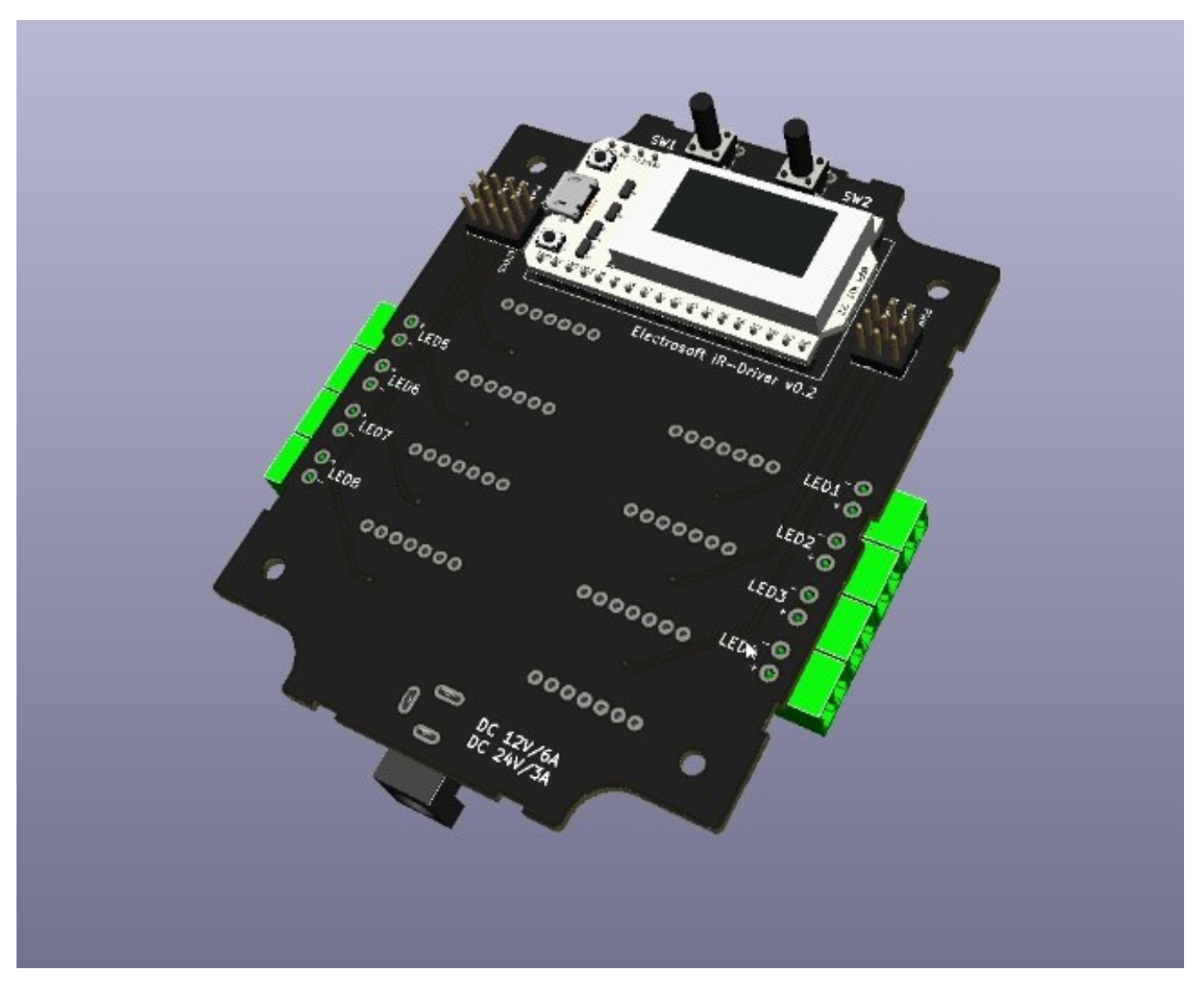
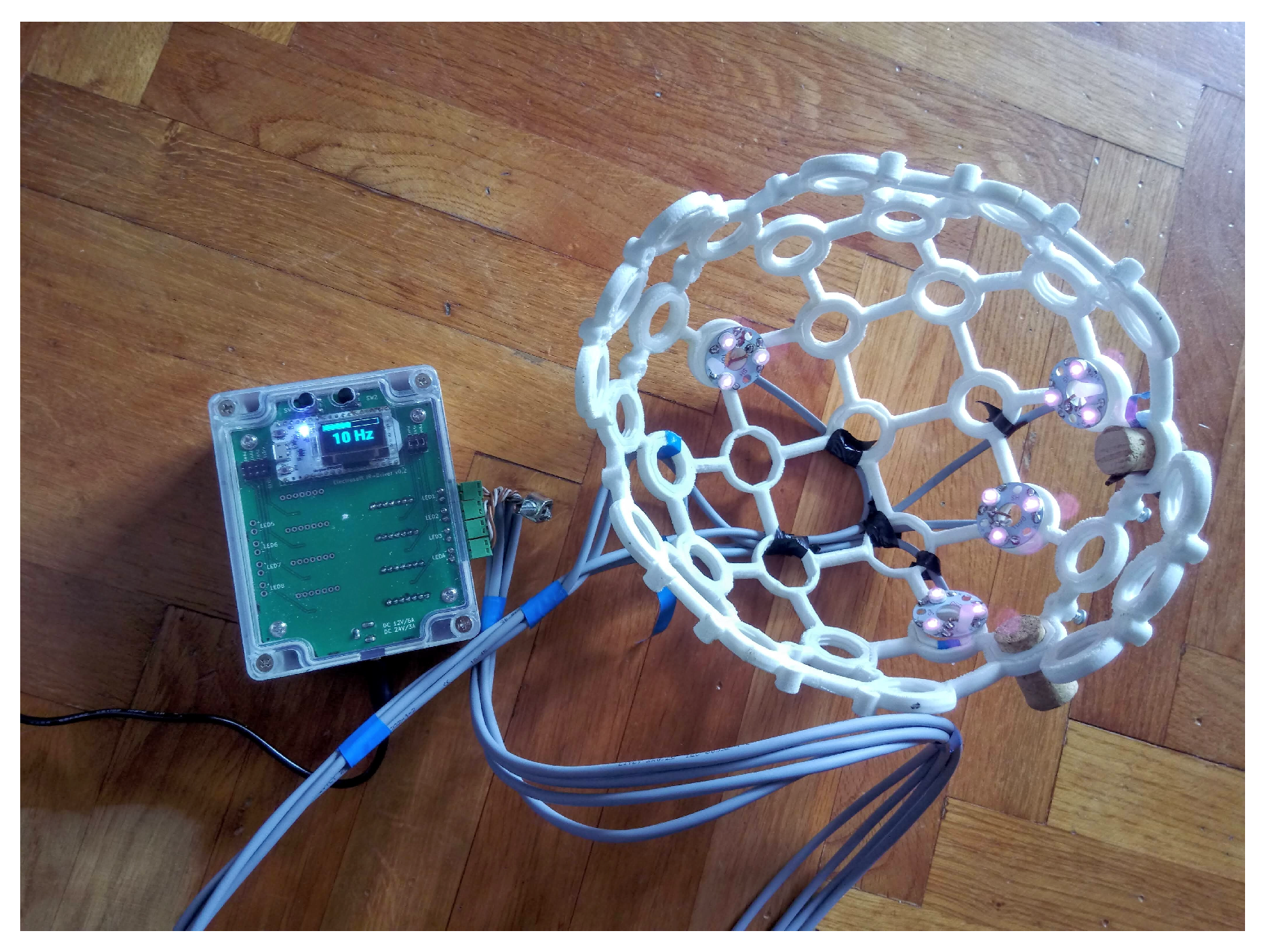
2.1. Near Infra Red (NIR) Device Prototype
- 1 W, 810 nm; DC IF: 700 mA; DC UF: typical: 1.6–2.2 V; beam angle 120–140.
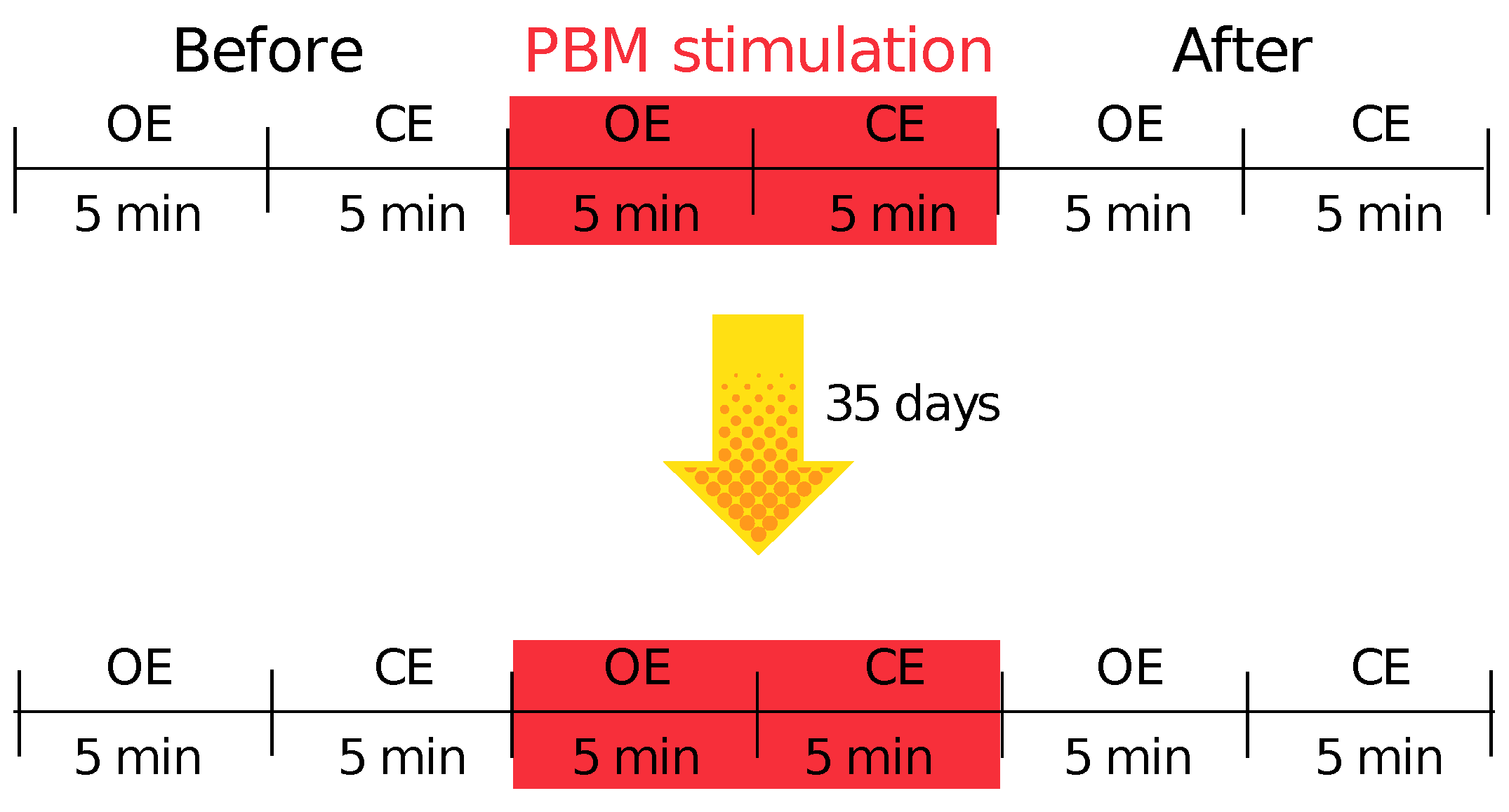
2.2. Stimulation Protocol
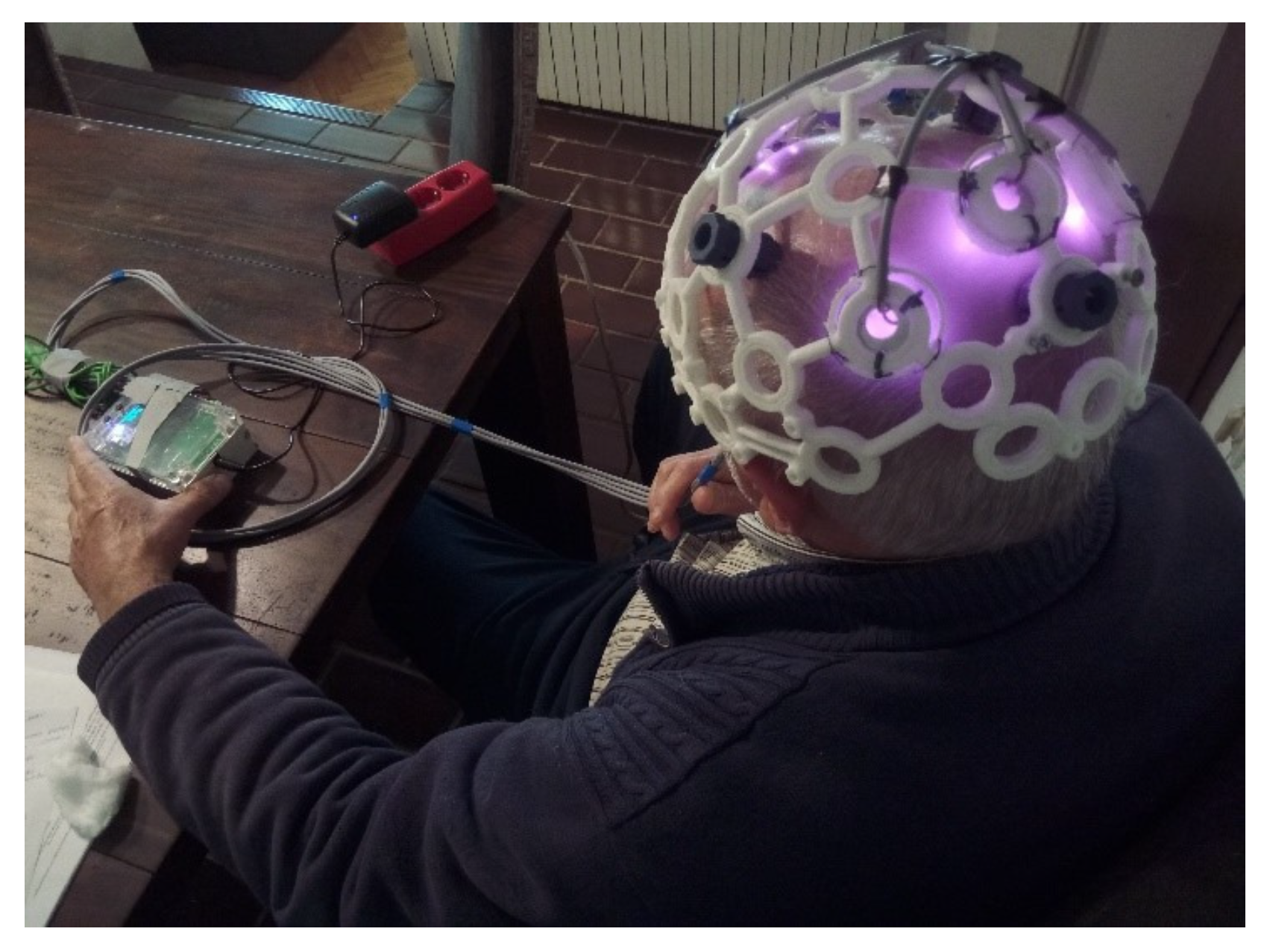
2.3. EEG Acquisition
2.4. Description of the Pilot Study Design
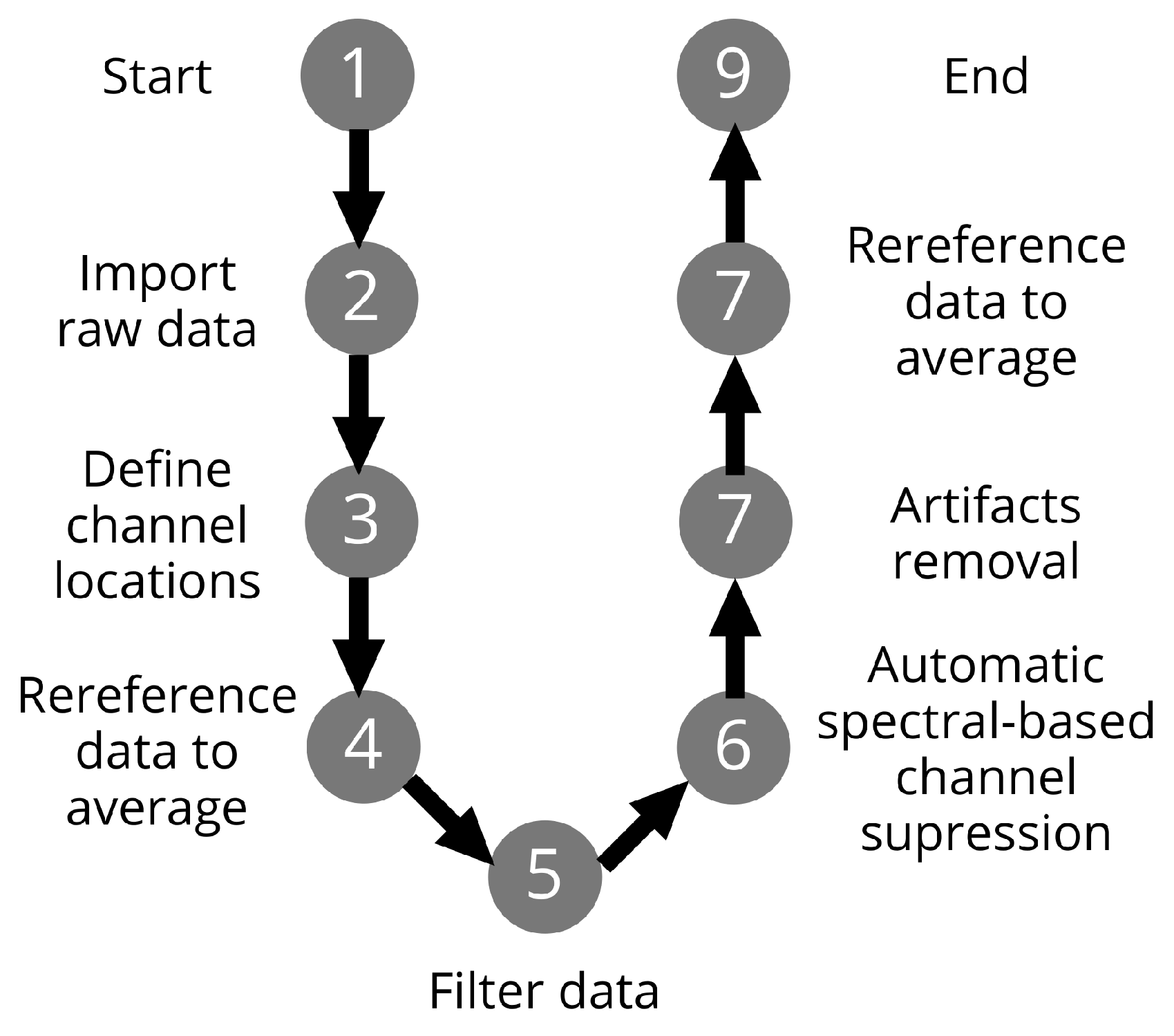
2.5. Offline Preprocessing
2.6. Analyses
2.6.1. Power Spectrum Analysis
2.6.2. Connectivity Analysis
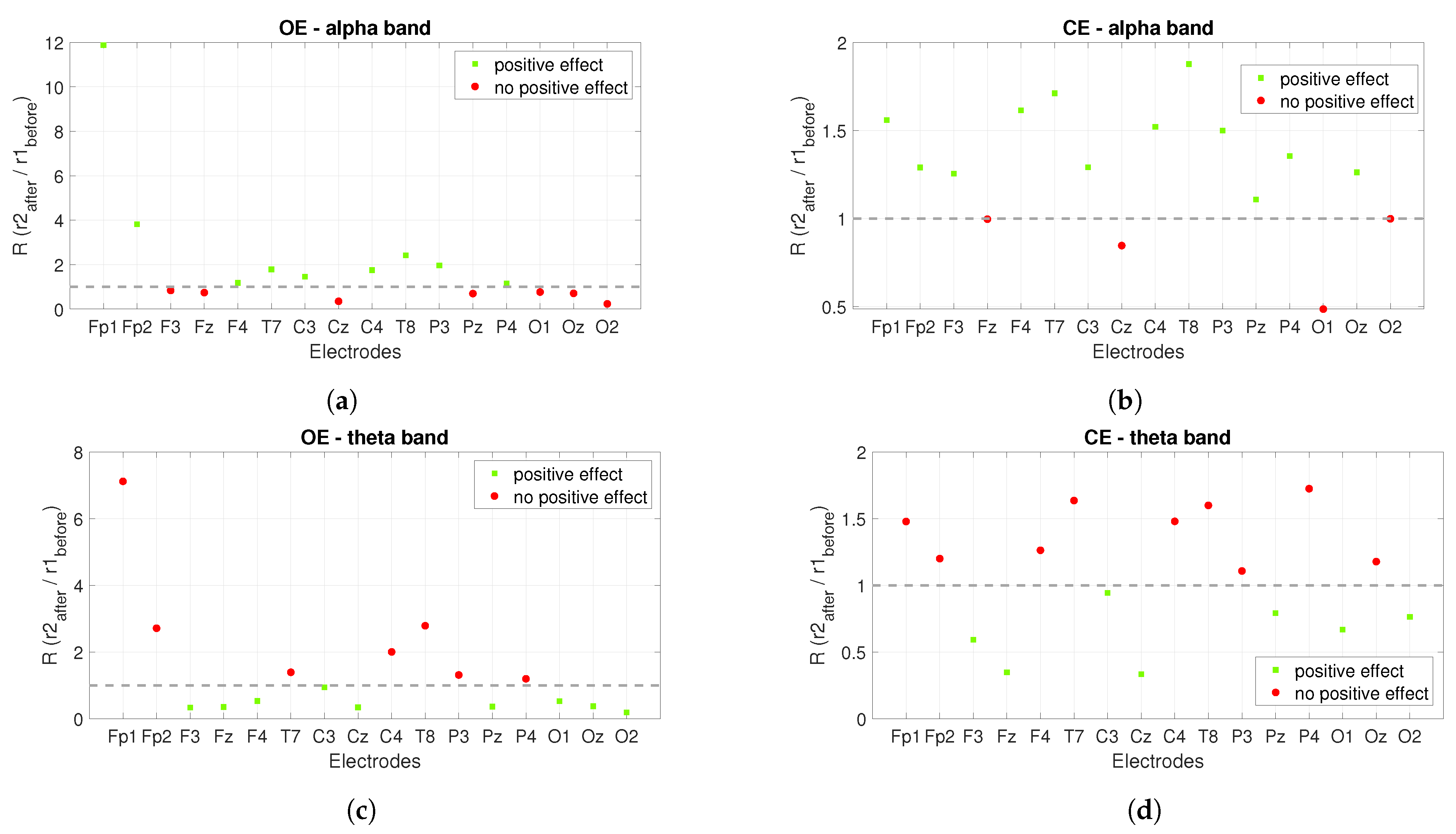
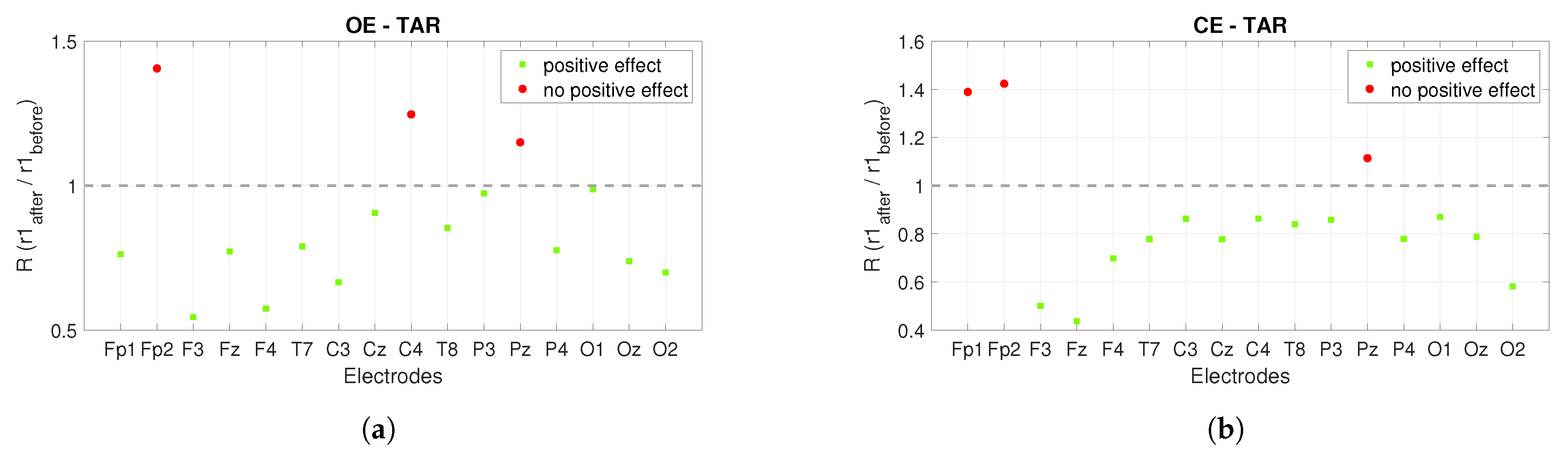
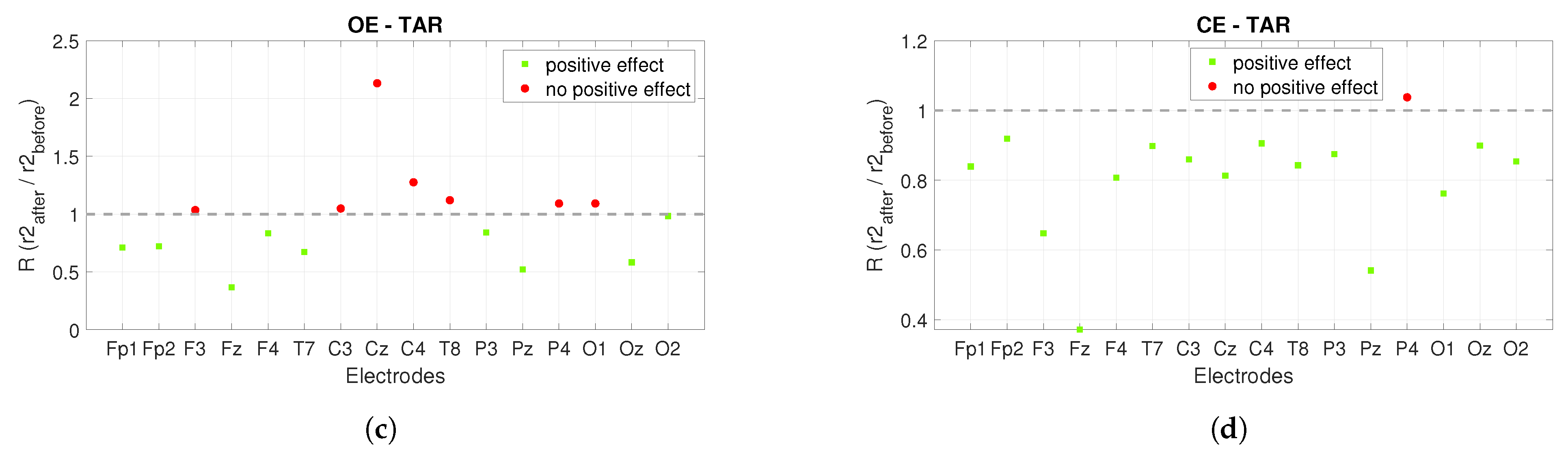
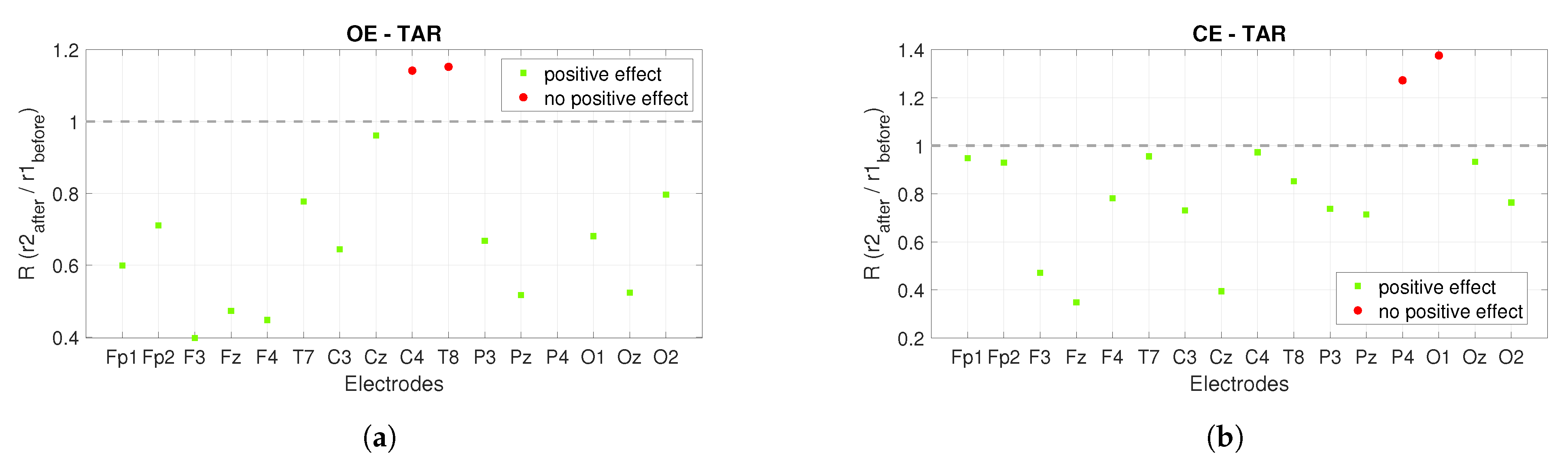
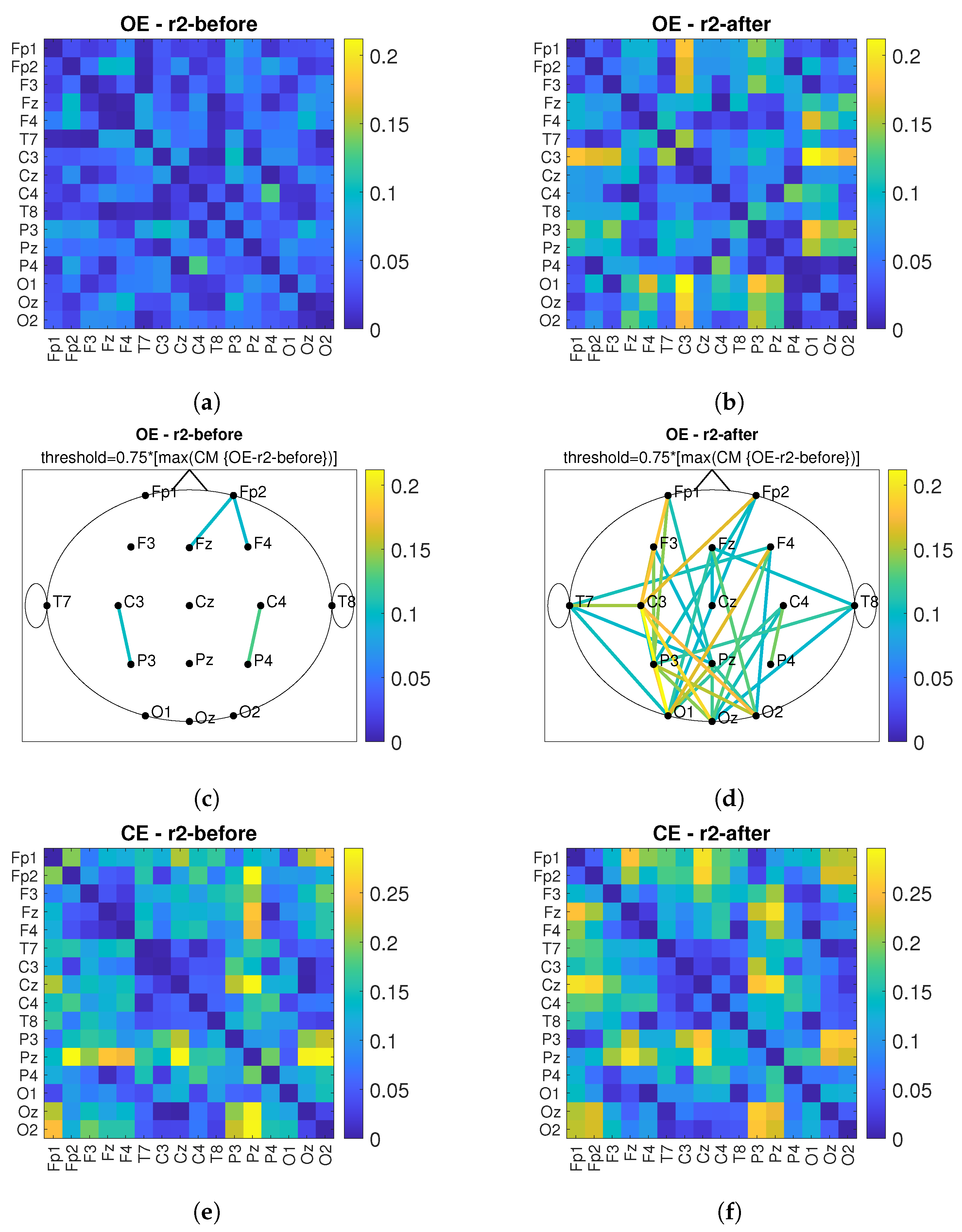
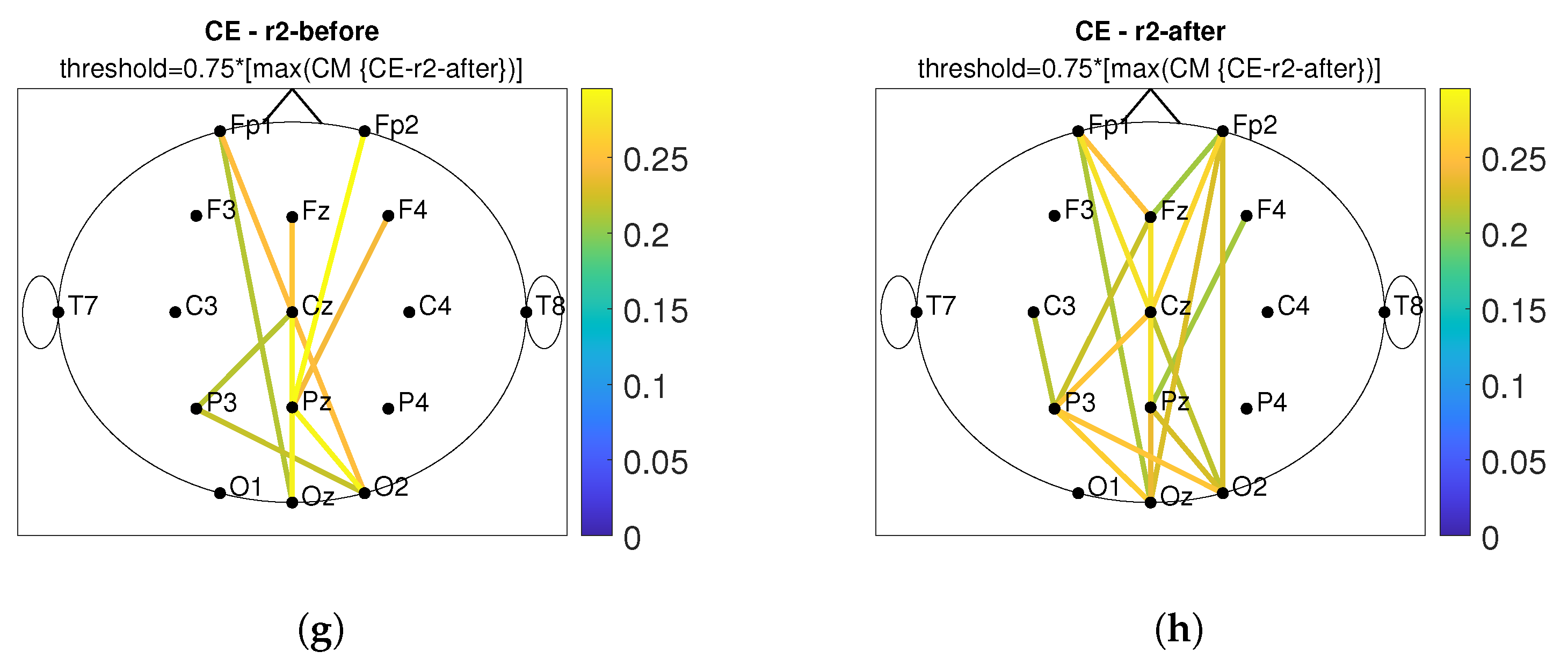
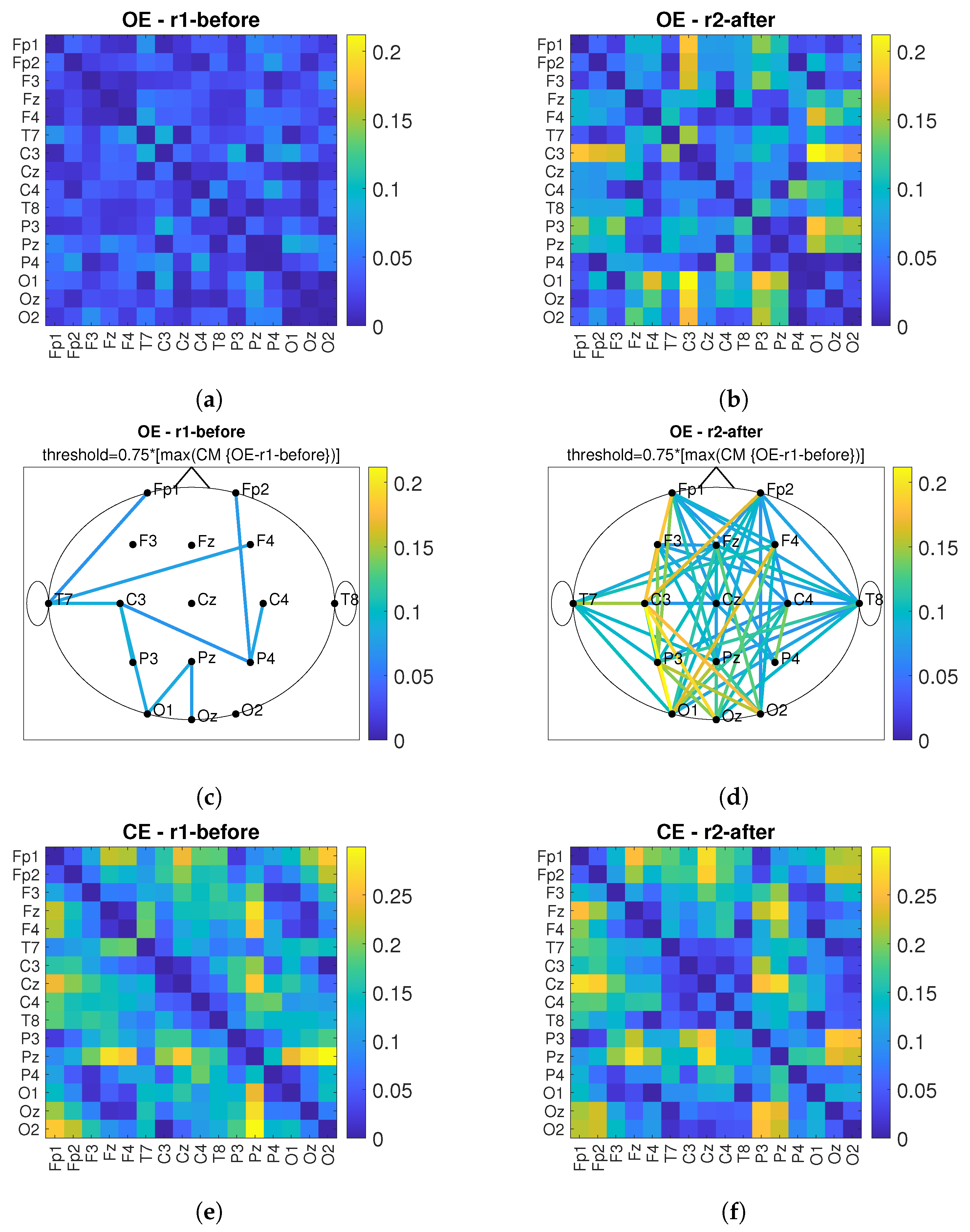
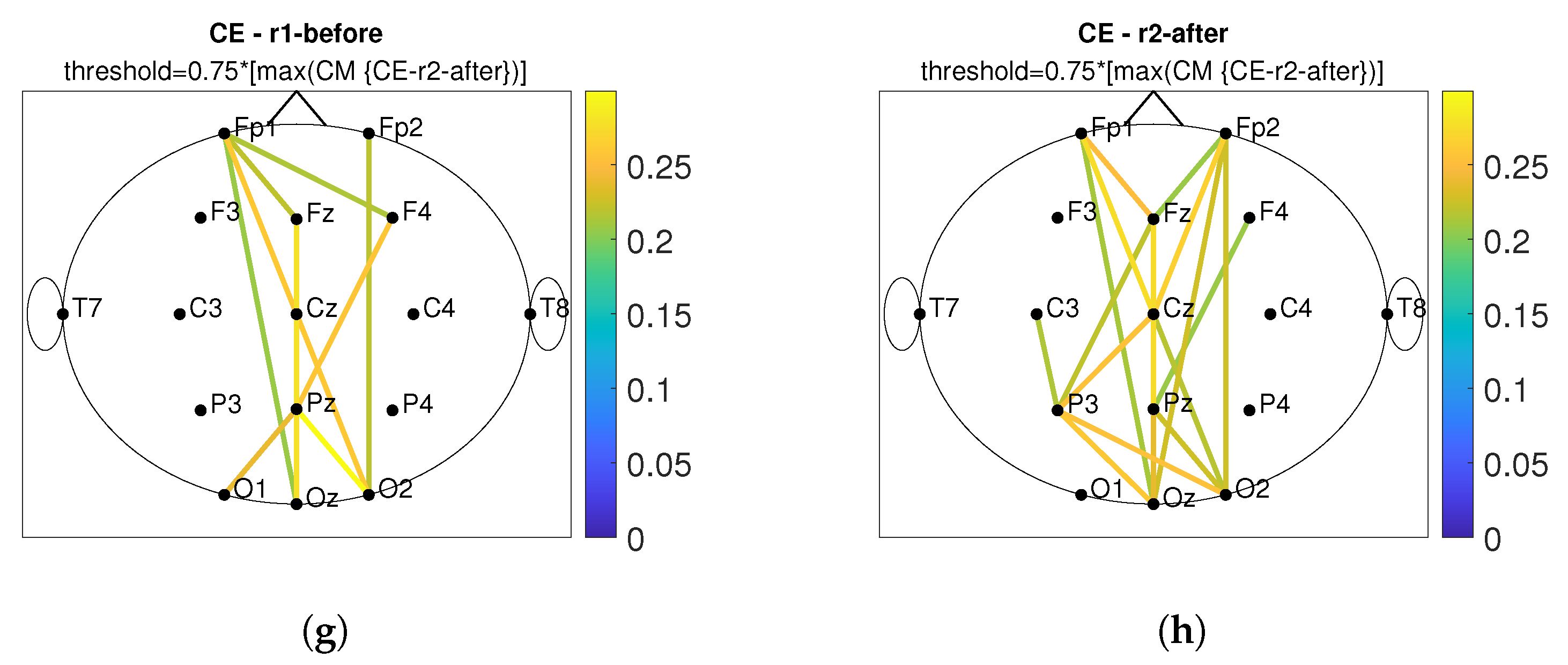
3. Results
3.1. Power Spectrum Analysis
3.2. Connectivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| absCPCC | absolute value of complex Pearson correlation coefficient |
| AD | Alzheimer’s disease |
| CE | closed eyes |
| CM | connectivity matrix |
| CPCC | Complex Pearson correlation coefficient |
| CT | Computed tomography |
| DBS | deep brain stimulation |
| EEG | Electroencephalography |
| FDA | U.S. Food and Drug Administration |
| FFT | Fast Fourier transform |
| GFS | Global Field Synchronization |
| IR LED | infra-red Light Emitting Diode |
| imCPCC | imaginary component of complex Pearson correlation coefficient |
| LED | Light Emitting Diode |
| MCI | mild cognitive impairment |
| MEG | Magnetoencephalography |
| MI | Mutual Information |
| MMSE | Mini-Mental State Exam |
| MRI | Magnetic Resonance Imaging |
| NIR | near-infrared |
| OE | opened eyes |
| PBM | photobiomodulation |
| PLI | Phase Lag Index |
| PLV | Phase Locking Value |
| r1 | First Recording |
| r2 | Second Recording |
| TAR | theta-alpha ratio |
| tDCS | transcranial direct current stimulation |
| TILS | transcranial infrared laser stimulation |
| TMS | transcranial magnetic stimulation |
| tPBM | transcranial photobiomodulation |
| SAGE | Self Administered Gerocognitive Exam |
| SCI | subjective cognitive impairment |
| wPLI | Weighted Phase Lag Index |
References
- Boccaletti, S.; Latora, V.; Moreno, Y.; Chavez, M.; Hwang, D.U. Complex networks: Structure and dynamics. Phys. Rep. 2006, 424, 175–308. [Google Scholar] [CrossRef]
- Callixte, K.T.; Clet, T.B.; Jacques, D.; Faustin, Y.; François, D.J.; Maturin, T.T. The pattern of neurological diseases in elderly people in outpatient consultations in Sub-Saharan Africa. BMC Res. Notes 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Alzheimer’s Association. What Is Alzheimer’s Disease? Available online: https://www.alz.org/alzheimers-dementia/what-is-alzheimers (accessed on 15 June 2022).
- Hogan, D.B.; Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Dykeman, J.; Pringsheim, T.; Steeves, T.; Smith, E.E.; Pearson, D.; Jetté, N. The prevalence and incidence of dementia with Lewy bodies: A systematic review. Can. J. Neurol. Sci. 2016, 43, S83–S95. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Emre, M.; Dubois, B. Parkinson’s disease dementia: Definitions, guidelines, and research perspectives in diagnosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2008, 64, S81–S92. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.S.; Neary, D.; Mann, D.M. Frontotemporal dementia. Br. J. Psychiatry 2002, 180, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Iwasaki, Y. Creutzfeldt-Jakob disease. Neuropathology 2017, 37, 174–188. [Google Scholar] [CrossRef] [PubMed]
- NHS. Tests for Diagnosing Dementia. Available online: https://www.nhs.uk/conditions/dementia/diagnosis-tests/ (accessed on 15 June 2022).
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef] [PubMed]
- Busse, E.W.; Barnes, R.H.; Friedman, E.L.; Kelty, E.J. Psychological functioning of aged individuals with normal and abnormal electroencephalograms. J. Nerv. Ment. Dis. 1956, 124, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.P.; Ulrich, R.F.; Spiker, D.G.; Sclabassi, R.J.; Reynolds III, C.F.; Marin, R.S.; Boller, F. Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 483–492. [Google Scholar] [CrossRef]
- Besthorn, C.; Förstl, H.; Geiger-Kabisch, C.; Sattel, H.; Gasser, T.; Schreiter-Gasser, U. EEG coherence in Alzheimer disease. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 242–245. [Google Scholar] [CrossRef]
- Houmani, N.; Vialatte, F.; Gallego-Jutglà, E.; Dreyfus, G.; Nguyen-Michel, V.H.; Mariani, J.; Kinugawa, K. Diagnosis of Alzheimer’s disease with Electroencephalography in a differential framework. PLoS ONE 2018, 13, e0193607. [Google Scholar] [CrossRef] [PubMed]
- Akrofi, K.; Pal, R.; Baker, M.C.; Nutter, B.S.; Schiffer, R.W. Classification of Alzheimer’s disease and mild cognitive impairment by pattern recognition of EEG power and coherence. In Proceedings of the 2010 IEEE International Conference on Acoustics, Speech and Signal Processing, Dallas, TX, USA, 15–19 March 2010; pp. 606–609. [Google Scholar]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s disease: Has the light dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef]
- Vlahinić, S.; Šverko, Z.; Bačnar, D.; Bebić, L. Analyses of IR stimulation influence on EEG. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; pp. 1–6. [Google Scholar]
- Jeong, J.; Gore, J.C.; Peterson, B.S. Mutual information analysis of the EEG in patients with Alzheimer’s disease. Clin. Neurophysiol. 2001, 112, 827–835. [Google Scholar] [CrossRef]
- König, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.; John, E.; Jelic, V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef]
- Stam, C.; De Haan, W.; Daffertshofer, A.; Jones, B.; Manshanden, I.; van Cappellen van Walsum, A.M.; Montez, T.; Verbunt, J.; De Munck, J.; Van Dijk, B.; et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 2009, 132, 213–224. [Google Scholar] [CrossRef]
- National Institute on Aging. How Is Alzheimer’s Disease Treated? Available online: https://www.nia.nih.gov/health/how-alzheimers-disease-treated (accessed on 15 June 2022).
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): Clinical applications and safety concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef]
- Gangemi, A.; Colombo, B.; Fabio, R.A. Effects of short-and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: Two randomized studies. Aging Clin. Exp. Res. 2021, 33, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.; Sadowsky, C.; Tousi, B.; Agronin, M.E.; Alva, G.; Armon, C.; Bernick, C.; Keegan, A.P.; Karantzoulis, S.; Baror, E.; et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, 641–650. [Google Scholar] [CrossRef]
- Laxton, A.W.; Lozano, A.M. Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg. 2013, 80, S28.e1–S28.e8. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rojas, R.; Liao, M.; Romero, J.; Huang, X.; Ou, K.L. Cortical network response to acupuncture and the effect of the hegu point: An fNIRS study. Sensors 2019, 19, 394. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BAA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef]
- Vargas, E.; Barrett, D.W.; Saucedo, C.L.; Huang, L.D.; Abraham, J.A.; Tanaka, H.; Haley, A.P.; Gonzalez-Lima, F. Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci. 2017, 32, 1153–1162. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of home photobiomodulation treatments on cognitive and behavioral function, cerebral perfusion, and resting-state functional connectivity in patients with dementia: A pilot trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- 2022 OpenBCI. OpenBCI. Available online: https://openbci.com/ (accessed on 15 June 2022).
- Scharre, D.W.; Chang, S.I.; Murden, R.A.; Lamb, J.; Beversdorf, D.Q.; Kataki, M.; Nagaraja, H.N.; Bornstein, R.A. Self-administered Gerocognitive Examination (SAGE): A brief cognitive assessment Instrument for mild cognitive impairment (MCI) and early dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 64–71. [Google Scholar] [CrossRef]
- Šverko, Z.; Vrankić, M.; Vlahinić, S.; Rogelj, P. Complex Pearson Correlation Coefficient for EEG Connectivity Analysis. Sensors 2022, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Šverko, Z.; Vrankic, M.; Vlahinić, S.; Rogelj, P. Dynamic Connectivity Analysis Using Adaptive Window Size. Sensors 2022, 22, 5162. [Google Scholar] [CrossRef]
- Costumero, V.; Bueichekú, E.; Adrián-Ventura, J.; Ávila, C. Opening or closing eyes at rest modulates the functional connectivity of V1 with default and salience networks. Sci. Rep. 2020, 10, 9137. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
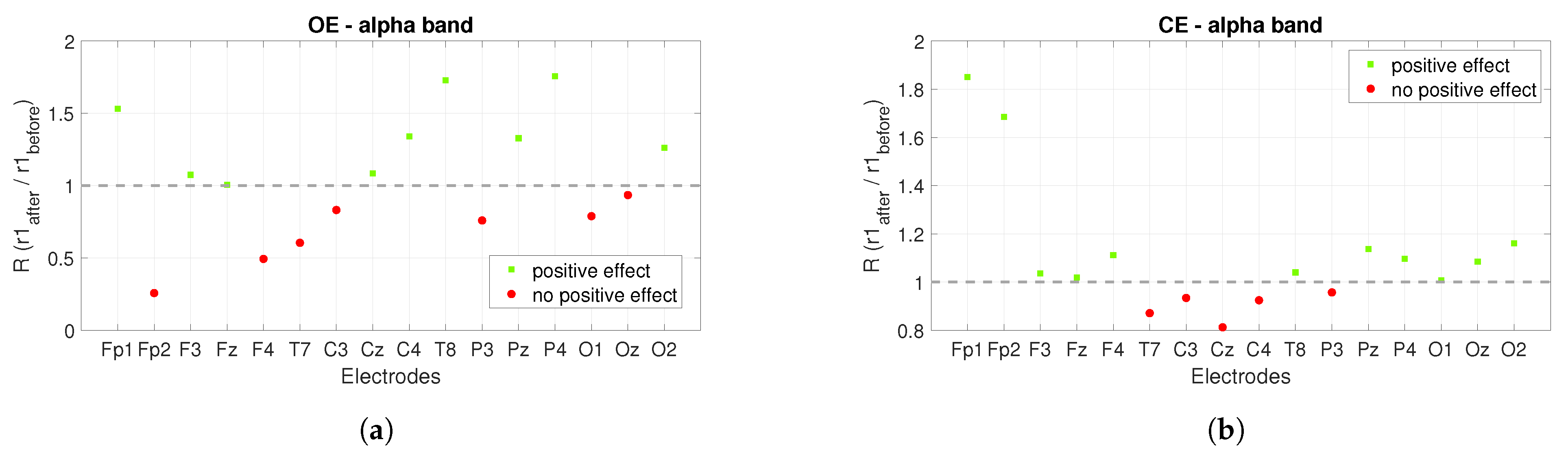
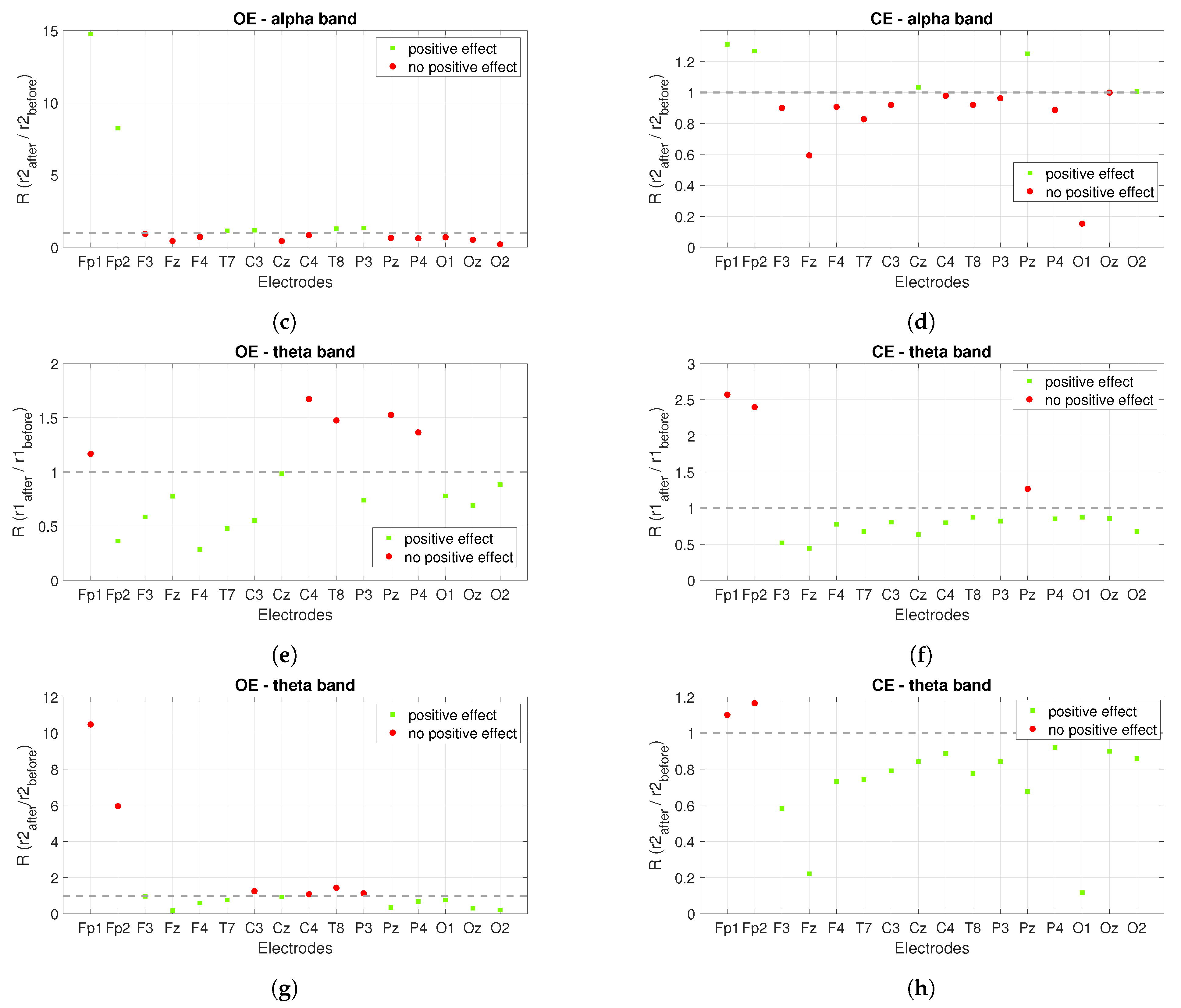


| Type/Measure | 10 Hz/PL1 | 10 Hz/PL2 | 10 Hz/PL3 | 10 Hz/PL4 | 40 Hz/PL2 | 40 Hz/PL4 | DC/PL4 |
|---|---|---|---|---|---|---|---|
| P(mW) | 1 | 10 | 39 | ||||
| (mW/cm |
| Recordings | E |
|---|---|
| r1-OE condition before stimulation | |
| r2-OE condition after stimulation | |
| r1-CE condition before stimulation | |
| r2-CE condition after stimulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrankic, M.; Vlahinić, S.; Šverko, Z.; Markovinović, I. EEG-Validated Photobiomodulation Treatment of Dementia—Case Study. Sensors 2022, 22, 7555. https://doi.org/10.3390/s22197555
Vrankic M, Vlahinić S, Šverko Z, Markovinović I. EEG-Validated Photobiomodulation Treatment of Dementia—Case Study. Sensors. 2022; 22(19):7555. https://doi.org/10.3390/s22197555
Chicago/Turabian StyleVrankic, Miroslav, Saša Vlahinić, Zoran Šverko, and Ivan Markovinović. 2022. "EEG-Validated Photobiomodulation Treatment of Dementia—Case Study" Sensors 22, no. 19: 7555. https://doi.org/10.3390/s22197555
APA StyleVrankic, M., Vlahinić, S., Šverko, Z., & Markovinović, I. (2022). EEG-Validated Photobiomodulation Treatment of Dementia—Case Study. Sensors, 22(19), 7555. https://doi.org/10.3390/s22197555








