Less Vibrotactile Feedback Is Effective to Improve Human Balance Control during Sensory Cues Alteration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Set-Up: Vibrotactile Feedback Device
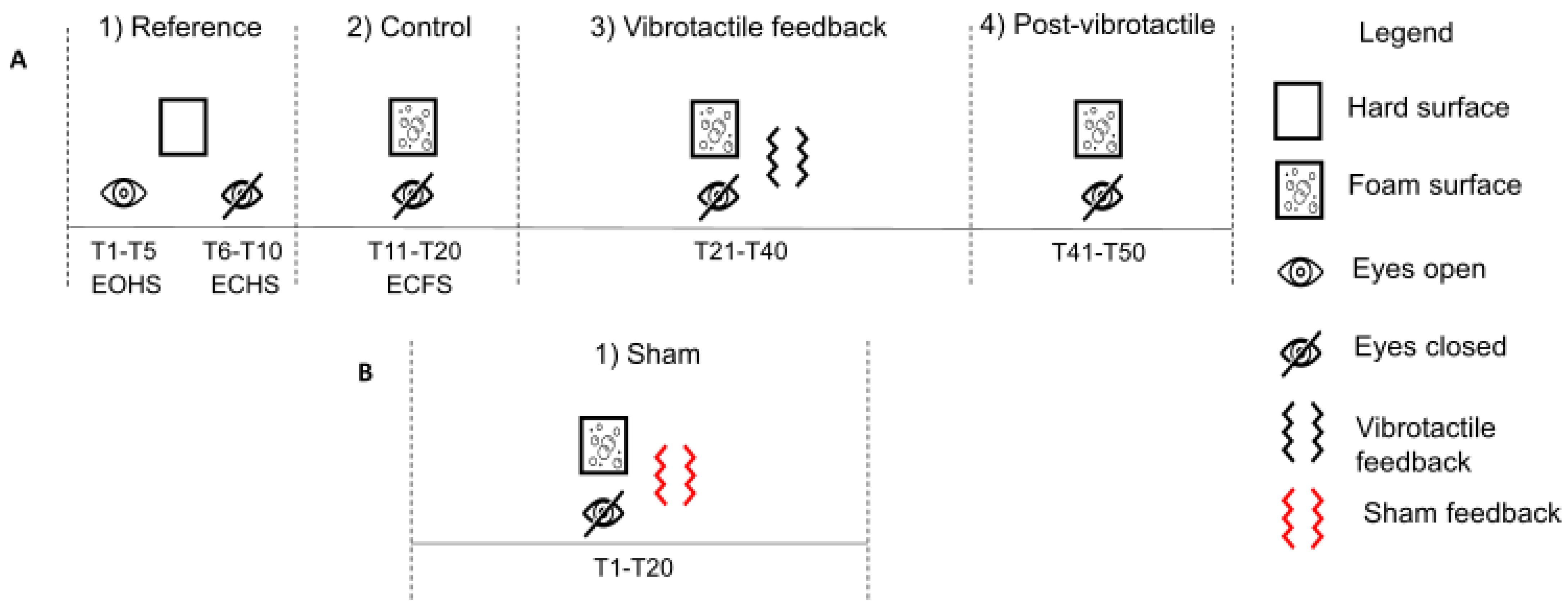
2.3. Protocol and Experimental Conditions
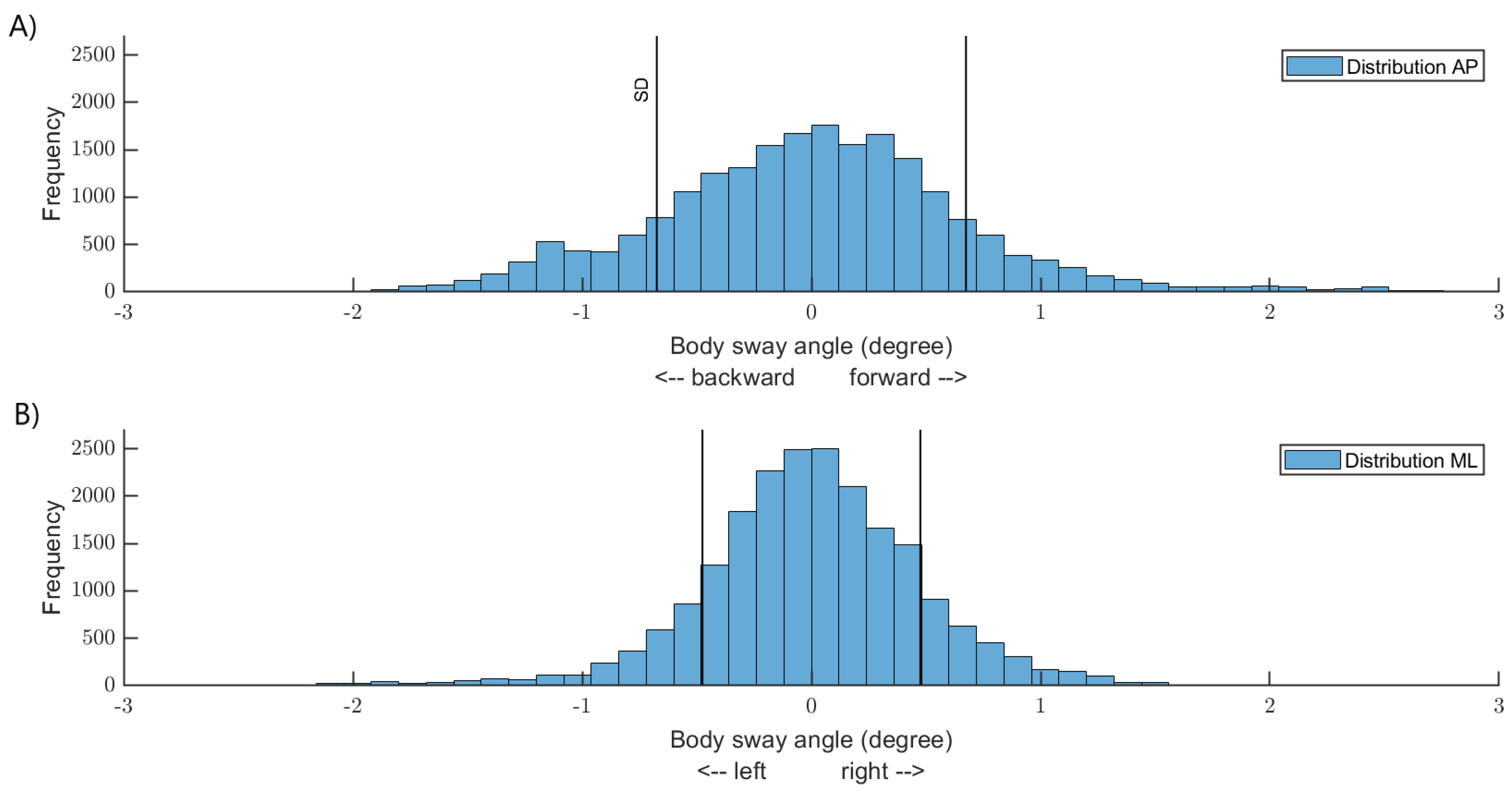
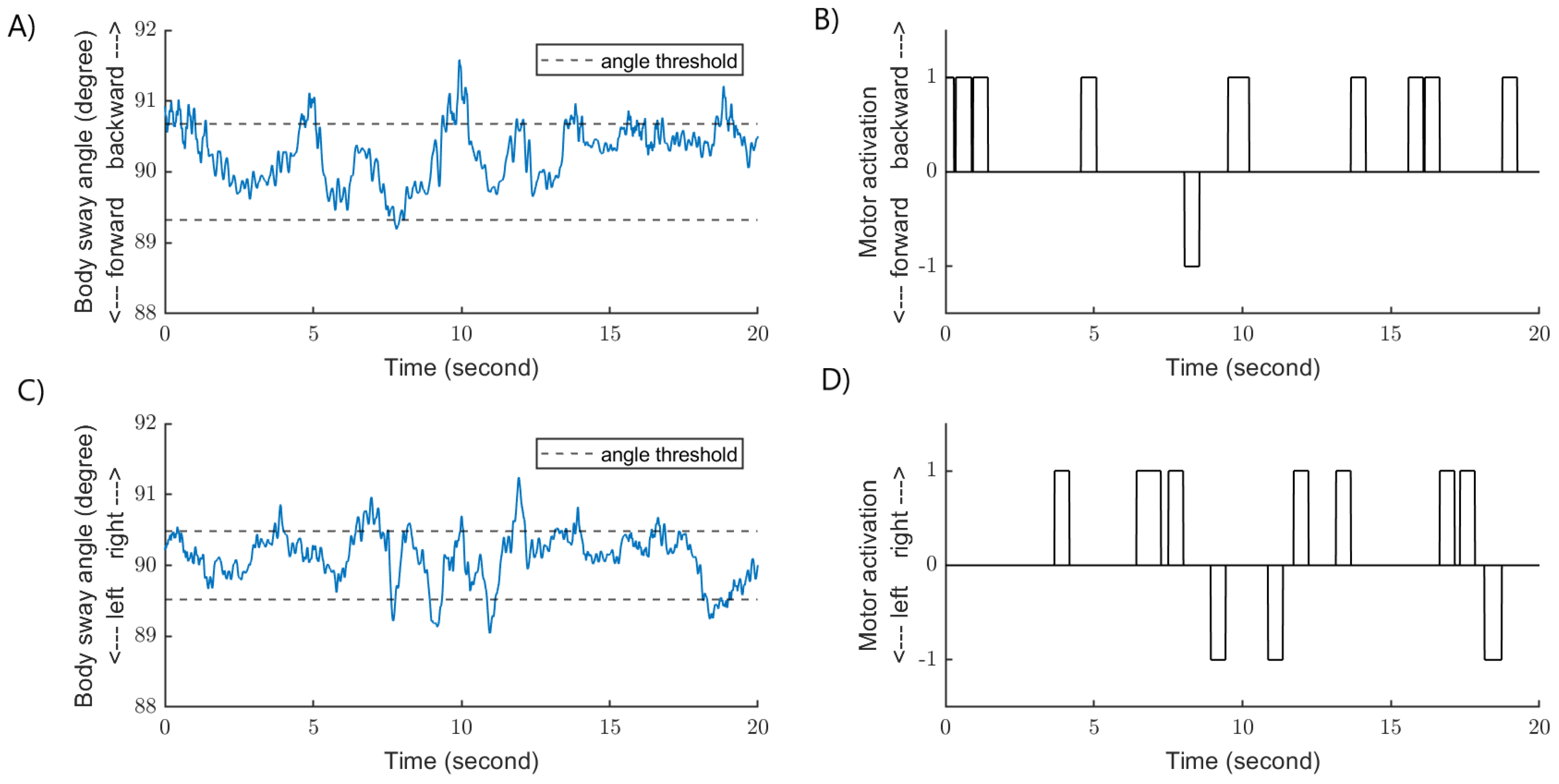
2.4. Data and Statistical Analysis
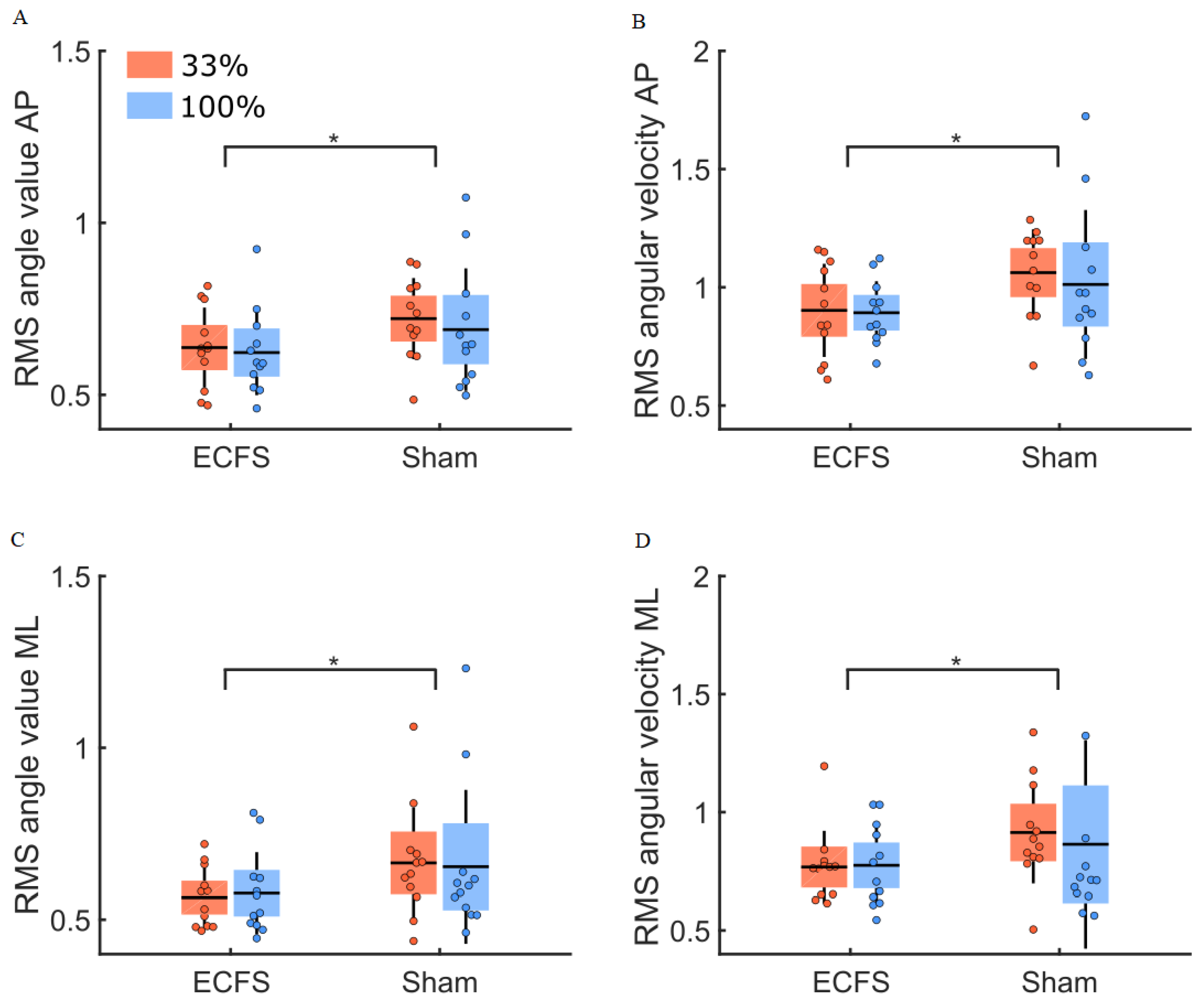
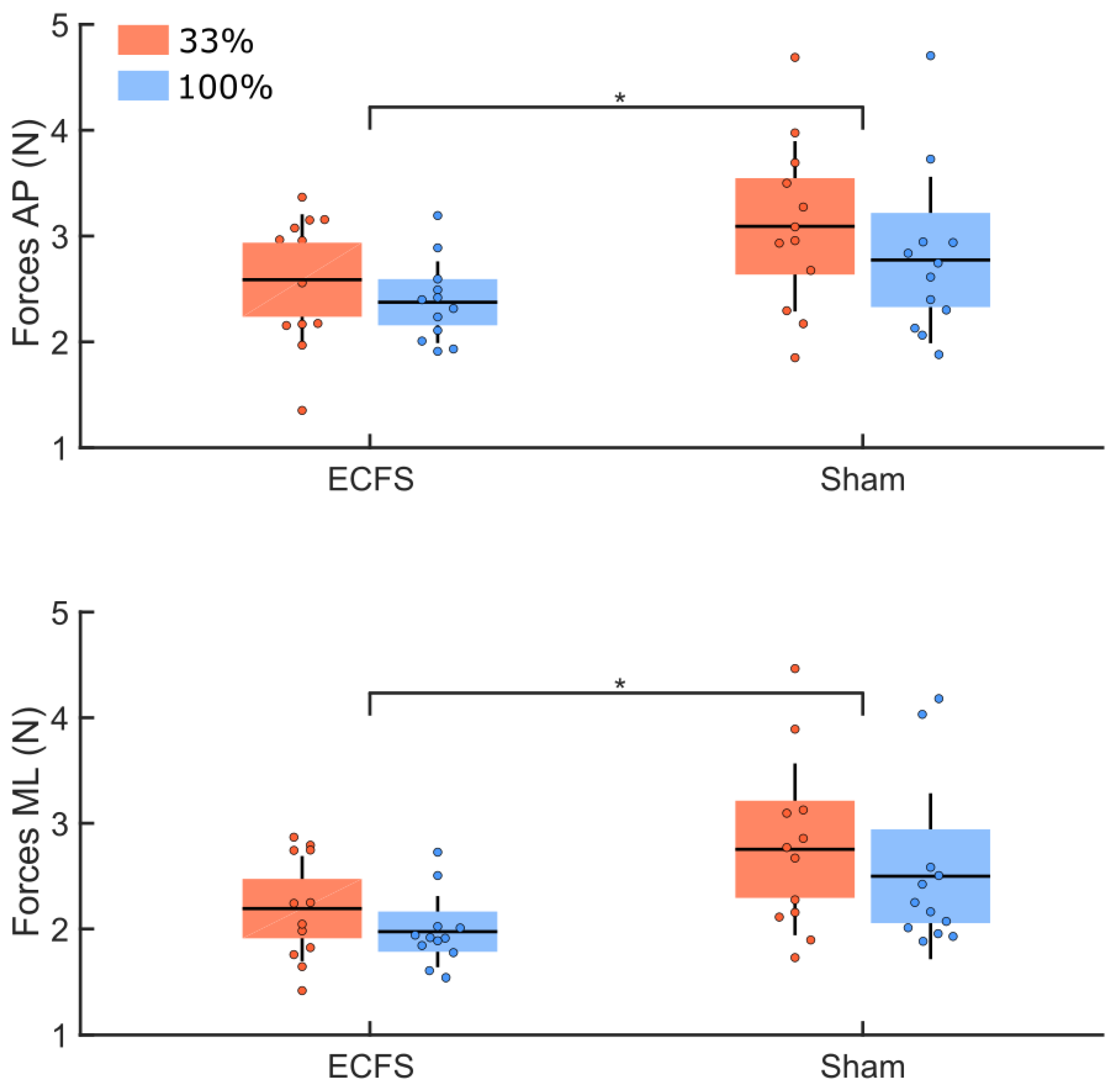
3. Results
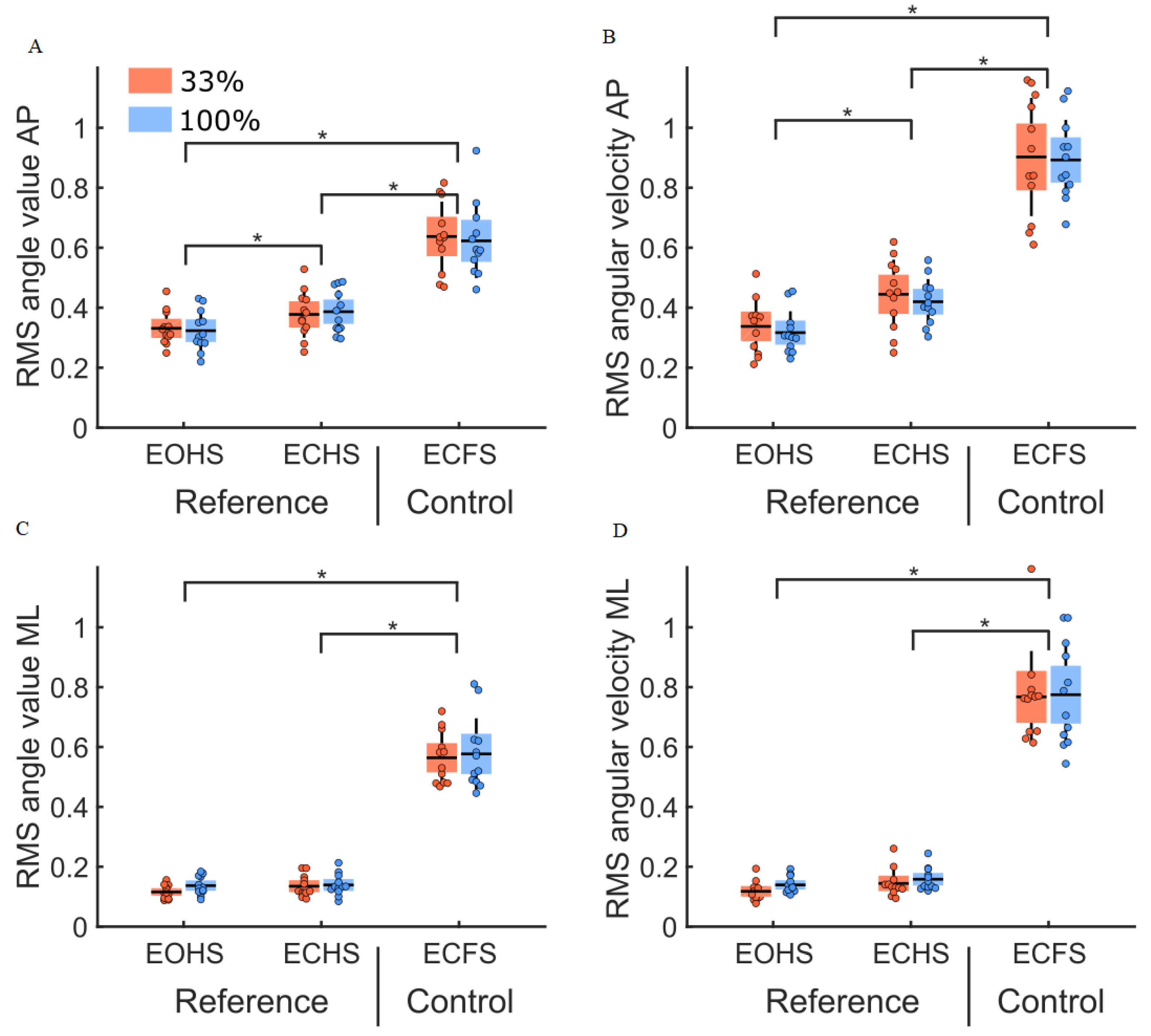
3.1. Initial Conditions (Reference and Control Conditions)
3.2. Vibrotactile Thresholds
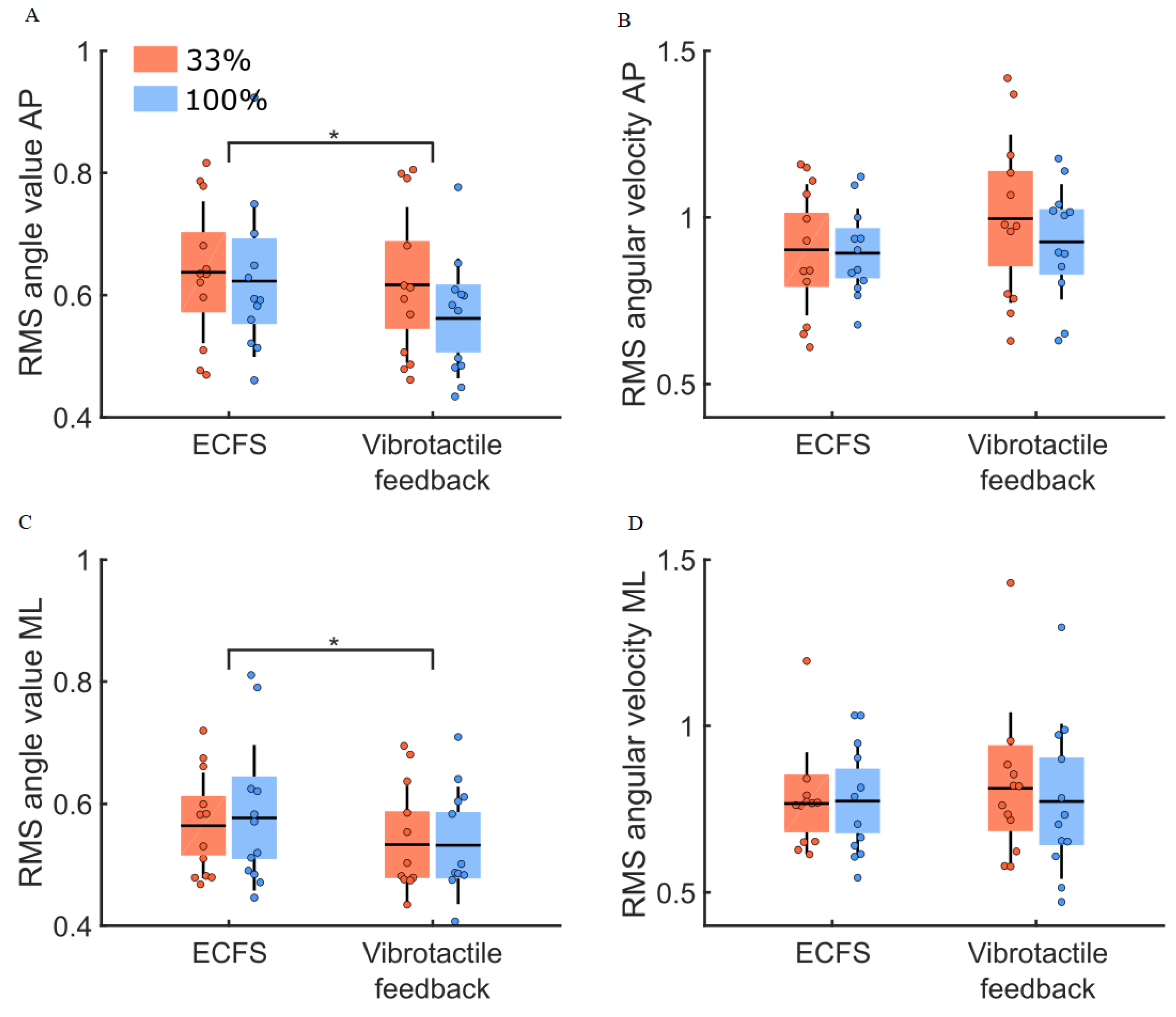
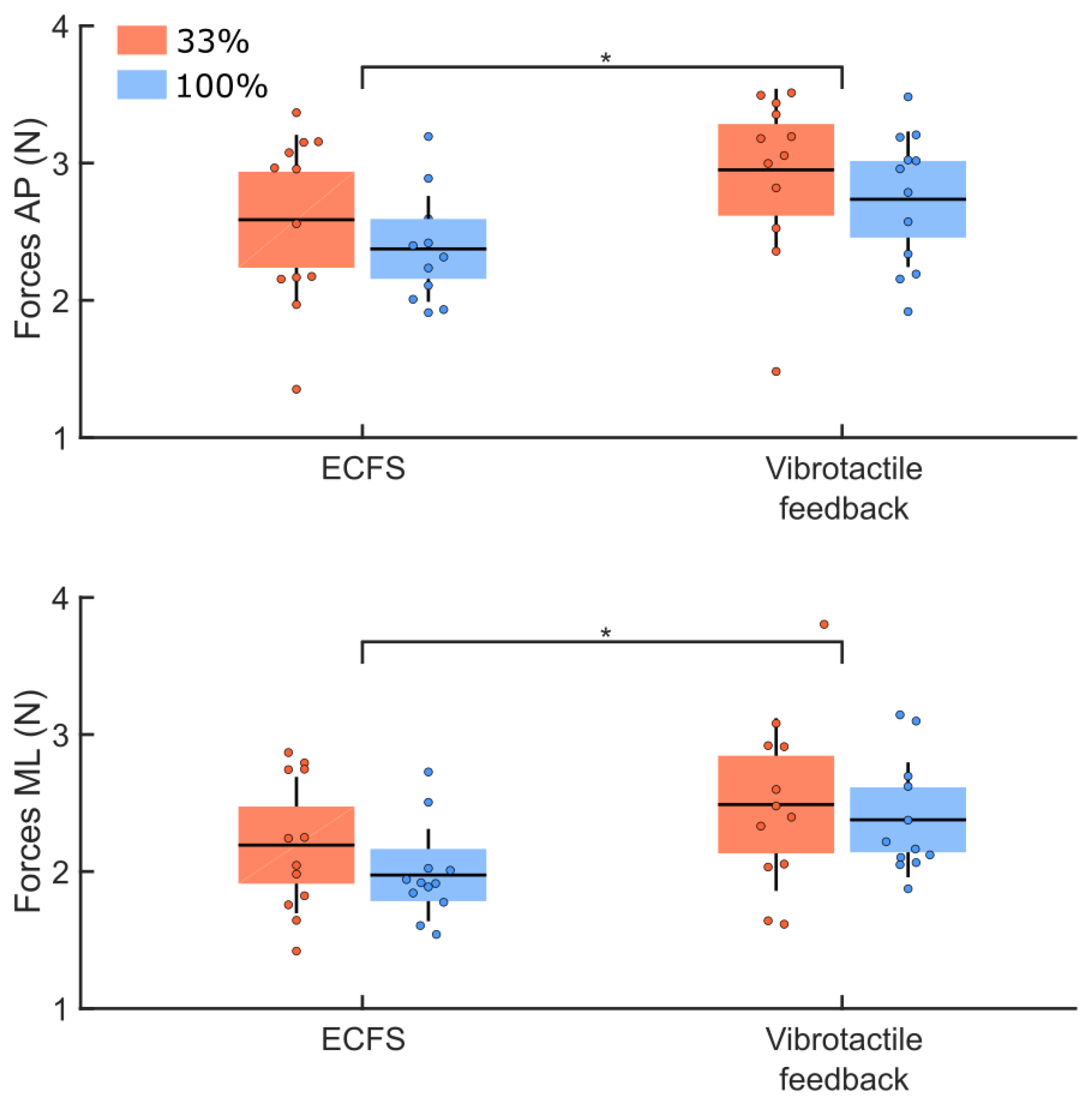
3.3. Vibrotactile Feedback Condition
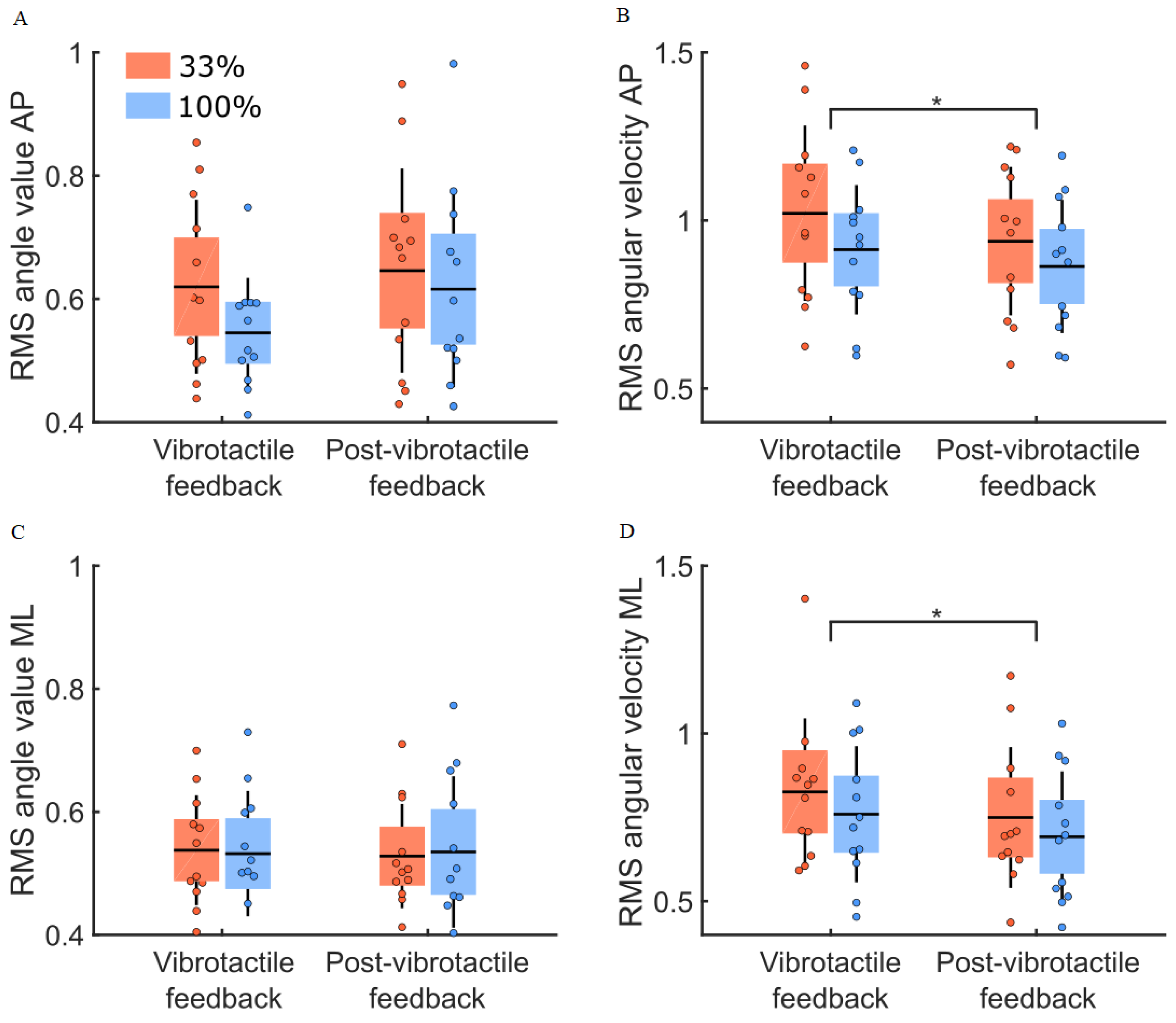
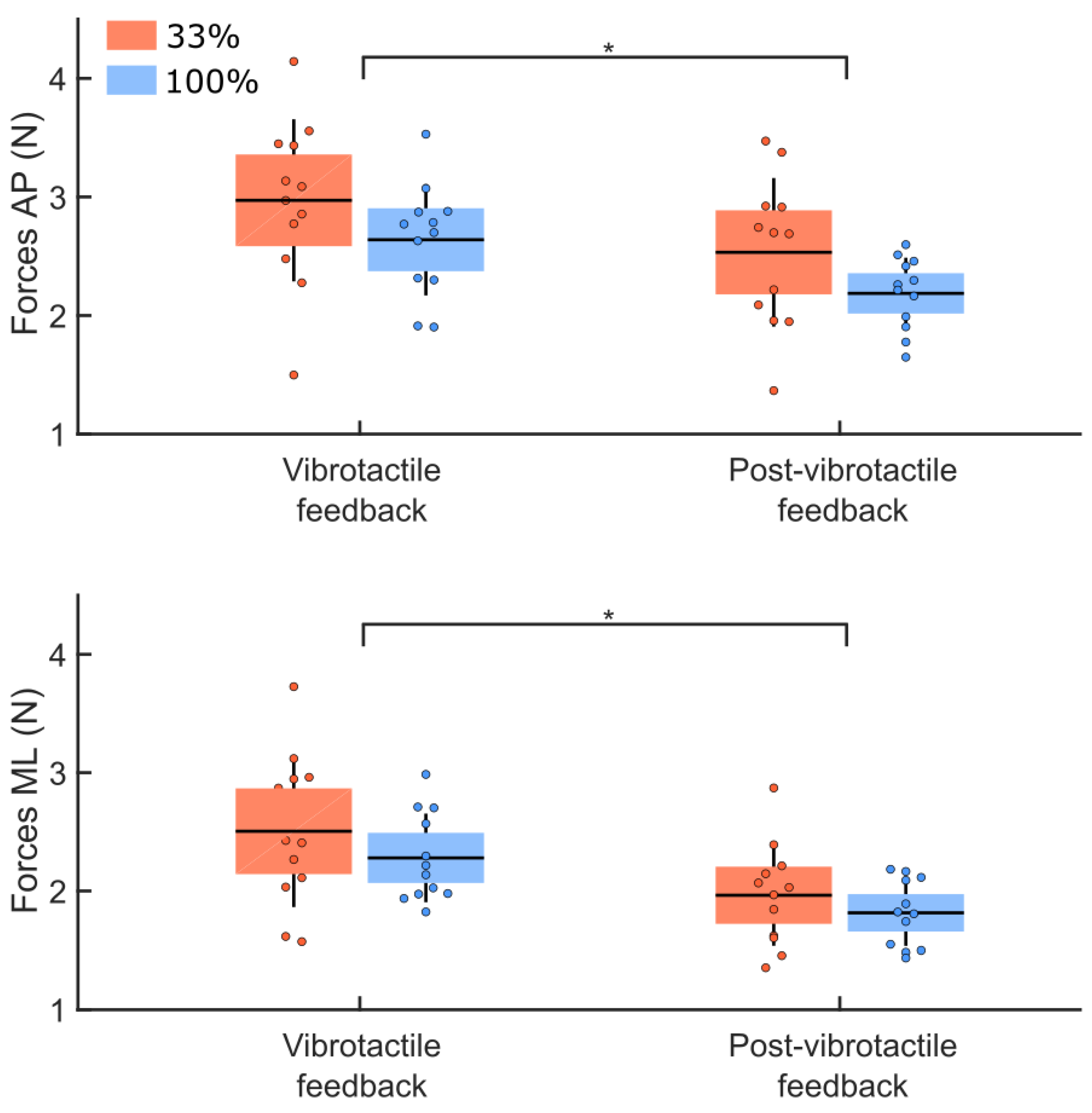
3.4. Post Vibrotactile Condition
3.5. Sham Condition
3.6. Ellipse Data
4. Discussion
4.1. Control Condition
4.2. Quantity of Vibrotactile Feedback
4.3. Post-Vibrotactile Feedback
4.4. Sham Vibrotactile Feedback
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 100% Feedback | 33% Feedback | ||
|---|---|---|---|
| Reference condition—EOHS (initial conditions) | Angle (°) | 0.50 ± 0.17 | 0.44 ± 0.12 |
| Angular velocity (°/s) | 0.53 ± 0.21 | 0.48 ± 0.20 | |
| Forces (N) | 3.06 ± 1.13 | 3.48 ± 1.18 | |
| Reference condition—ECHS (initial conditions) | Angle (°) | 0.62 ± 0.23 | 0.58 ± 0.17 |
| Angular velocity (°/s) | 0.82 ± 0.33 | 0.78 ± 0.32 | |
| Forces (N) | 5.28 ± 2.52 | 7.04 ± 3.18 | |
| Control condition—ECFS (initial conditions) | Angle (°) | 4.29 ± 1.67 | 4.26 ± 1.31 |
| Angular velocity (°/s) | 8.45 ± 2.81 | 8.46 ± 3.29 | |
| Forces (N) | 57.22 ± 20.00 | 71.57 ± 30.28 | |
| Vibrotactile feedback condition | Angle (°) | 3.58 ± 1.11 | 3.96 ± 1.35 |
| Angular velocity (°/s) | 8.85 ± 3.95 | 10.10 ± 4.59 | |
| Forces (N) | 79.36 ± 26.16 | 91.45 ± 35.61 | |
| Post-vibrotactile condition | Angle (°) | 4.00 ± 1.98 | 4.04 ± 1.40 |
| Angular velocity (°/s) | 7.55 ± 3.75 | 8.86 ± 4.05 | |
| Forces (N) | 48.01 ± 12.01 | 62.03 ± 26.89 | |
| Sham condition | Angle (°) | 5.76 ± 3.82 | 5.88 ± 2.13 |
| Angular velocity (°/s) | 12.34 ± 12.29 | 12.01 ± 3.56 | |
| Forces (N) | 89.78 ± 59.01 | 109.59 ± 61.38 |
| Comparisons | Measures | Group | Condition | Interaction |
|---|---|---|---|---|
| EOHS vs. EOFS vs. ECFS (initial conditions) | Angle | 0.85 | <0.001 * | 1.00 |
| Angular velocity | 0.95 | <0.001 * | 1.00 | |
| Forces | 0.16 | <0.001 * | 0.19 | |
| Vibrotactile feedback vs. control conditions | Angle | 0.75 | 0.004 * | 0.20 |
| Angular velocity | 0.67 | 0.03 * | 0.18 | |
| Forces | 0.23 | <0.001 * | 0.81 | |
| Post-vibrotactile vs. vibrotactile conditions | Angle | 0.65 | 0.15 | 0.21 |
| Angular velocity | 0.34 | 0.001 * | 0.44 | |
| Forces | 0.11 | <0.001 * | 0.51 | |
| Sham vs. control conditions | Angle | 0.95 | 0.046 * | 0.92 |
| Angular velocity | 0.94 | 0.047 * | 0.92 | |
| Forces | 0.28 | 0.004 * | 0.81 |
References
- Fitzpatrick, R.; McCloskey, D.I. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J. Physiol. 1994, 478, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, G.G.; Ulbrecht, J.S.; Derr, J.A.; Cavanaugh, P.R. Role of somatosensory input in the control of human posture. Gait Posture 1995, 3, 115–122. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Melzer, I.; Benjuya, N.; Kaplanski, J. Postural stability in the elderly: A comparison between fallers and non-fallers. Age Ageing 2004, 33, 602–607. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef]
- Couture, E.; Simoneau, M. Estimate of body motion during voluntary body sway movements. Gait Posture 2014, 39, 70–74. [Google Scholar] [CrossRef]
- Xu, I.; Laurendeau, S.; Teasdale, N.; Simoneau, M. Change in the natural head-neck orientation momentarily altered sensorimotor control during sensory transition. Gait Posture 2017, 53, 80–85. [Google Scholar] [CrossRef]
- Cyr, J.P.; Anctil, N.; Simoneau, M. Balance control mechanisms do not benefit from successive stimulation of different sensory systems. PLoS ONE 2019, 14, e0226216. [Google Scholar] [CrossRef]
- Dozza, M.; Chiari, L.; Hlavacka, F.; Cappello, A.; Horak, F.B. Effects of linear versus sigmoid coding of visual or audio biofeedback for the control of upright stance. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 505–512. [Google Scholar] [CrossRef]
- Sienko, K.H.; Seidler, R.D.; Carender, W.J.; Goodworth, A.D.; Whitney, S.L.; Peterka, R.J. Potential Mechanisms of Sensory Augmentation Systems on Human Balance Control. Front. Neurol. 2018, 9, 944. [Google Scholar] [CrossRef] [Green Version]
- Wall, C., 3rd; Weinberg, M.S.; Schmidt, P.B.; Krebs, D.E. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans. Biomed. Eng. 2001, 48, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Chiari, L.; Dozza, M.; Cappello, A.; Horak, F.B.; Macellari, V.; Giansanti, D. Audio-biofeedback for balance improvement: An accelerometry-based system. IEEE Trans. Biomed. Eng. 2005, 52, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Dozza, M.; Chiari, L.; Chan, B.; Rocchi, L.; Horak, F.B.; Cappello, A. Influence of a portable audio-biofeedback device on structural properties of postural sway. J. Neuroeng. Rehabil. 2005, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D.; Dozza, M.; Chiari, L.; Maccioni, G.; Cappello, A. Energetic assessment of trunk postural modifications induced by a wearable audio-biofeedback system. Med. Eng. Phys. 2009, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Halická, Z.; Lobotková, J.; Bučková, K.; Hlavačka, F. Effectiveness of different visual biofeedback signals for human balance improvement. Gait Posture 2014, 39, 410–414. [Google Scholar] [CrossRef]
- Ballardini, G.; Florio, V.; Canessa, A.; Carlini, G.; Morasso, P.; Casadio, M. Vibrotactile Feedback for Improving Standing Balance. Front. Bioeng. Biotechnol. 2020, 8, 94. [Google Scholar] [CrossRef]
- Haggerty, S.; Jiang, L.T.; Galecki, A.; Sienko, K.H. Effects of biofeedback on secondary-task response time and postural stability in older adults. Gait Posture 2012, 35, 523–528. [Google Scholar] [CrossRef]
- Kentala, E.; Vivas, J.; Wall, C., 3rd. Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann. Otol. Rhinol. Laryngol. 2003, 112, 404–409. [Google Scholar] [CrossRef]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.I.; Wall, C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J. Vestib. Res. 2008, 18, 273–285. [Google Scholar] [CrossRef]
- Bechly, K.E.; Carender, W.J.; Myles, J.D.; Sienko, K.H. Determining the preferred modality for real-time biofeedback during balance training. Gait Posture 2013, 37, 391–396. [Google Scholar] [CrossRef]
- Goebel, J.A.; Sinks, B.C.; Parker, B.E.; Richardson, N.T.; Olowin, A.B.; Cholewiak, R.W. Effectiveness of head-mounted vibrotactile stimulation in subjects with bilateral vestibular loss: A phase 1 clinical trial. Otol. Neurotol. 2009, 30, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Dozza, M.; Horak, F.B.; Chiari, L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 2007, 178, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Sienko, K.H.; Balkwill, M.D.; Wall, C., 3rd. Biofeedback improves postural control recovery from multi-axis discrete perturbations. J. Neuroeng. Rehabil. 2012, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kingma, H.; Felipe, L.; Gerards, M.C.; Gerits, P.; Guinand, N.; Perez-Fornos, A.; Demkin, V.; van de Berg, R. Vibrotactile feedback improves balance and mobility in patients with severe bilateral vestibular loss. J. Neurol. 2019, 266, 19–26. [Google Scholar] [CrossRef]
- Gill, J.; Allum, J.H.; Carpenter, M.G.; Held-Ziolkowska, M.; Adkin, A.L.; Honegger, F.; Pierchala, K. Trunk sway measures of postural stability during clinical balance tests: Effects of age. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M438–M447. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.; Adkin, A.L.; Carpenter, M.G.; Held-Ziolkowska, M.; Honegger, F.; Pierchala, K. Trunk sway measures of postural stability during clinical balance tests: Effects of a unilateral vestibular deficit. Gait Posture 2001, 14, 227–237. [Google Scholar] [CrossRef]
- Van Erp, J.B. Presenting directions with a vibrotactile torso display. Ergonomics 2005, 48, 302–313. [Google Scholar] [CrossRef]
- Garcia-Valle, G.; Arranz-Paraiso, S.; Serrano-Pedraza, I.; Ferre, M. Estimation of Torso Vibrotactile Thresholds Using Eccentric Rotating Mass Motors. IEEE Trans. Haptics 2021, 14, 538–550. [Google Scholar] [CrossRef]
- Scalera, L.; Seriani, S.; Gallina, P.; Di Luca, M.; Gasparetto, A. An experimental setup to test dual-joystick directional responses to vibrotactile stimuli. IEEE Trans. Haptics 2018, 11, 378–387. [Google Scholar] [CrossRef]
- van der Linden, J.; Schoonderwaldt, E.; Bird, J.; Johnson, R. MusicJacket—Combining Motion Capture and Vibrotactile Feedback to Teach Violin Bowing. IEEE Trans. Inst. Meas. 2011, 60, 104–113. [Google Scholar] [CrossRef]
- Held, J.P.; Klaassen, B.; van Beijnum, B.F.; Luft, A.R.; Veltink, P.H. Usability Evaluation of a VibroTactile Feedback System in Stroke Subjects. Front. Bioeng. Biotechnol. 2016, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Yunus, R.; Ali, S.; Ayaz, Y.; Khan, M.; Kanwal, S.; Akhlaque, U.; Nawaz, R. Development and Testing of a Wearable Vibrotactile Haptic Feedback System for Proprioceptive Rehabilitation. IEEE Access 2020, 8, 35172–35184. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Teasdale, N.; Simoneau, M. Attentional demands for postural control: The effects of aging and sensory reintegration. Gait Posture 2001, 14, 203–210. [Google Scholar] [CrossRef]
- Teasdale, N.; Bard, C.; LaRue, J.; Fleury, M. On the cognitive penetrability of posture control. Exp. Aging Res. 1993, 19, 1–13. [Google Scholar] [CrossRef]
- Lin, C.C.; Whitney, S.L.; Loughlin, P.J.; Furman, J.M.; Redfern, M.S.; Sienko, K.H.; Sparto, P.J. The effect of age on postural and cognitive task performance while using vibrotactile feedback. J. Neurophysiol. 2015, 113, 2127–2136. [Google Scholar] [CrossRef]
- Kinnaird, C.; Lee, J.; Carender, W.J.; Kabeto, M.; Martin, B.; Sienko, K.H. The effects of attractive vs. repulsive instructional cuing on balance performance. J. Neuroeng. Rehabil. 2016, 13, 29. [Google Scholar] [CrossRef]
- Kuo, A.D. An optimal control model for analyzing human postural balance. IEEE Trans. Biomed. Eng. 1995, 42, 87–101. [Google Scholar] [CrossRef]
- Wall, C.; Kentala, E. Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J. Vestib. Res. 2005, 15, 313–325. [Google Scholar] [CrossRef]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Salmoni, A.W.; Schmidt, R.A.; Walter, C.B. Knowledge of results and motor learning: A review and critical reappraisal. Psychol. Bull. 1984, 95, 355–386. [Google Scholar] [CrossRef]
- Bao, T.; Carender, W.J.; Kinnaird, C.; Barone, V.J.; Peethambaran, G.; Whitney, S.L.; Kabeto, M.; Seidler, R.D.; Sienko, K.H. Effects of long-term balance training with vibrotactile sensory augmentation among community-dwelling healthy older adults: A randomized preliminary study. J. Neuroeng. Rehabil. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.A.; Wulf, G. Continuous concurrent feedback degrades skill learning: Implications for training and simulation. Hum. Factors 1997, 39, 509–525. [Google Scholar] [CrossRef]
- Simoneau, M.; Teasdale, N.; Bourdin, C.; Bard, C.; Fleury, M.; Nougier, V. Aging and postural control: Postural perturbations caused by changing the visual anchor. J. Am. Geriatr. Soc. 1999, 47, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.; Honegger, F. Vibro-tactile and auditory balance biofeedback changes muscle activity patterns: Possible implications for vestibular implants. J. Vestib. Res. 2017, 27, 77–87. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J. Gerontol. Biol. A Sci. Med. Sci 2003, 58, M446–M452. [Google Scholar] [CrossRef]
- Janssen, L.J.; Verhoeff, L.L.; Horlings, C.G.; Allum, J.H. Directional effects of biofeedback on trunk sway during gait tasks in healthy young subjects. Gait Posture 2009, 29, 575–581. [Google Scholar] [CrossRef]
- Lin, C.C.; Whitney, S.L.; Loughlin, P.J.; Furman, J.M.; Redfern, M.S.; Sienko, K.H.; Sparto, P.J. The Use of Vibrotactile Feedback During Dual-Task Standing Balance Conditions in People With Unilateral Vestibular Hypofunction. Otol. Neurotol. 2018, 39, e349–e356. [Google Scholar] [CrossRef]


| Group | 100% Feedback | 33% Feedback | p |
|---|---|---|---|
| Men:Women | 6:6 | 6:6 | |
| Age (yrs) | 23.8 ± 2.5 | 24.6 ± 2.2 | 0.69 |
| Weight (kg) | 65.3 ± 12.0 | 74.3 ± 14.2 | 0.60 |
| Height (m) | 168.2 ± 7.7 | 171.7 ± 10.4 | 0.32 |
| Score IPAQ (High:Moderate:Low) | 9:1:2 | 9:2:1 |
| 100% Feedback | 33% Feedback | |
|---|---|---|
| AP axis (°) | 0.65 ± 0.13 | 0.67 ± 0.13 |
| ML axis (°) | 0.60 ± 0.12 | 0.58 ± 0.10 |
| Group | 100% Feedback | 33% Feedback | t-Value | p |
|---|---|---|---|---|
| Number of tactile vibrations | 22.74 ± 3.86 | 9.10 ± 0.93 | t(22) = 11.91 | <0.001 |
| Duration of tactile vibration (s) | 13.68 ± 1.65 | 5.80 ± 0.71 | t(22) = 15.24 | <0.001 |
| Group | 100% Feedback | 33% Feedback | t-Value | p |
|---|---|---|---|---|
| Number of vibrations | 18.97 ± 2.97 | 9.21 ± 0.79 | t(22) = 10.97 | <0.001 |
| Duration of vibration (s) | 15.38 ± 2.21 | 7.67 ± 1.12 | t(22) = 10.78 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anctil, N.; Malenfant, Z.; Cyr, J.-P.; Turcot, K.; Simoneau, M. Less Vibrotactile Feedback Is Effective to Improve Human Balance Control during Sensory Cues Alteration. Sensors 2022, 22, 6432. https://doi.org/10.3390/s22176432
Anctil N, Malenfant Z, Cyr J-P, Turcot K, Simoneau M. Less Vibrotactile Feedback Is Effective to Improve Human Balance Control during Sensory Cues Alteration. Sensors. 2022; 22(17):6432. https://doi.org/10.3390/s22176432
Chicago/Turabian StyleAnctil, Noémie, Zachary Malenfant, Jean-Philippe Cyr, Katia Turcot, and Martin Simoneau. 2022. "Less Vibrotactile Feedback Is Effective to Improve Human Balance Control during Sensory Cues Alteration" Sensors 22, no. 17: 6432. https://doi.org/10.3390/s22176432
APA StyleAnctil, N., Malenfant, Z., Cyr, J.-P., Turcot, K., & Simoneau, M. (2022). Less Vibrotactile Feedback Is Effective to Improve Human Balance Control during Sensory Cues Alteration. Sensors, 22(17), 6432. https://doi.org/10.3390/s22176432






