The Development of an Innovative Embedded Sensor for the Optical Measurement of Ex-Vivo Engineered Muscle Tissue Contractility
Abstract
:1. Introduction
2. Materials and Methods
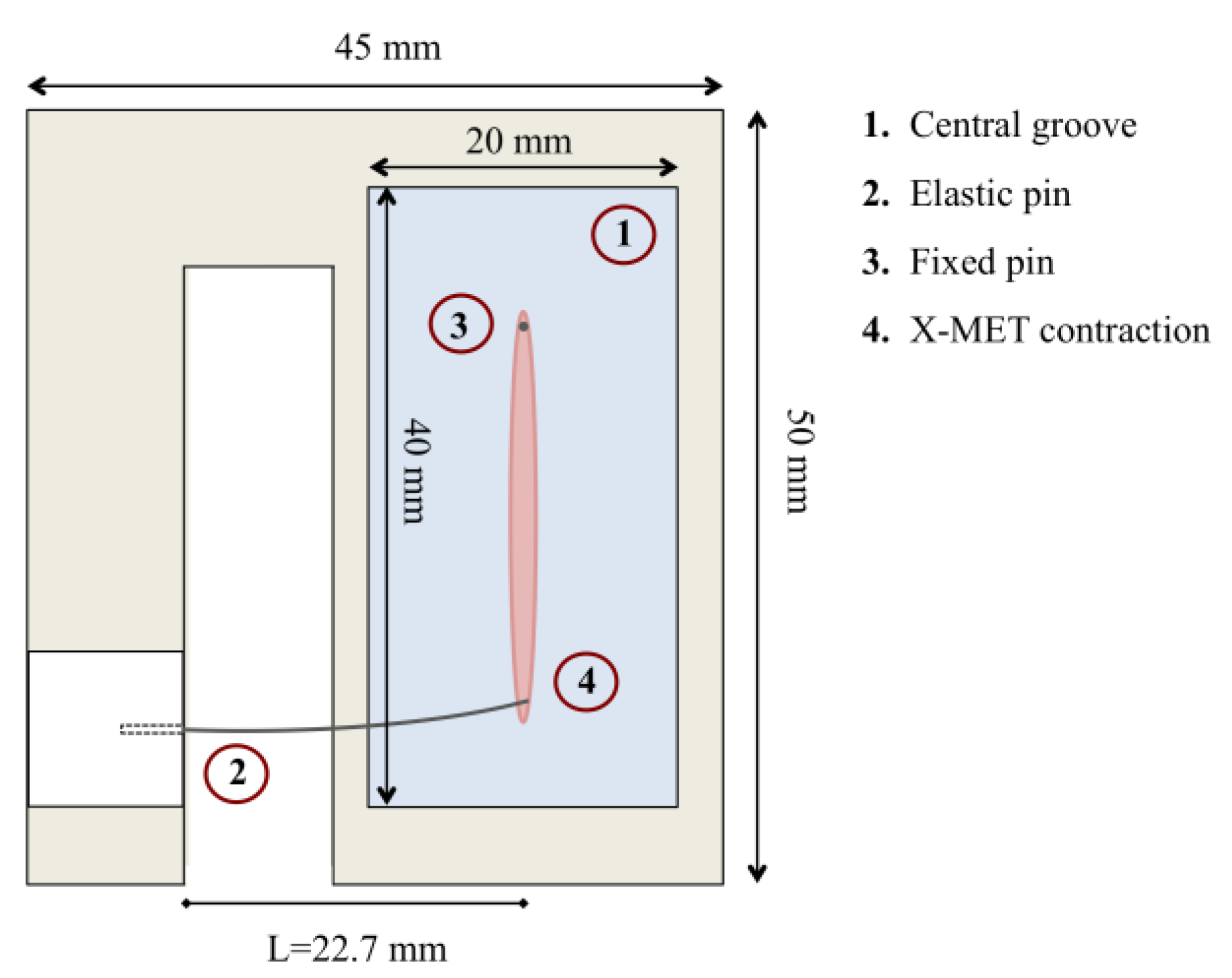
2.1. Sensor Design
2.2. Elastic Pin Calibration
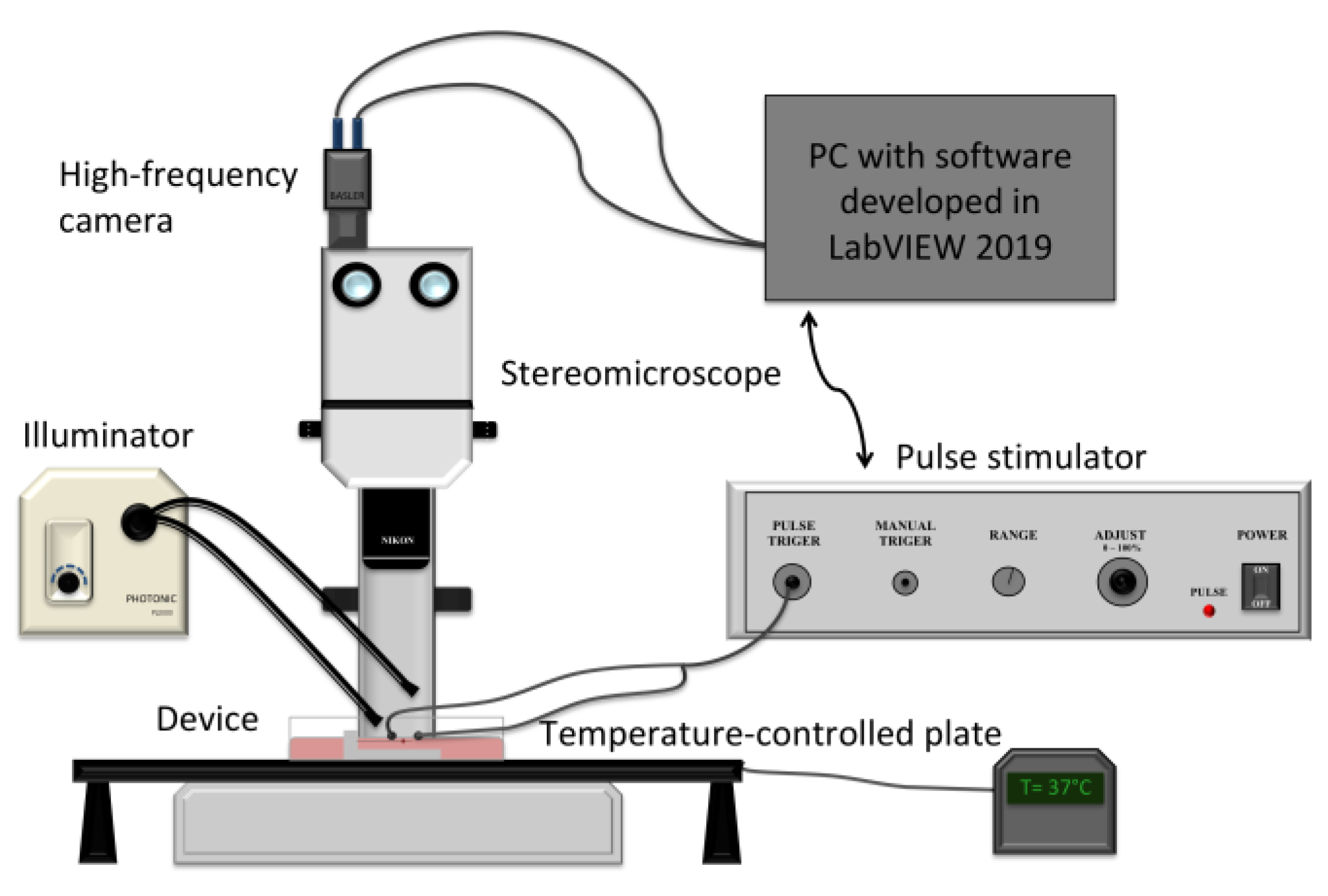
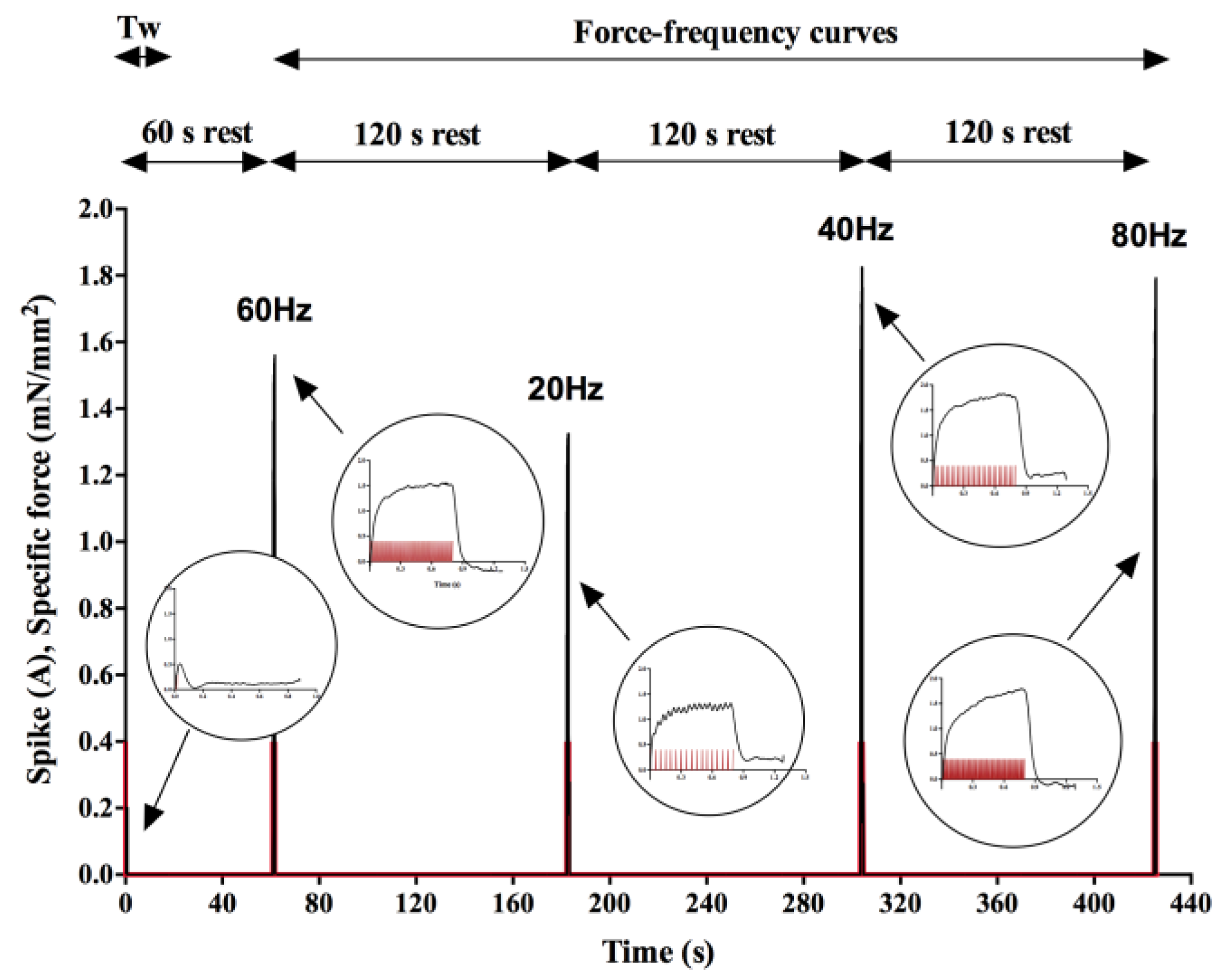
2.3. Experimental System and Stimulation Protocol
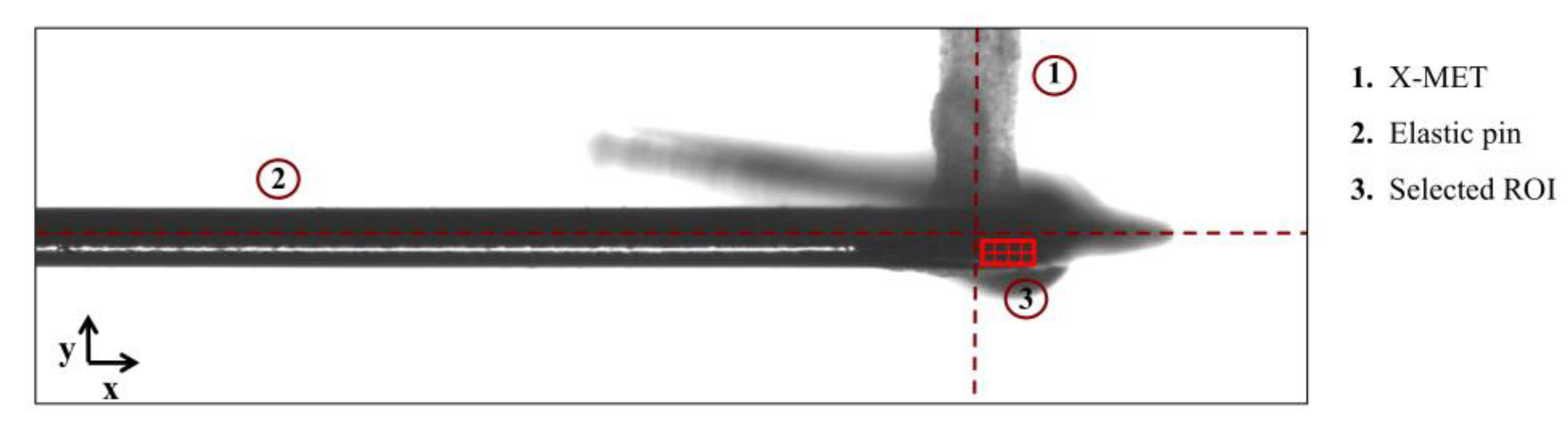
2.4. Contraction Force Measurement through the Optical Tracking Method
2.5. Statistical Analysis
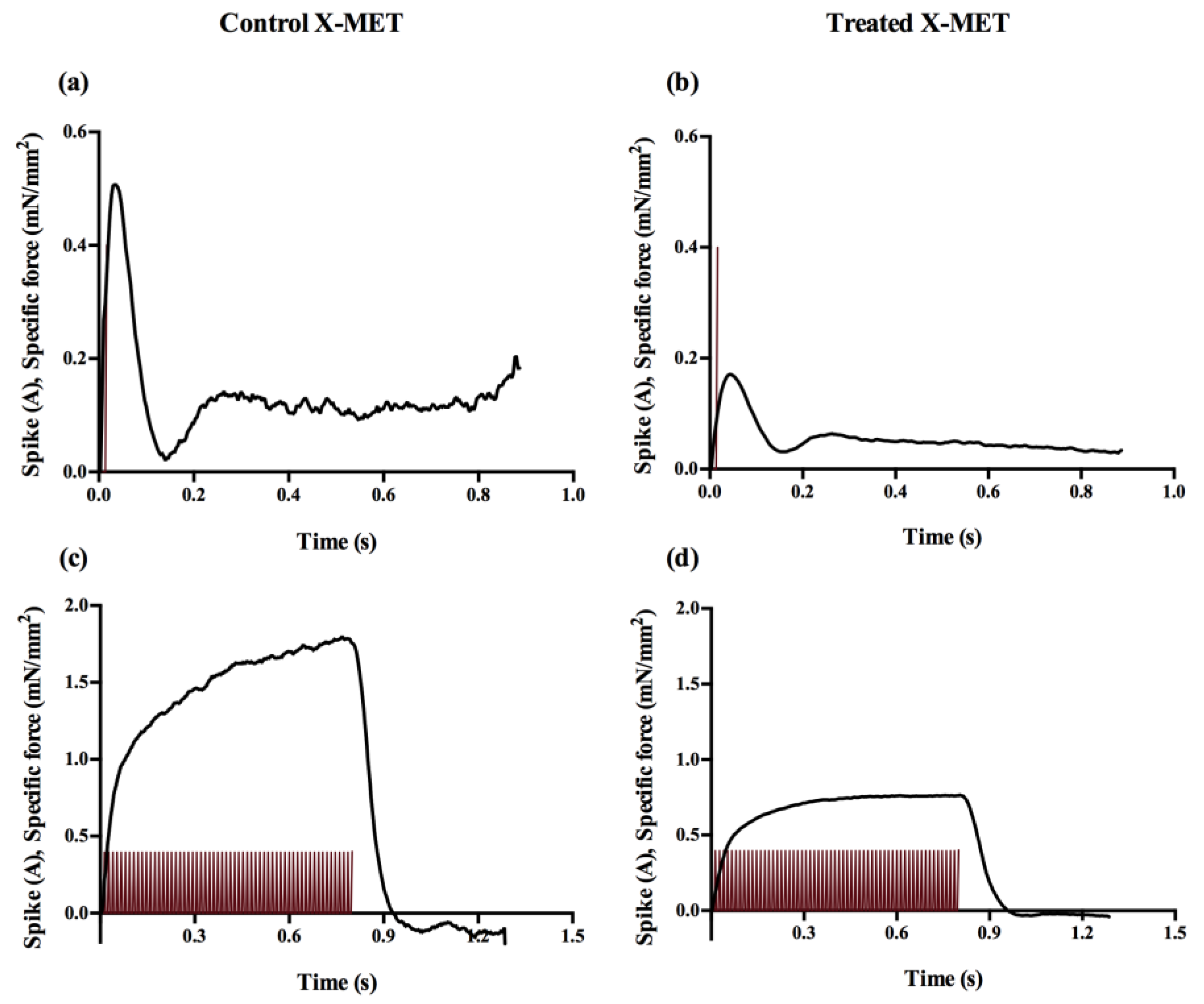
3. Results
3.1. Force-Displacement Calibration
3.2. Contractility Measurements
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue Engineering: Current Strategies and Future Directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, F. Tissue Mechanics and Tissue Engineering. Appl. Sci. 2022, 12, 6664. [Google Scholar] [CrossRef]
- Karagounis, L.G.; Hawley, J.A. Skeletal muscle: Increasing the size of the locomotor cell. Int. J. Biochem. Cell Biol. 2010, 42, 1376–1379. [Google Scholar] [CrossRef]
- Liu, G.; Mac Gabhann, F.; Popel, A.S. Effects of Fiber Type and Size on the Heterogeneity of Oxygen Distribution in Exercising Skeletal Muscle. PLoS ONE 2012, 7, e44375. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.H.; Park, W.J.; Lee, J.E.; Kim, B.; Han, D.W. Advanced techniques for skeletal muscle tissue engineering and regeneration. Bioengineering 2020, 7, 99. [Google Scholar] [CrossRef]
- Guettier-Sigrist, S.; Coupin, G.; Braun, S.; Warter, J.M.; Poindron, P. Muscle could be the therapeutic target in SMA treatment. J. Neurosci. Res. 1998, 53, 663–669. [Google Scholar] [CrossRef]
- Khodabukus, A.; Kaza, A.; Wang, J.; Prabhu, N.; Goldstein, R.; Vaidya, V.S.; Bursac, N. Tissue-engineered human myobundle system as a platform for evaluation of skeletal muscle injury biomarkers. Toxicol. Sci. 2020, 176, 124–136. [Google Scholar] [CrossRef]
- Khodabukus, A. Tissue-Engineered Skeletal Muscle Models to Study Muscle Function, Plasticity, and Disease. Front. Physiol. 2021, 12, 619710. [Google Scholar] [CrossRef]
- Kim, J.; Kong, J.S.; Han, W.; Kim, B.S.; Cho, D.W. 3d cell printing of tissue/organ-mimicking constructs for therapeutic and drug testing applications. Int. J. Mol. Sci. 2020, 21, 7757. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Carosio, S.; Barberi, L.; Rizzuto, E.; Nicoletti, C.; Del Prete, Z.; Musarò, A. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET). Sci. Rep. 2013, 3, 1420. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, E.; Carosio, S.; Faraldi, M.; Pisu, S.; Musarò, A.; Del Prete, Z. A DIC Based Technique to Measure the Contraction of a Skeletal Muscle Engineered Tissue. Appl. Bionics Biomech. 2016, 2016, 7465095. [Google Scholar] [CrossRef]
- Pisu, S.; Cosentino, M.; Apa, L.; Musarò, A.; Rizzuto, E.; Del Prete, Z. Measuring the Maximum Power of an ex vivo Engineered Muscle Tissue with Isovelocity Shortening Technique. IEEE Trans. Instrum. Meas. 2019, 68, 2404–2411. [Google Scholar] [CrossRef]
- Pisu, S.; Apa, L.; Cosentino, M.; Musarò, A.; Rizzuto, E.; Del Prete, Z. Measuring the X-MET’s maximum power: A preliminary study. In Proceedings of the MeMeA 2018 IEEE International Symposium on Medical Measurements and Applications, Rome, Italy, 11–13 June 2018. [Google Scholar]
- Alave Reyes-Furrer, A.; De Andrade, S.; Bachmann, D.; Jeker, H.; Steinmann, M.; Accart, N.; Dunbar, A.; Rausch, M.; Bono, E.; Rimann, M.; et al. Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses. Commun. Biol. 2021, 4, 1183. [Google Scholar] [CrossRef]
- Turner, M.C.; Rimington, R.P.; Martin, N.R.W.; Fleming, J.W.; Capel, A.J.; Hodson, L.; Lewis, M.P. Physiological and pathophysiological concentrations of fatty acids induce lipid droplet accumulation and impair functional performance of tissue engineered skeletal muscle. J. Cell. Physiol. 2021, 236, 7033–7044. [Google Scholar] [CrossRef]
- Sasaki, D.; Matsuura, K.; Seta, H.; Haraguchi, Y.; Okano, T.; Shimizu, T. Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS ONE 2018, 13, e0198026. [Google Scholar] [CrossRef]
- Juhas, M.; Bursac, N. Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials 2014, 35, 9438–9446. [Google Scholar] [CrossRef]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Dennis, R.G.; Kosnik, P.E.; Gilbert, M.E.; Faulkner, J.A. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am. J. Physiol.-Cell Physiol. 2001, 280, C288–C295. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, M.; Perlingeiro, R.C.R.; Shen, W. Skeletal Muscle Constructs Engineered from Human Embryonic Stem Cell Derived Myogenic Progenitors Exhibit Enhanced Contractile Forces When Differentiated in a Medium Containing EGM-2 Supplements. Adv. Biosyst. 2019, 3, 1900005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Das, M.; Wahl, K.J.; Colton, R.J.; Hickman, J. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS ONE 2010, 5, e11042. [Google Scholar] [CrossRef]
- Santoso, J.W.; Li, X.; Gupta, D.; Suh, G.C.; Hendricks, E.; Lin, S.; Perry, S.; Ichida, J.K.; Dickman, D.; McCain, M.L. Engineering skeletal muscle tissues with advanced maturity improves synapse formation with human induced pluripotent stem cell-derived motor neurons. APL Bioeng. 2021, 5, 036101. [Google Scholar] [CrossRef]
- Guo, X.; Badu-Mensah, A.; Thomas, M.C.; McAleer, C.W.; Hickman, J.J. Characterization of functional human skeletal myotubes and neuromuscular junction derived-from the same induced pluripotent stem cell source. Bioengineering 2020, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Hadiwidjaja, S.; Ohsumi, S.; Murata, A.; Hisada, T.; Kato, R.; Okada, Y.; Honda, H.; Shimizu, K. In Vitro Model of Human Skeletal Muscle Tissues with Contractility Fabricated by Immortalized Human Myogenic Cells. Adv. Biosyst. 2020, 4, 2000121. [Google Scholar] [CrossRef]
- Rausch, M.; Böhringer, D.; Steinmann, M.; Schubert, D.W.; Schrüfer, S.; Mark, C.; Fabry, B. Measurement of Skeletal Muscle Fiber Contractility with High-Speed Traction Microscopy. Biophys. J. 2020, 118, 657–666. [Google Scholar] [CrossRef]
- Pasqualini, F.S.; Agarwal, A.; O’Connor, B.B.; Liu, Q.; Sheehy, S.P.; Parker, K.K. Traction force microscopy of engineered cardiac tissues. PLoS ONE 2018, 13, e0194706. [Google Scholar] [CrossRef]
- Pasquarelli, A. Microelectrode Arrays, Implants, and Organs-on-a-Chip. Biosens. Biochips 2021, 291–322. [Google Scholar] [CrossRef]
- Sala, L.; Van Meer, B.J.; Tertoolen, L.G.J.; Bakkers, J.; Bellin, M.; Davis, R.P.; Denning, C.; Dieben, M.A.E.; Eschenhagen, T.; Giacomelli, E.; et al. Musclemotion: A versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circ. Res. 2018, 122, e5–e16. [Google Scholar] [CrossRef]
- Bruegmann, T.; Malan, D.; Hesse, M.; Beiert, T.; Fuegemann, C.J.; Fleischmann, B.K.; Sasse, P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 2010, 7, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L. Albumin and mammalian cell culture: Implications for biotechnology applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ham, R.G. Albumin replacement by fatty acids in clonal growth of mammalian cells. Science 1963, 140, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Romero, L.; Garry, P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996, 64, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Moustogiannis, A.; Philippou, A.; Taso, O.; Zevolis, E.; Pappa, M.; Chatzigeorgiou, A.; Koutsilieris, M. The effects of muscle cell aging on myogenesis. Int. J. Mol. Sci. 2021, 22, 3721. [Google Scholar] [CrossRef]
- Dennis, R.G.; Kosnik, P.E. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. Vitr. Cell. Dev. Biol.-Anim. 2000, 36, 327–335. [Google Scholar] [CrossRef]
- Apa, L.; Martelli, F.; Rizzuto, E.; Del Prete, Z. Design of a new device to measure skeletal muscle engineered tissues’ contractile force by using an optical tracking technique. In Proceedings of the Medical Measurements and Applications, MeMeA, İstanbul, Turkey, 26–28 June 2019; pp. 1–5. [Google Scholar]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Forconi, F.; Apa, L.; Marianna, C.; Antonio, M.; Emanuele, R.; Zaccaria, D.P. Effects of ROI positioning on the measurement of engineered muscle tissue contractility with an optical tracking method. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022. [Google Scholar]
- Forconi, F.; Apa, L.; Pisu, S.; Casola, I.; Musarò, A.; Rizzuto, E.; Del Prete, Z. Development of a Novel Technique for the Measurement of Neuromuscular Junction Functionality in Isotonic Conditions. Cell. Mol. Bioeng. 2022, 15, 255–265. [Google Scholar] [CrossRef]
- Del Prete, Z.; Musarò, A.; Rizzuto, E. Measuring mechanical properties, including isotonic fatigue, of fast and slow MLC/mIgf-1 transgenic skeletal muscle. Ann. Biomed. Eng. 2008, 36, 1281–1290. [Google Scholar] [CrossRef]
- Pisu, S.; Rizzuto, E.; Musaro, A.; Del Prete, Z. Identification of the best stimulation parameters to measure in situ the comunication between muscle and nerve in mouse Tibialis muscle. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications, MeMeA, Rochester, MN, USA, 7–10 May 2017. [Google Scholar]
- Horn, B.K.P.; Schunck, B.G. Determining optical flow. Artif. Intell. 1981, 17, 185–203. [Google Scholar] [CrossRef]
- Cheng, C.S.; Davis, B.N.J.; Madden, L.; Bursac, N.; Truskey, G.A. Physiology and metabolism of tissue-engineered skeletal muscle. Exp. Biol. Med. 2014, 239, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Rizzuto, E.; Carosio, S.; Musaro, A.; Del Prete, Z. A Digital Image Correlation based technique to control the development of a skeletal muscle engineered tissue by measuring its surface strain field. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications, MeMeA, Turin, Italy, 7–9 May 2015. [Google Scholar]
- Vesga-Castro, C.; Aldazabal, J.; Vallejo-Illarramendi, A.; Paredes, J. Contractile force assessment methods for in vitro skeletal muscle tissues. Elife 2022, 11, e77204. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.K.; Russ, D.W.; Binder-Macleod, S.A. Force-Frequency Relation of Skeletal Muscle. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Shimizu, K.; Genma, R.; Gotou, Y.; Nagasaka, S.; Honda, H. Three-dimensional culture model of skeletal muscle tissue with atrophy induced by dexamethasone. Bioengineering 2017, 4, 56. [Google Scholar] [CrossRef]
- Vandenburgh, H.; Shansky, J.; Benesch-Lee, F.; Barbata, V.; Reid, J.; Thorrez, L.; Valentini, R.; Crawford, G. Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 2008, 37, 438–447. [Google Scholar] [CrossRef]
- Urciuoli, E.; Peruzzi, B. Involvement of the fak network in pathologies related to altered mechanotransduction. Int. J. Mol. Sci. 2020, 21, 9426. [Google Scholar] [CrossRef] [PubMed]





| TTP (ms) | 1/2RT (ms) | FTW/CSA (mN/mm2) | ||||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | Control | Treated | |
| Day 0 | 40.7 ± 8.9 | 42.5 ± 5.0 | 59.3 ± 18.2 | 57.5 ± 12.6 | 0.7 ± 0.5 | 0.5 ± 0.3 |
| Day 1 | 34.0 ± 8.0 | 32.2 ± 1.9 | 70.0 ± 9.4 | 45.0 ± 16.6 | 1.6 ± 0.7 *** | 0.3 ± 0.3 |
| Day 2 | 47.3 ± 13.2 | 35.0 ± 16.6 | 72.0 ± 16.8 | 70.0 ± 18.8 | 0.8 ± 0.6 | 0.1 ± 0.1 + |
| Day 3 | 41.3 ± 10.7 | 50.0 | 64.7 ± 16.4 | 73.3 | 1.1 ± 0.5 ** | 0.0 ± 0.1 + |
| Day 4 | 37.7 ± 18.2 | 13.3 | 64.0 ± 17.5 | 16.7 | 1.3 ± 0.5 ** | 0.1 ± 0.1 + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apa, L.; Cosentino, M.; Forconi, F.; Musarò, A.; Rizzuto, E.; Del Prete, Z. The Development of an Innovative Embedded Sensor for the Optical Measurement of Ex-Vivo Engineered Muscle Tissue Contractility. Sensors 2022, 22, 6878. https://doi.org/10.3390/s22186878
Apa L, Cosentino M, Forconi F, Musarò A, Rizzuto E, Del Prete Z. The Development of an Innovative Embedded Sensor for the Optical Measurement of Ex-Vivo Engineered Muscle Tissue Contractility. Sensors. 2022; 22(18):6878. https://doi.org/10.3390/s22186878
Chicago/Turabian StyleApa, Ludovica, Marianna Cosentino, Flavia Forconi, Antonio Musarò, Emanuele Rizzuto, and Zaccaria Del Prete. 2022. "The Development of an Innovative Embedded Sensor for the Optical Measurement of Ex-Vivo Engineered Muscle Tissue Contractility" Sensors 22, no. 18: 6878. https://doi.org/10.3390/s22186878
APA StyleApa, L., Cosentino, M., Forconi, F., Musarò, A., Rizzuto, E., & Del Prete, Z. (2022). The Development of an Innovative Embedded Sensor for the Optical Measurement of Ex-Vivo Engineered Muscle Tissue Contractility. Sensors, 22(18), 6878. https://doi.org/10.3390/s22186878









