A Signal Processing Method for Assessing Ankle Torque with a Custom-Made Electronic Dynamometer in Participants Affected by Diabetic Peripheral Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
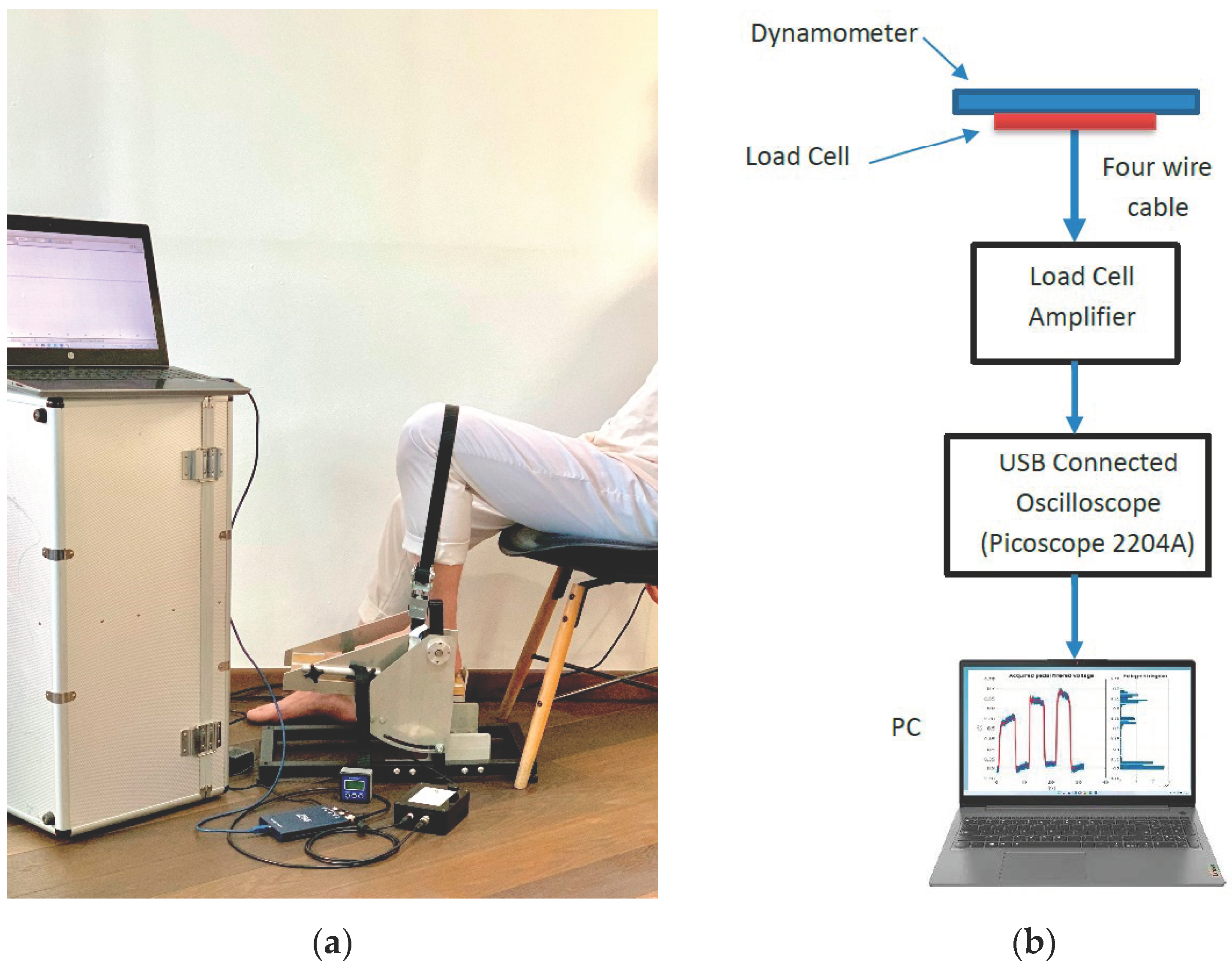
2.1. System Hardware Components
2.2. System Software Components
2.2.1. Data Acquisition Software
2.2.2. Data Processing Software
2.3. Data Processing
2.3.1. Acquired Data Format
2.3.2. Data Collection and the Index File
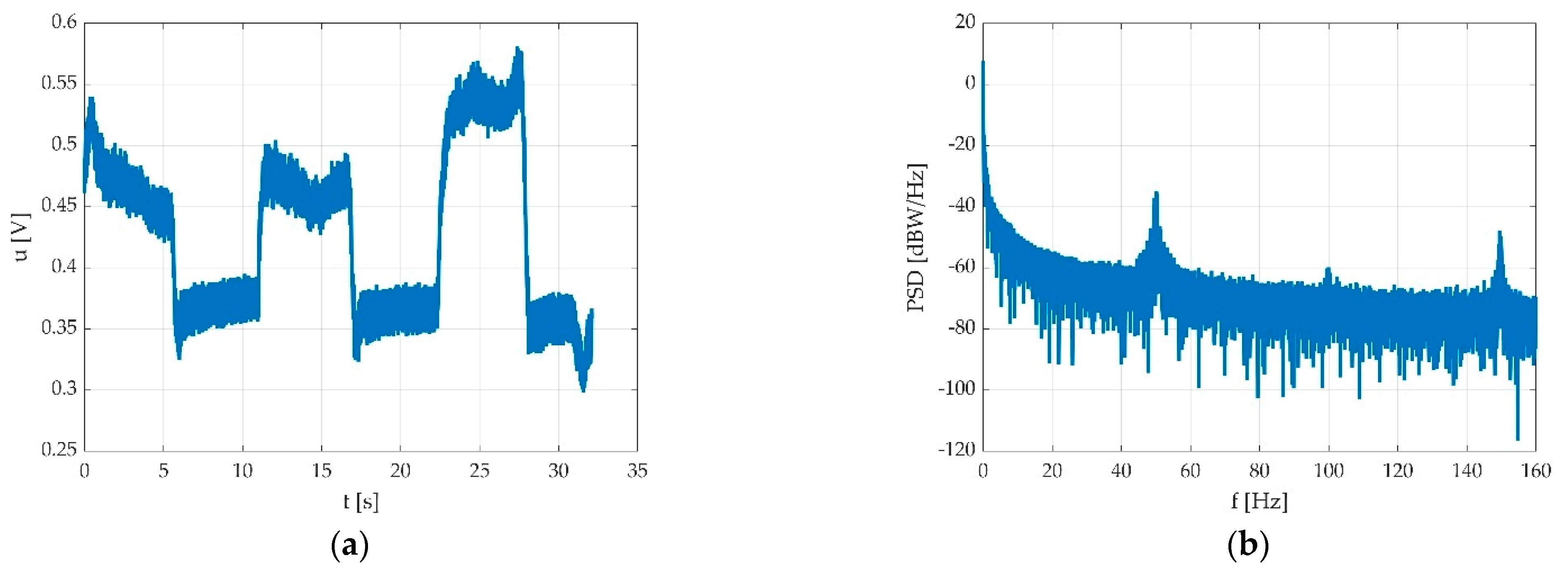
2.3.3. The Pedal Signal Analysis
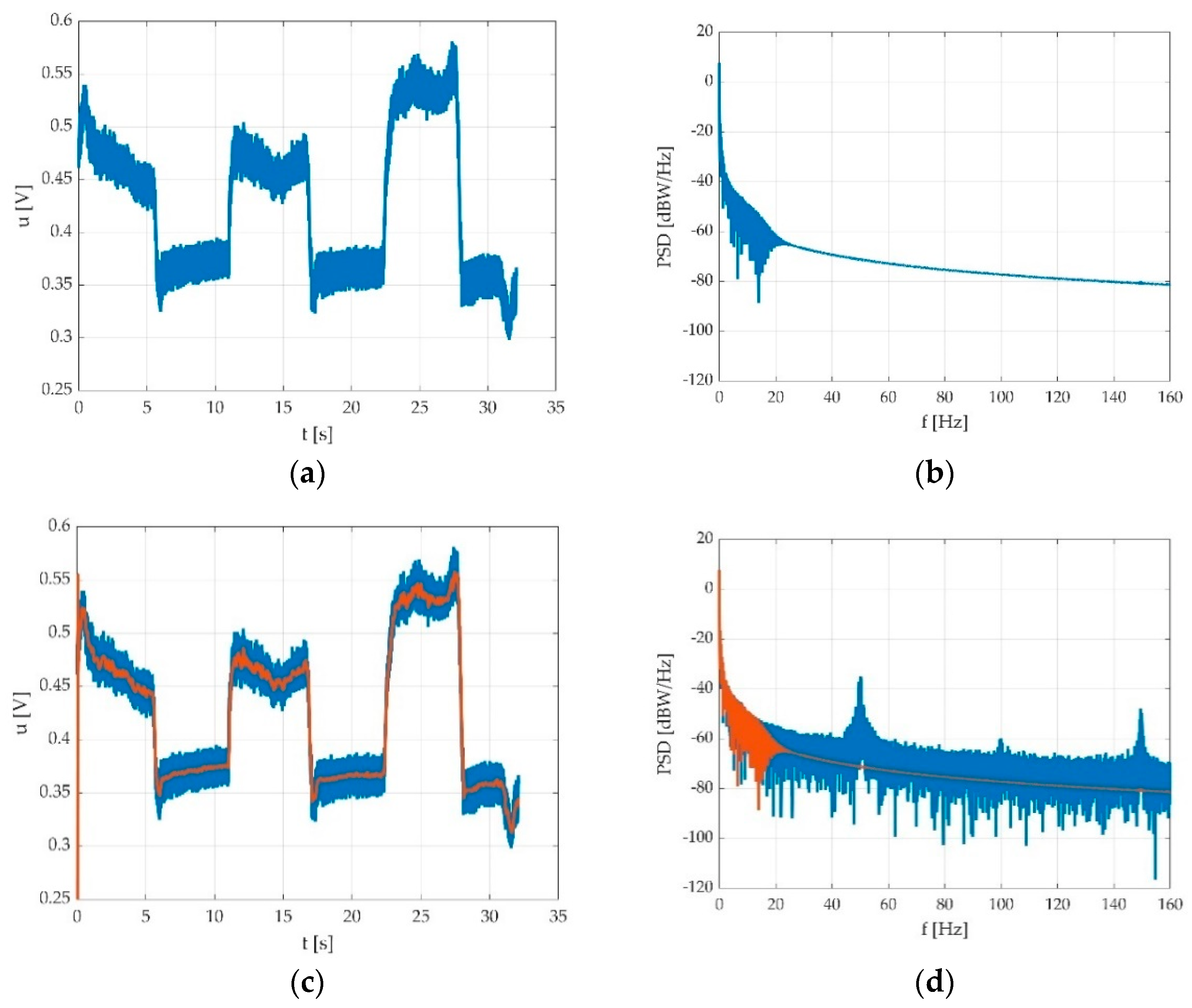
2.3.4. The Low Pass Filtering
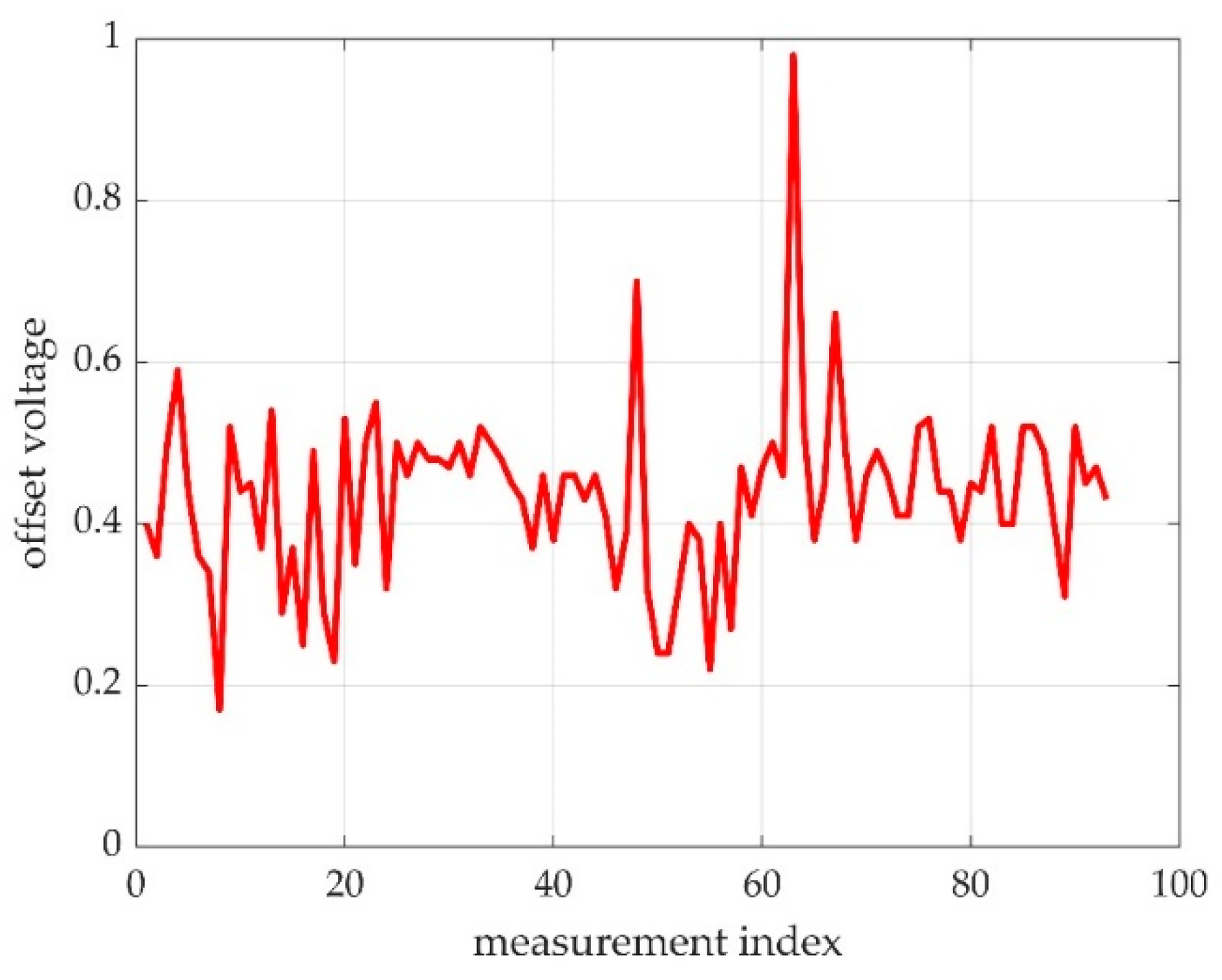
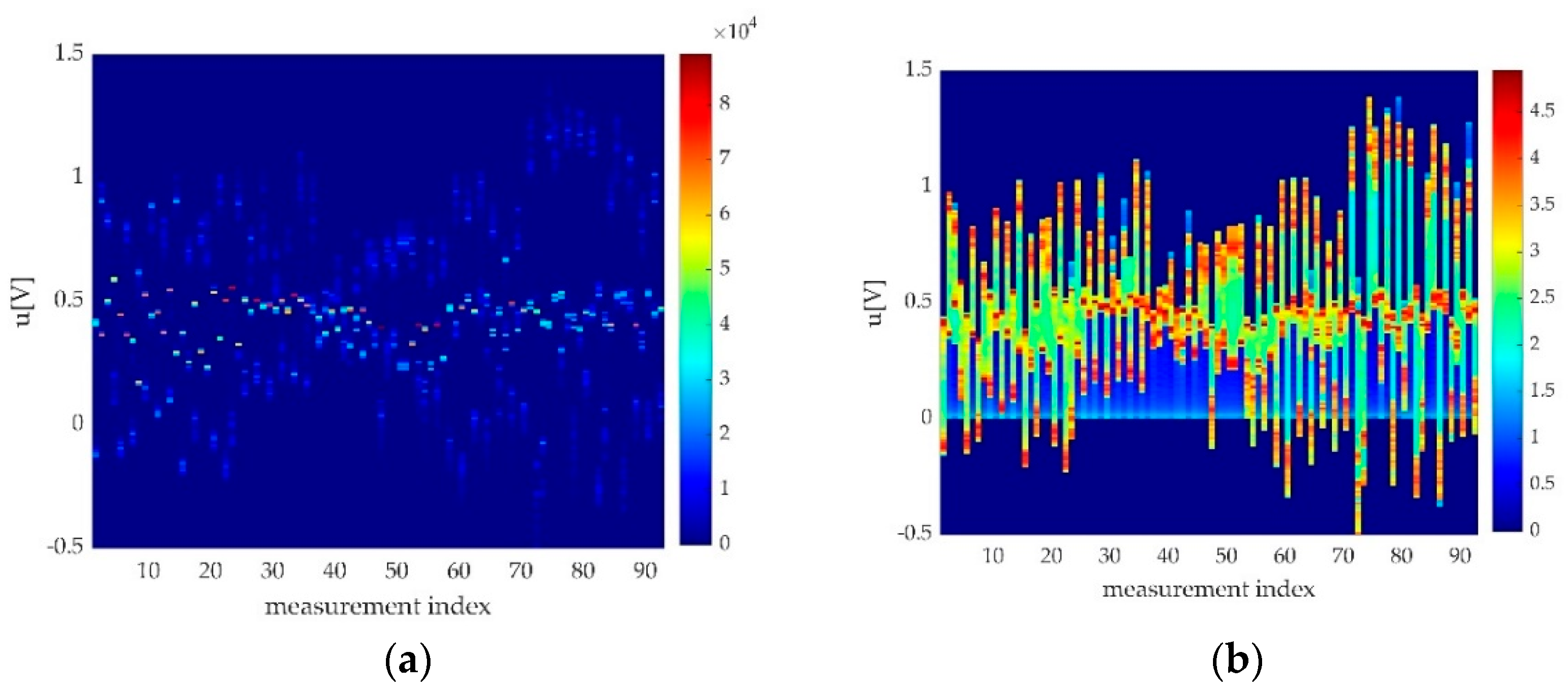
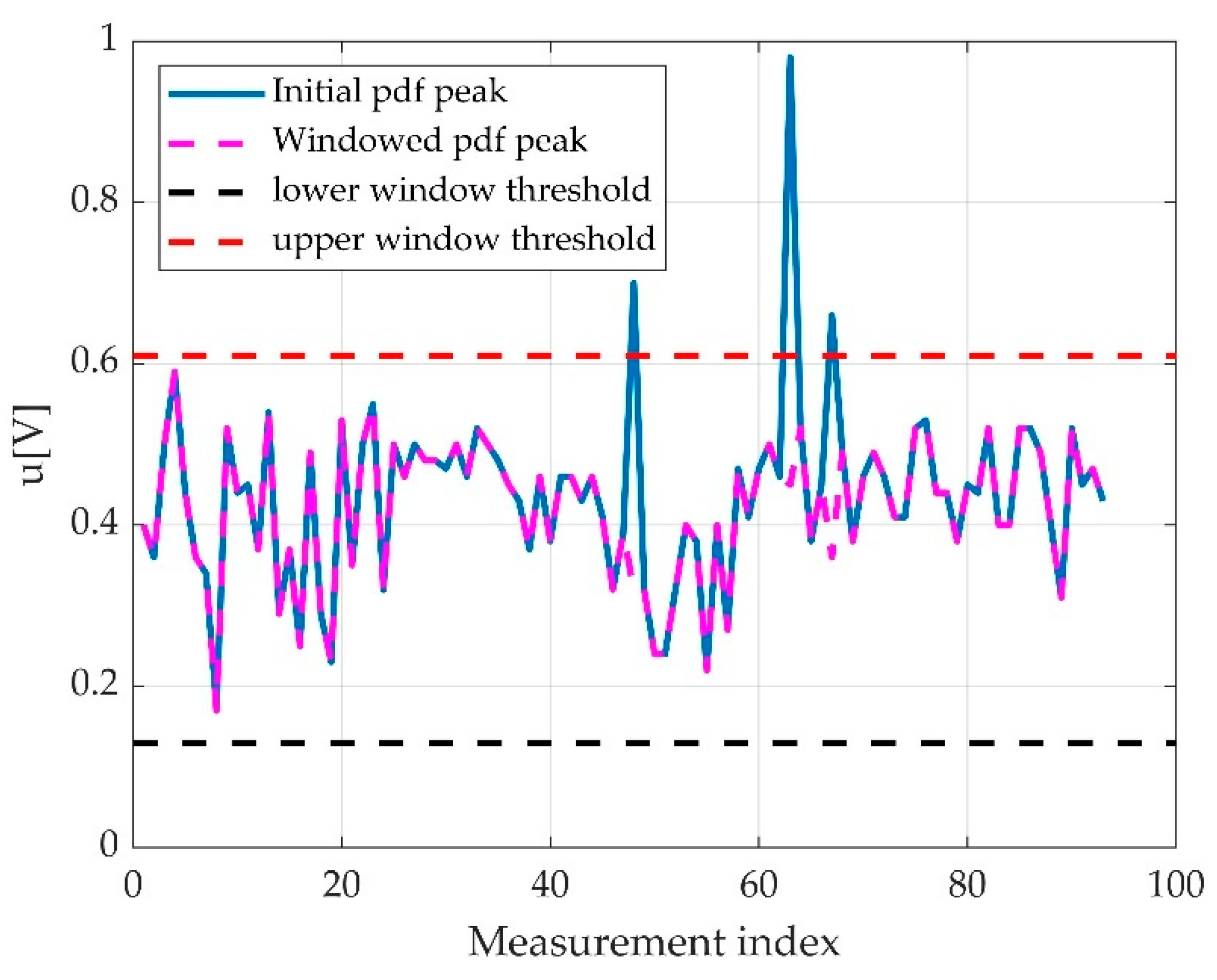
2.3.5. The Offset Detection
2.3.6. Feature Data Export
2.4. Primary Mathematical Calculus
3. Results
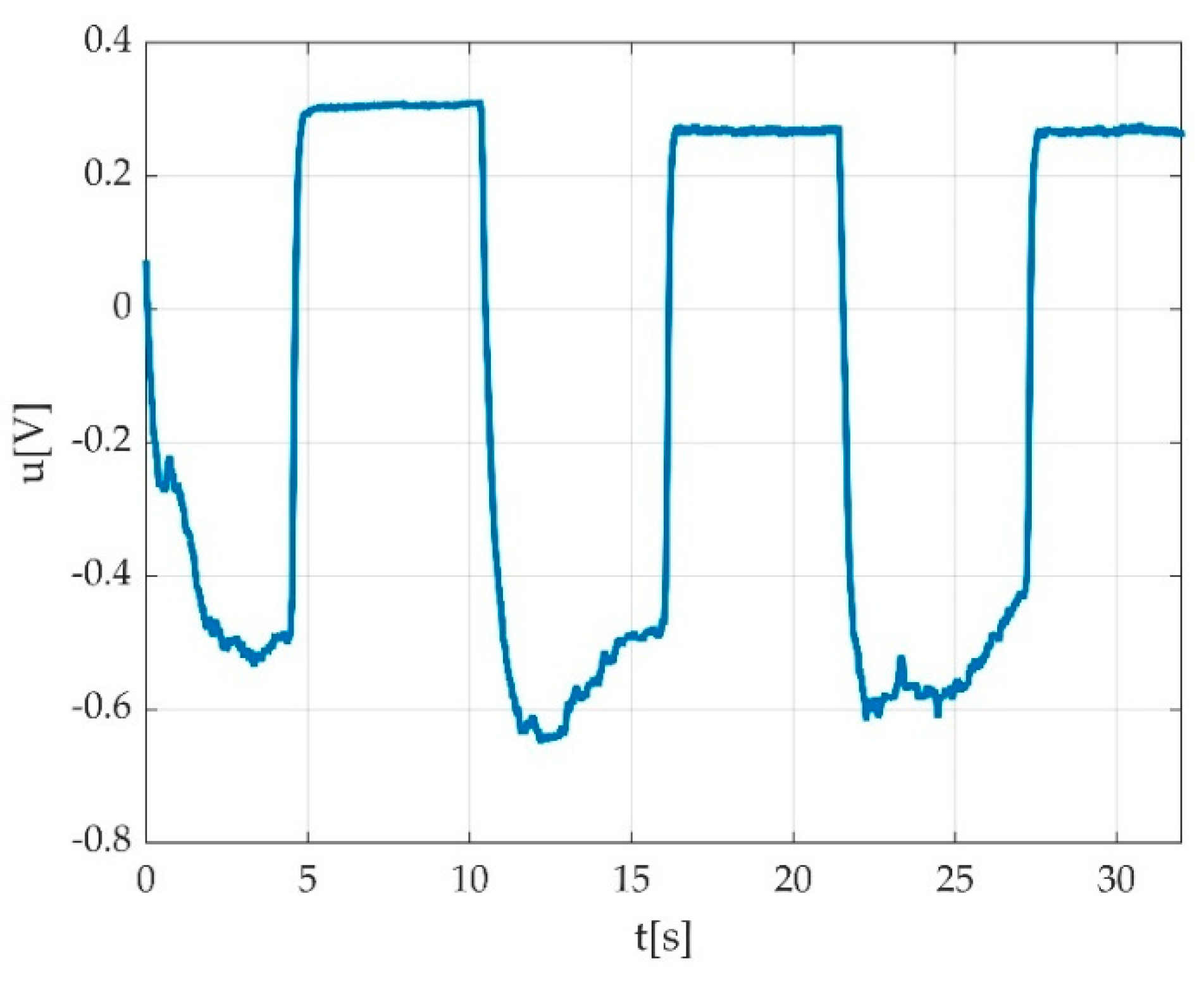
3.1. The Set-Up for the Voltage Signal Acquisition
3.2. The Digital Signal Processing
3.2.1. Data Collection and the Index File
3.2.2. The Low Pass Filtering
3.2.3. The Offset Detection
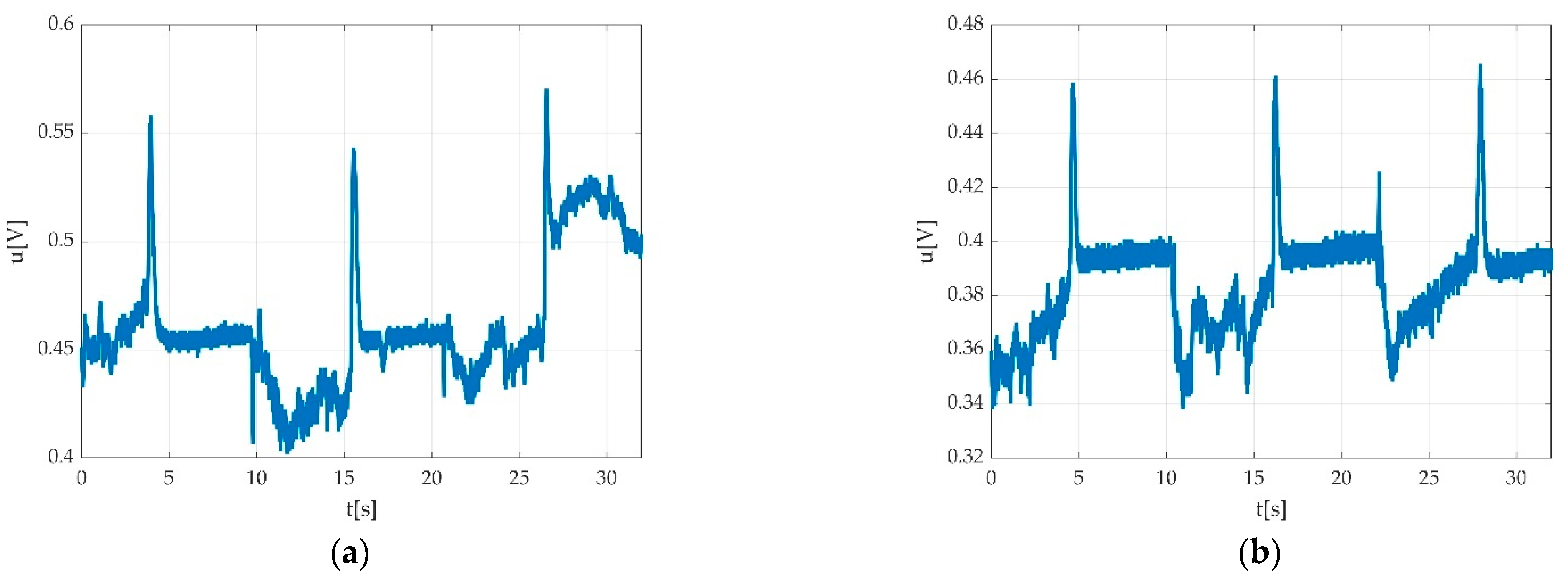
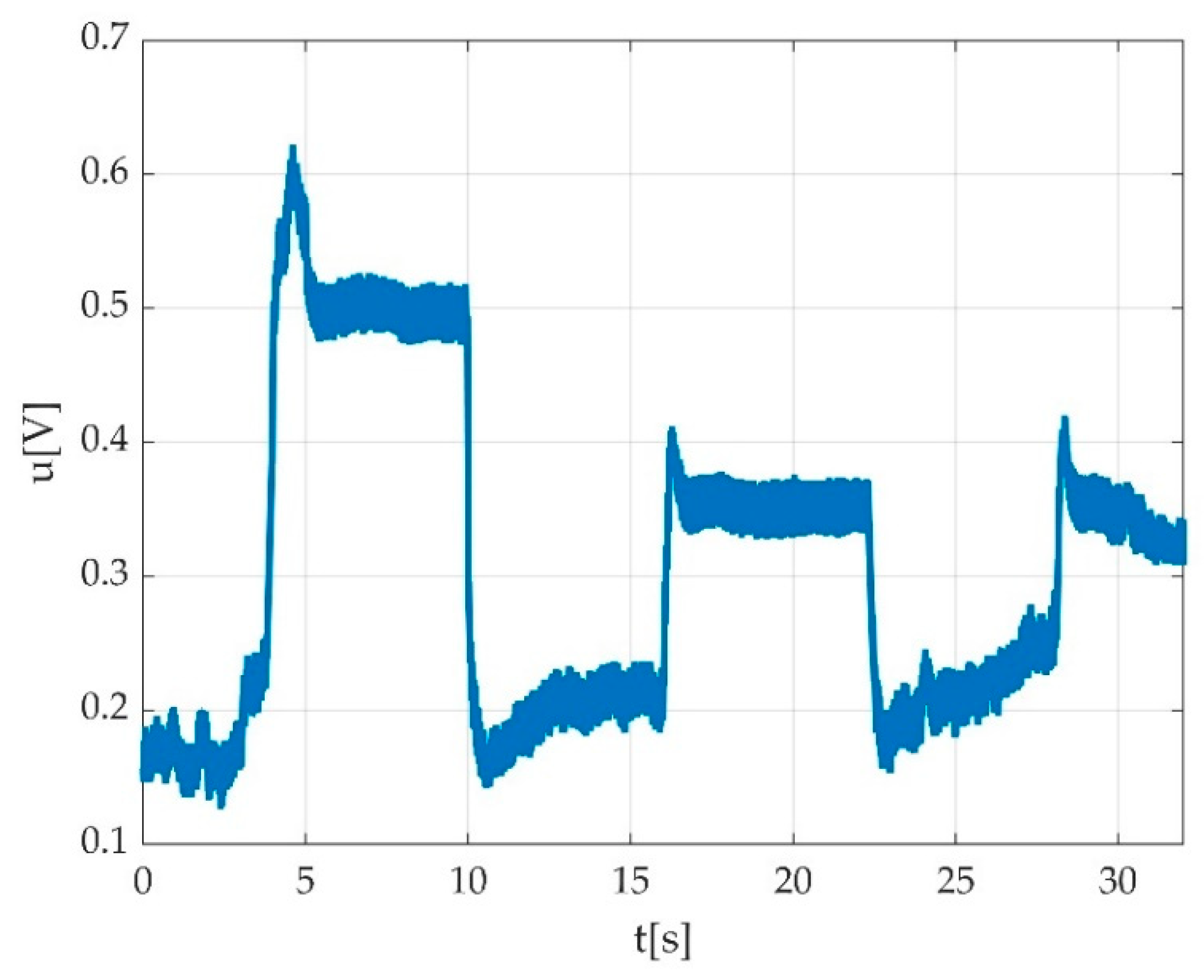
3.2.4. Data Processing
3.3. Errors
3.3.1. Participant-Related Errors
3.3.2. Tester Command-Related Errors
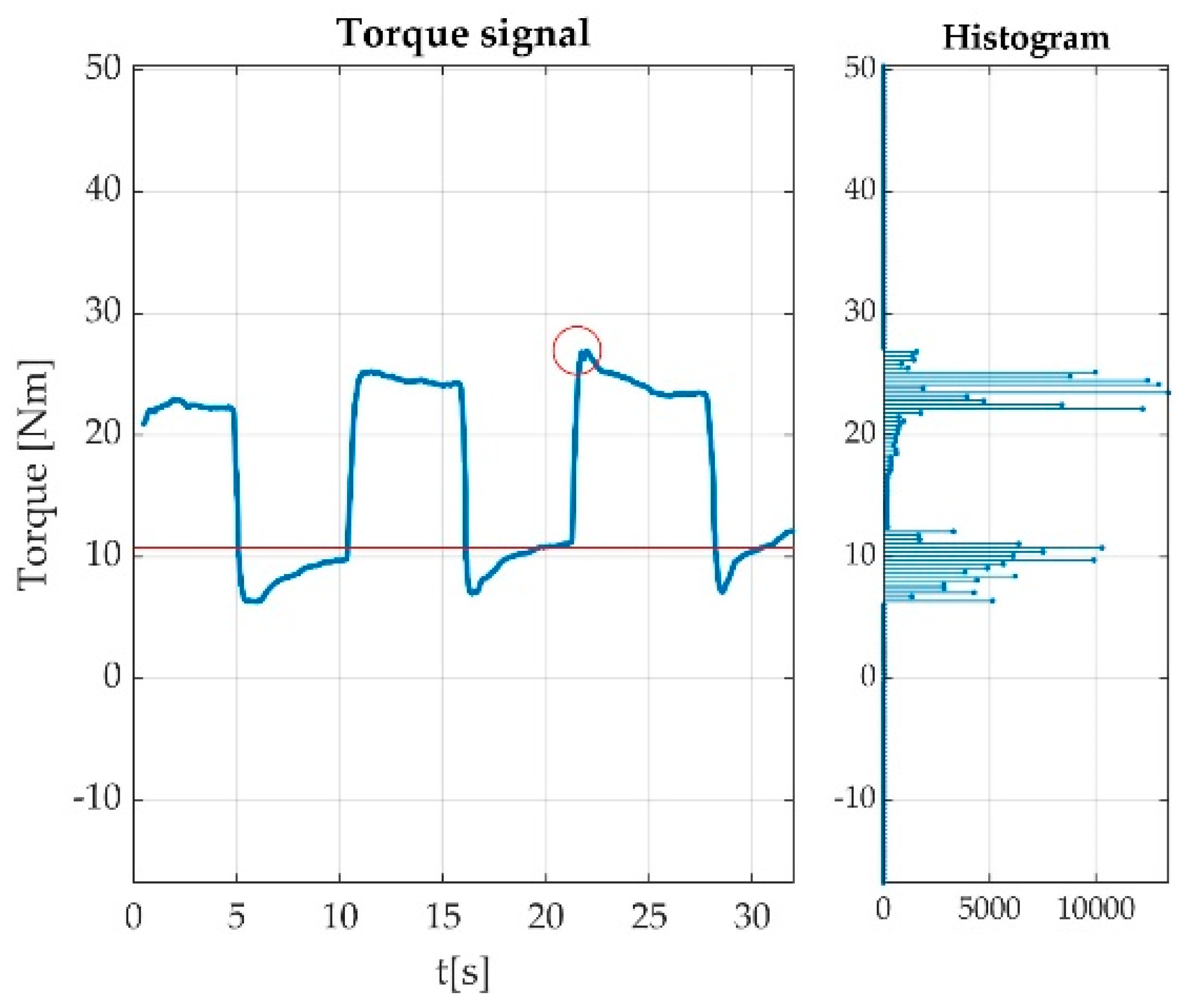
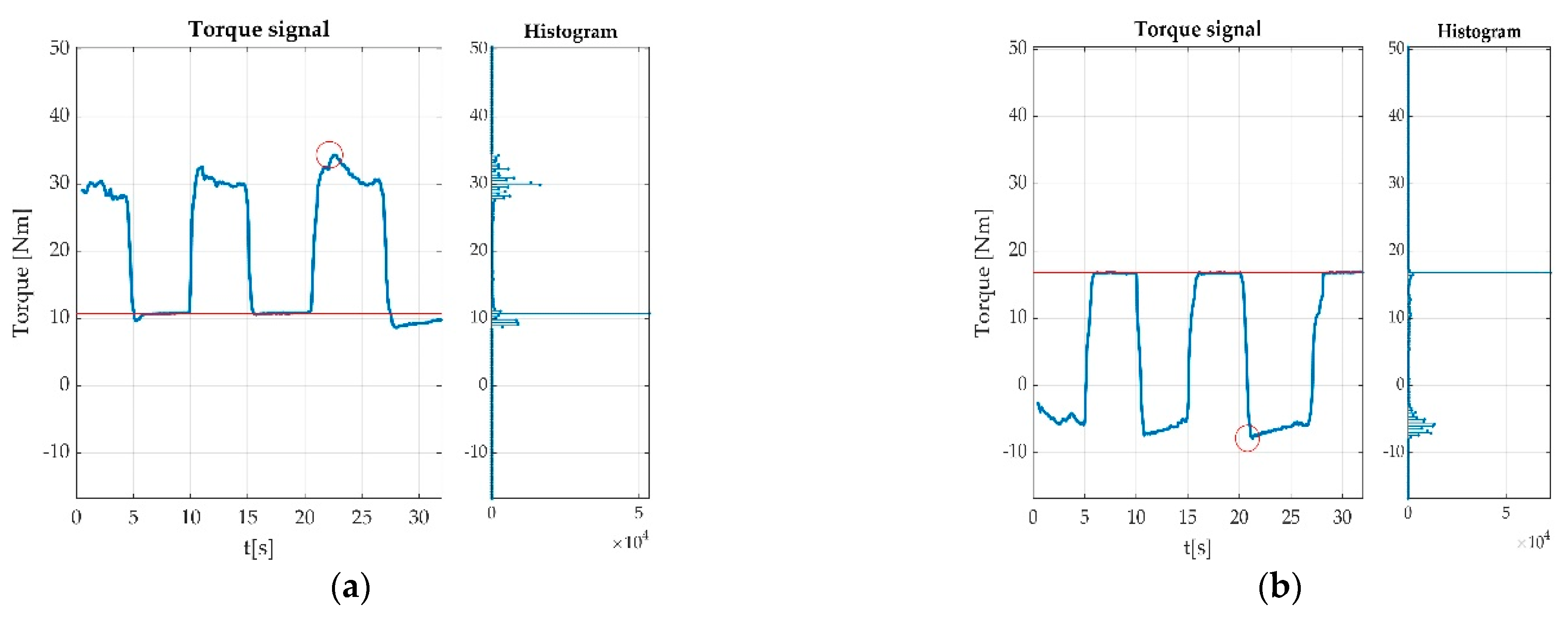
3.3.3. Automatically Detected Errors
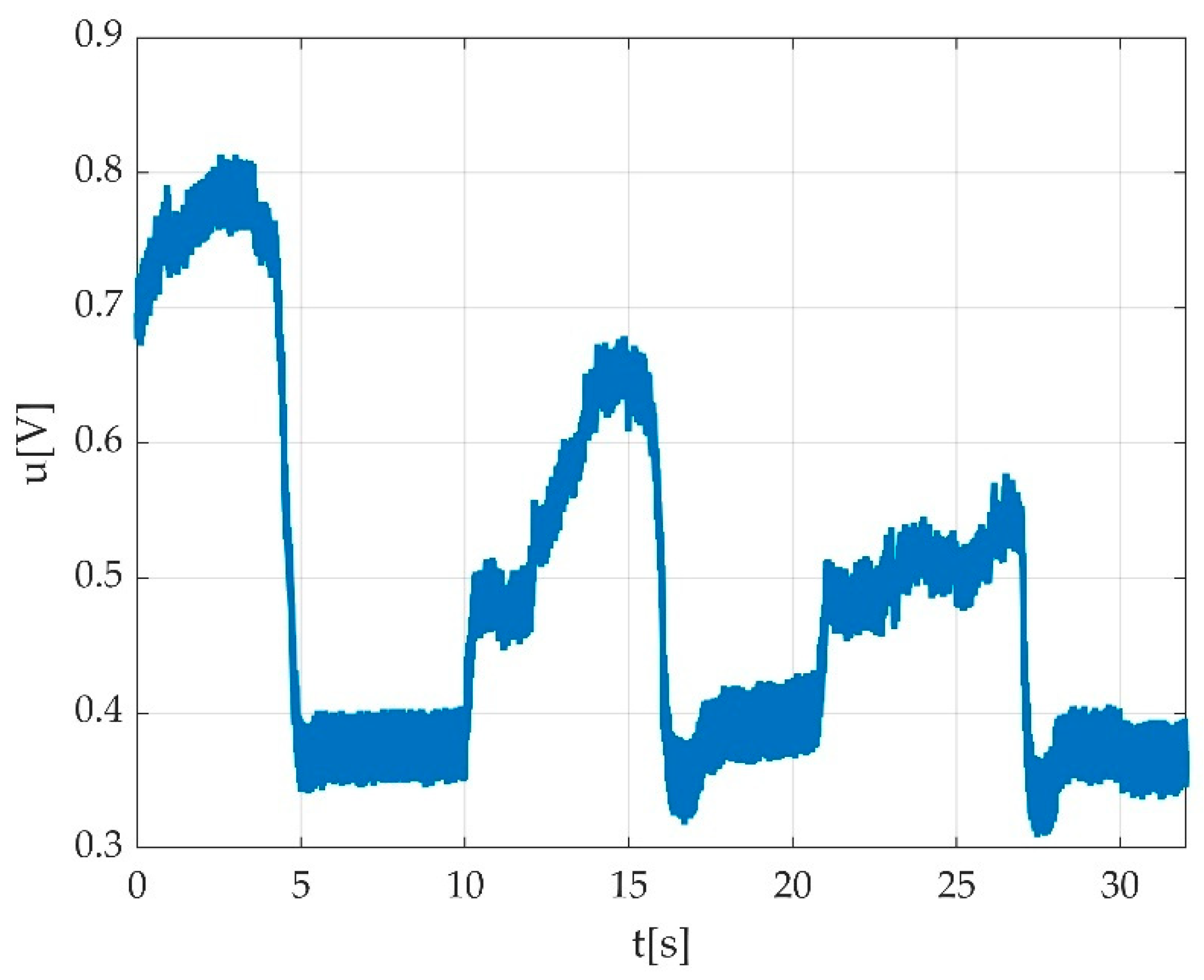
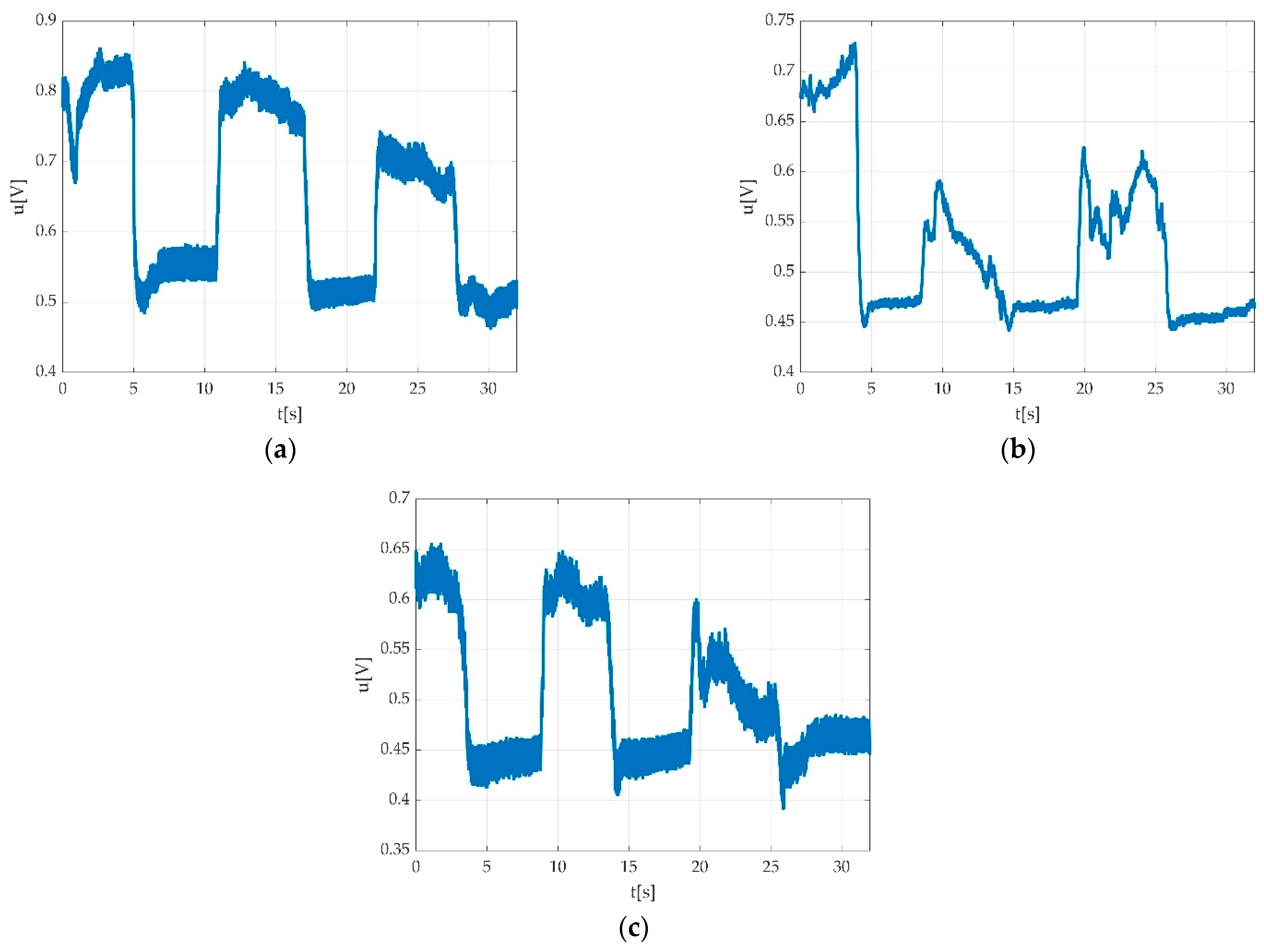
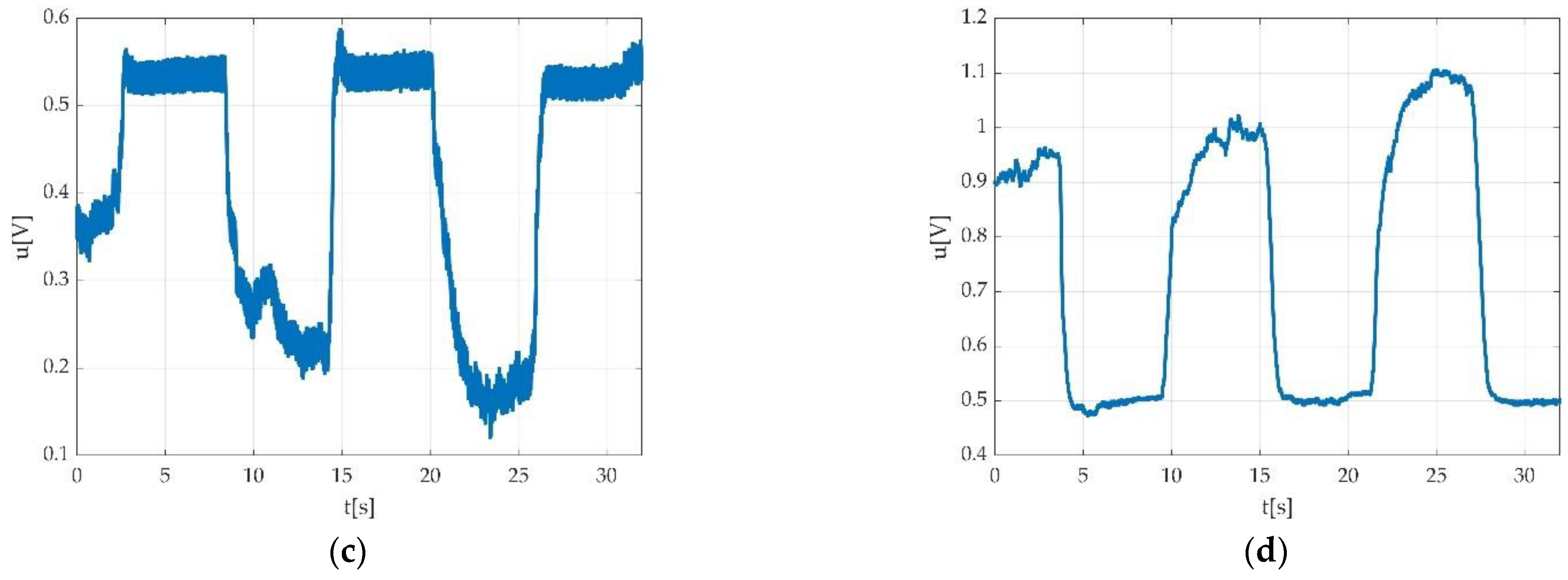
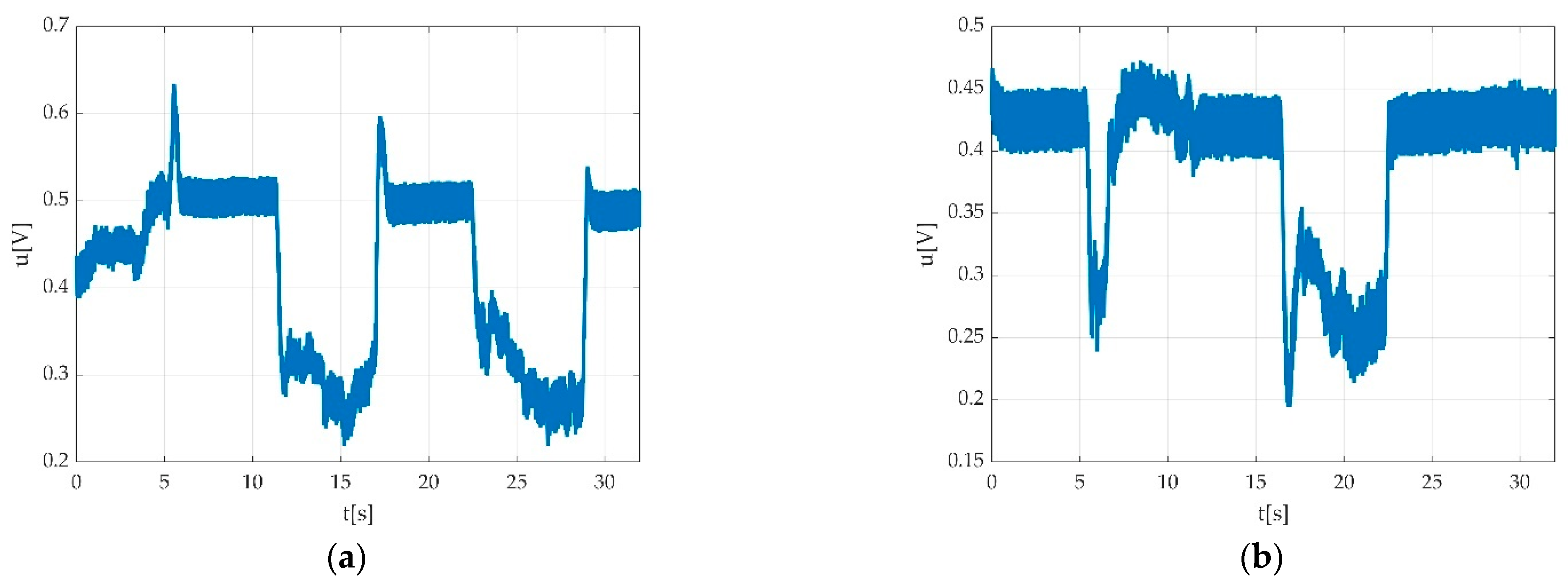
3.4. Resulted Parameters Derived from Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almekinders, L.C.; Oman, J. Isokinetic Muscle Testing: Is It Clinically Useful? J. Am. Acad. Orthop. Surg. 1994, 2, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Drăgoi, I.I.; Popescu, F.G.; Petrița, T.; Tatu, R.F.; Bondor, C.I.; Tatu, C.; Bowling, F.L.; Reeves, N.D.; Ionac, M. A Custom-Made Lower Limb Dynamometer for Assessing Ankle Joint Torque in Humans: Calibration and Measurement Procedures. Sensors 2022, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Luca, C.J.D. Surface Electromyography: Detection and Recording. 2002. Available online: https://www.delsys.com/Attachments_pdf/WP_SEMGintro.pdf (accessed on 2 January 2019).
- Thompson, B.J. Influence of signal filtering and sample rate on isometric torque-time parameters using a traditional isokinetic dynamometer. J. Biomech. 2019, 83, 235–242. [Google Scholar] [CrossRef]
- Healy, A.; Naemi, R.; Sundar, L.; Chatzistergos, P.; Ramachandran, A.; Chockalingam, N. Hallux plantar flexor strength in people with diabetic neuropathy: Validation of a simple clinical test. Diabetes Res. Clin. Pract. 2018, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Błażkiewicz, M.; Sundar, L.; Healy, A.; Ramachandran, A.; Chockalingam, N.; Naemi, R. Assessment of lower leg muscle force distribution during isometric ankle dorsi and plantar flexion in patients with diabetes: A preliminary study. J. Diabetes Complicat. 2015, 29, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eetvelde, B.L.M.; Lapauw, B.; Proot, P.; Vanden Wyngaert, K.; Celie, B.; Cambier, D.; Calders, P. The impact of sensory and/or sensorimotor neuropathy on lower limb muscle endurance, explosive and maximal muscle strength in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2020, 34, 107562. [Google Scholar] [CrossRef]
- Pandy, M.G.; Andriacchi, T.P. Muscle and Joint Function in Human Locomotion. Annu. Rev. Biomed. Eng. 2010, 12, 401–433. [Google Scholar] [CrossRef]
- Marsh, E.; Sale, D.; McComas, A.J.; Quinlan, J. Influence of joint position on ankle dorsiflexion in humans. J. Appl. Physiol. 1981, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Maganaris, C.N.; Ferretti, G.; Narici, M.V. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J. Appl. Physiol. 2005, 98, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Moraux, A.; Canal, A.; Ollivier, G.; LeDoux, I.; Doppler, V.; Payan, C.; Hogrel, J.-Y. Ankle dorsi- and plantar-flexion torques measured by dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet. Disord. 2013, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, J.-P.; Brüggemann, G.-P. The potential of human toe flexor muscles to produce force. J. Anat. 2012, 221, 187–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoi, I.I.; Popescu, F.G.; Petrita, T.; Alexa, F.; Tatu, R.F.; Bondor, C.I.; Tatu, C.; Bowling, F.L.; Reeves, N.D.; Ionac, M. A Custom-Made Electronic Dynamometer for Evaluation of Peak Ankle Torque after COVID-19. Sensors 2022, 22, 2073. [Google Scholar] [CrossRef] [PubMed]
- Research Solutions (Alsager) LTD Overview—Find and Update Company Information—GOV.UK. Available online: https://find-and-update.company-information.service.gov.uk/company/07746832 (accessed on 6 March 2022).
- Dragoi, I.I.; Popescu, F.G.; Petrita, T.; Alexa, F.; Barac, S.; Bondor, C.I.; Pauncu, E.-A.; Bowling, F.L.; Reeves, N.D.; Ionac, M. Acute Effects of Sedentary Behavior on Ankle Torque Assessed with a Custom-Made Electronic Dynamometer. J. Clin. Med. 2022, 11, 2474. [Google Scholar] [CrossRef] [PubMed]
- Benxu Electronics. BX-CZL601 Parallel Beam Load Cell Sensor—Shanghai Benxu Electronics Technology Co., Ltd. Available online: http://www.benxu-tech.com/pd.jsp?id=43 (accessed on 21 March 2022).
- PicoScope Model 2204A, Manufactured by Pico Technology, St Neots, UK. Available online: https://www.picotech.com/oscilloscope/2000/picoscope-2000-overview (accessed on 20 March 2022).
- PC Oscilloscope, Data Logger & RF Products|Pico Technology. Available online: https://www.picotech.com/downloads/_lightbox/picoscope-6 (accessed on 10 March 2022).
- MATLAB, Version 9.9.0.1570001 (R2020b); Update 4. 2020. Available online: https://www.mathworks.com/products/matlab.html (accessed on 12 January 2022).
- Eaton, J.W.; Bateman, D.; Rik Wehbring, S.H. Gnu Octave Version 6.1.0 Manual: A High-Level Interactive Language for Numerical Computations. 2020. Available online: https://octave.org/doc/v6.1.0/ (accessed on 16 April 2022).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel (accessed on 26 March 2022).
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.; Bussonnier, M.; Frederic, J.; Kelley, K.; Hamrick, J.; Grout, J.; Corlay, S.; et al. Jupyter Notebooks—A Publishing Format for Reproducible Computational Workflows. In Positioning and Power in Academic Publishing: Players, Agents and Agendas; Loizides, F., Schmidt, B., Eds.; IOS Press: Amsterdam, The Netherlands, 2016; pp. 87–90. [Google Scholar]
- Anaconda Software Distribution, version Vers. 2-2.4.0; Anaconda Inc.: Austin, TX, USA, 2020; Available online: https://docs.anaconda.com (accessed on 30 April 2022).
- Install Jupyter-MATLAB—AM111 0.1 Documentation. Available online: https://am111.readthedocs.io/en/latest/jmatlab_install.html (accessed on 30 April 2022).
- Orfandis, S.J. Introduction to Signal Processing; Prentice-Hall: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Vinay, K.I.; Proakis, J.G. Digital Signal Processing Using MATLAB, 3rd ed.; CL Engineering: Stamford, CT, USA, 2012; pp. 411–419. [Google Scholar]
- Thomas, C.W.; Huebner, W.P.; Leigh, R.J. A low-pass notch filter for bioelectric signals. IEEE Trans. Biomed. Eng. 1988, 35, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.S.; Agarwala, R.A.; Mahadev, D.U. A comparison of Chebyshev I and Chebyshev II filter applied for noise suppression in ECG signal. In Proceedings of the 7th WSEAS International Conference on SIGNAL PROCESSING (SIP’08), Istanbul, Turkey, 27–30 May 2008. [Google Scholar]
- Octave Forge-The ‘signal’ Package. Available online: https://octave.sourceforge.io/signal/ (accessed on 30 April 2022).
- Signal Processing Toolbox-MATLAB. Available online: https://www.mathworks.com/products/signal.html (accessed on 30 April 2022).
- Vanderlinden, D.W.; Kukulka, C.G.; Soderberg, G.L. The effect of muscle length on motor unit dis-charge characteristics in human tibialis anterior muscle. Exp. Brain Res. 1991, 84, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, A.A.; Chesworth, B.M.; Cunningham, D.A.; Paterson, D.H.; Rechnitzer, P.A.; Koval, J.J. Age and sex effects on mobility of the human ankle. J. Gerontol. 1992, 47, M17–M21. [Google Scholar] [CrossRef] [PubMed]
- Vanschaik, C.S.; Hicks, A.L.; McCartney, N. An evaluation of the length-tension relationship in elderly human ankle dorsiflexors. J. Gerontol. 1994, 49, B121–B127. [Google Scholar] [CrossRef] [PubMed]
- Geboers, J.F.M.; van Tuijl, J.H.; Seelen, H.A.M.; Drost, M.R. Effect of immobilization on ankle dorsi-flexion strength. Scand. J. Rehabil. Med. 2000, 32, 66–71. [Google Scholar] [PubMed]
- Ortqvist, M.; Gutierrez-Farewik, E.M.; Farewik, M.; Jansson, A. Bartonek A, Brostrom E: Reliability of a new instrument for measuring plantarflexor muscle strength. Arch. Phys. Med. Rehabil. 2007, 88, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Brostrom, E.; Nordlund, M.M.; Cresswell, A.G. Plantar- and dorsiflexor strength in prepubertal girls with juvenile idiopathic arthritis. Arch. Phys. Med. Rehabil. 2004, 85, 1224–1230. [Google Scholar]
- Todd, G.; Gorman, R.B.; Gandevia, S.C. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 2004, 29, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Solari, A.; Laura, M.; Salsano, E.; Radice, D.; Pareyson, D. Grp C-TS: Reliability of clinical outcome measures in Charcot-Marie-Tooth disease. Neuromuscul. Dis. 2008, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Belanger, A.Y.; McComas, A.J.; Elder, G.B.C. Physiological properties of two antagonistic human muscle groups. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 51, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.G.; Loscher, W.N.; Thorstensson, A. Influence of gastrocnemiusmuscle length on triceps surae torque development and electromyographic activity in Man. Exp. Brain Res. 1995, 105, 283–290. [Google Scholar] [CrossRef]
- Kawakami, Y.; Sale, D.G.; MacDougall, J.D.; Moroz, J.S. Bilateral deficit in plantar flexion: Relation to knee joint position, muscle activation, and reflex excitability. Eur. J. App. Physiol. Occup. Physiol. 1998, 77, 212–216. [Google Scholar] [CrossRef]
- Sale, D.; Quinlan, J.; Marsh, E.; Mccomas, A.J.; Belanger, A.Y. Influence of joint position on ankle plantar-flexion in humans. J. Appl. Physiol. 1982, 52, 1636–1642. [Google Scholar] [CrossRef]
- Amrutha, N.; Arul, V.H. A Review on Noises in EMG Signal and its Removal. IJSRP 2017, 7, 23–27. [Google Scholar]
- Luca, C.J.D.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]








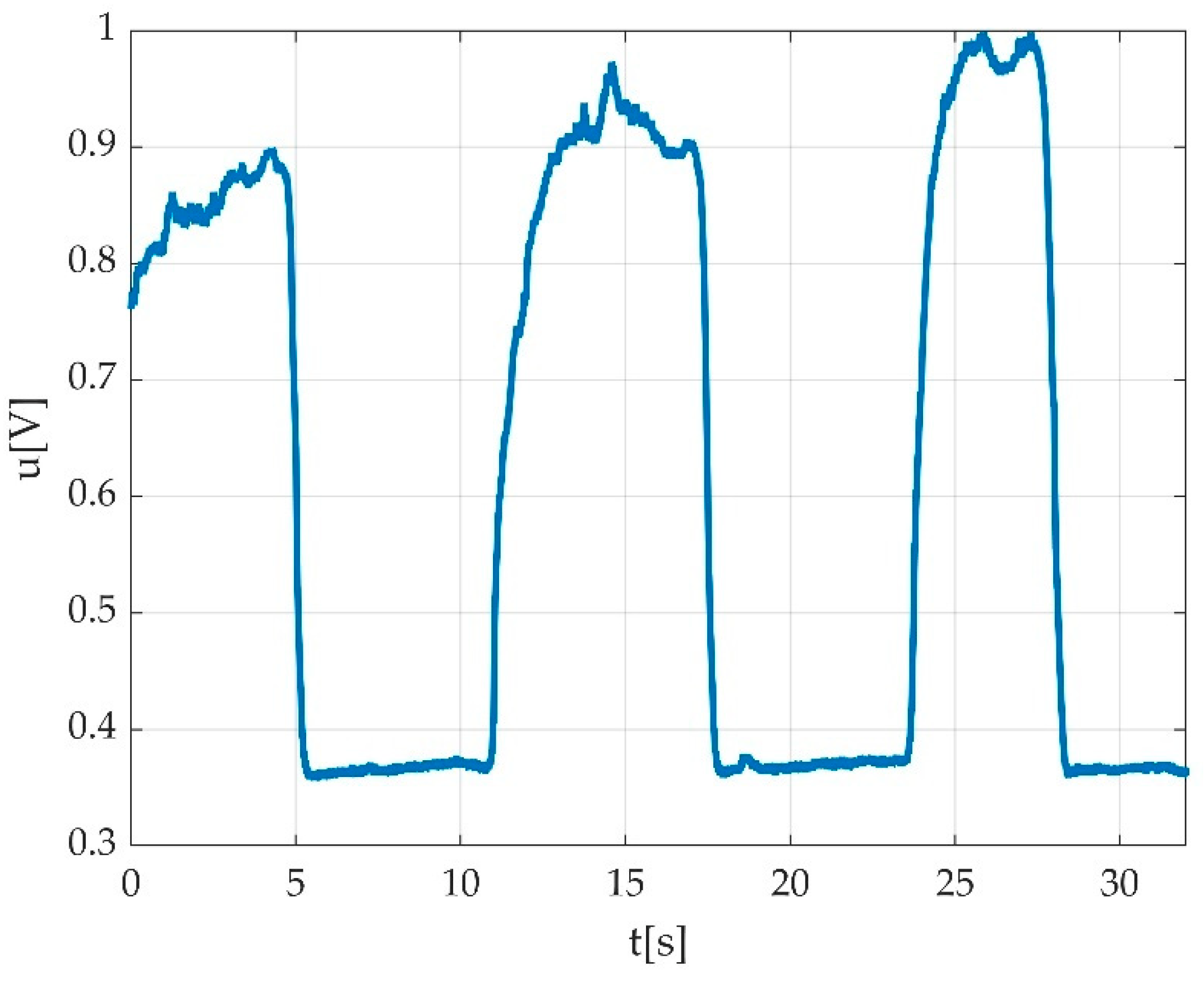
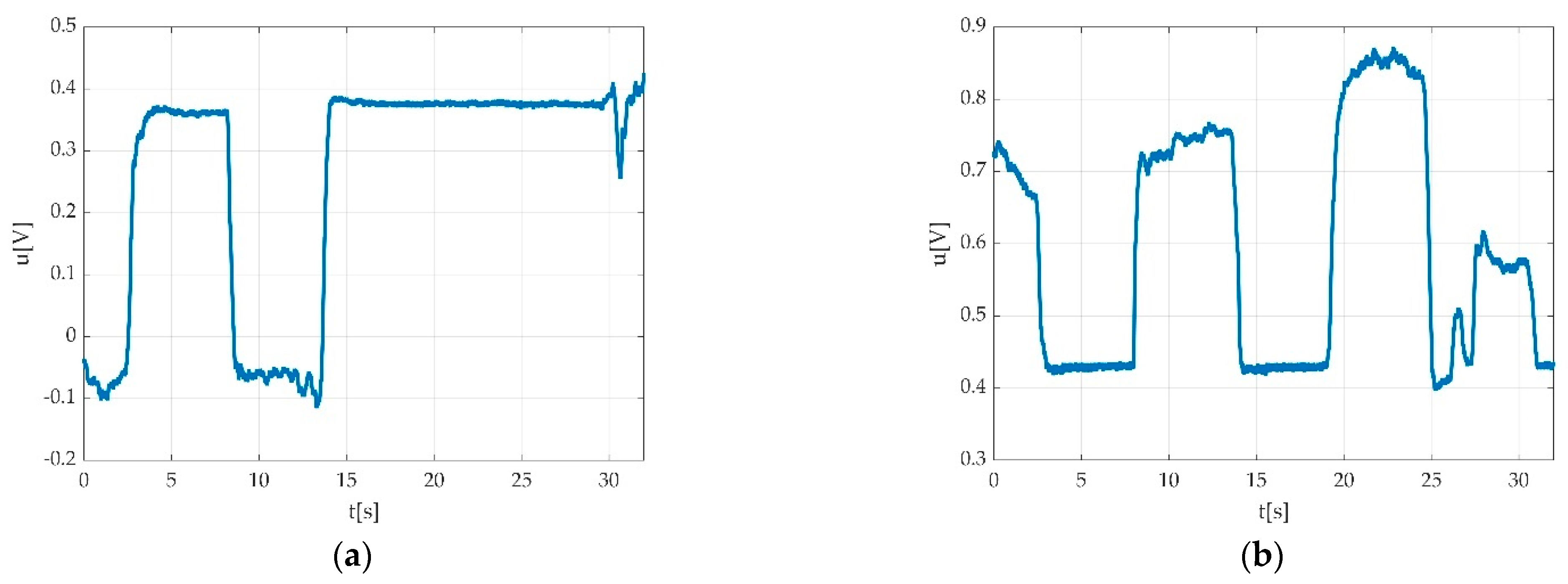
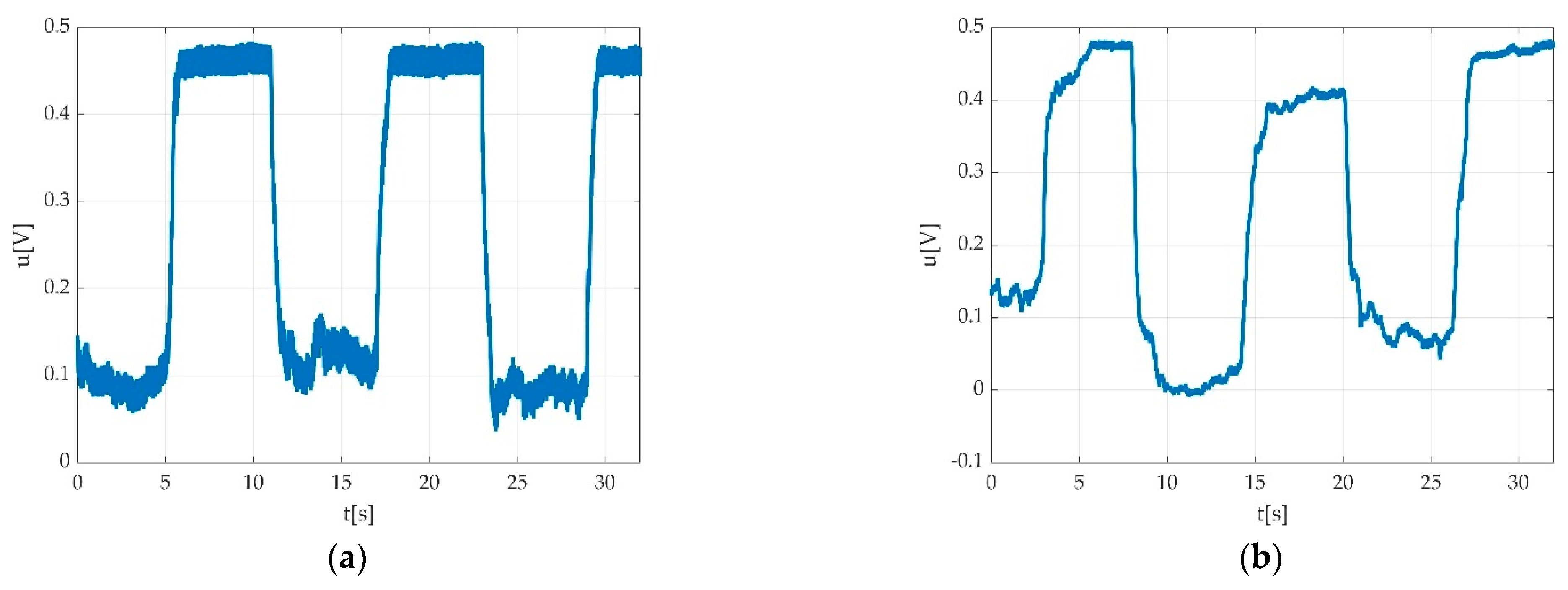
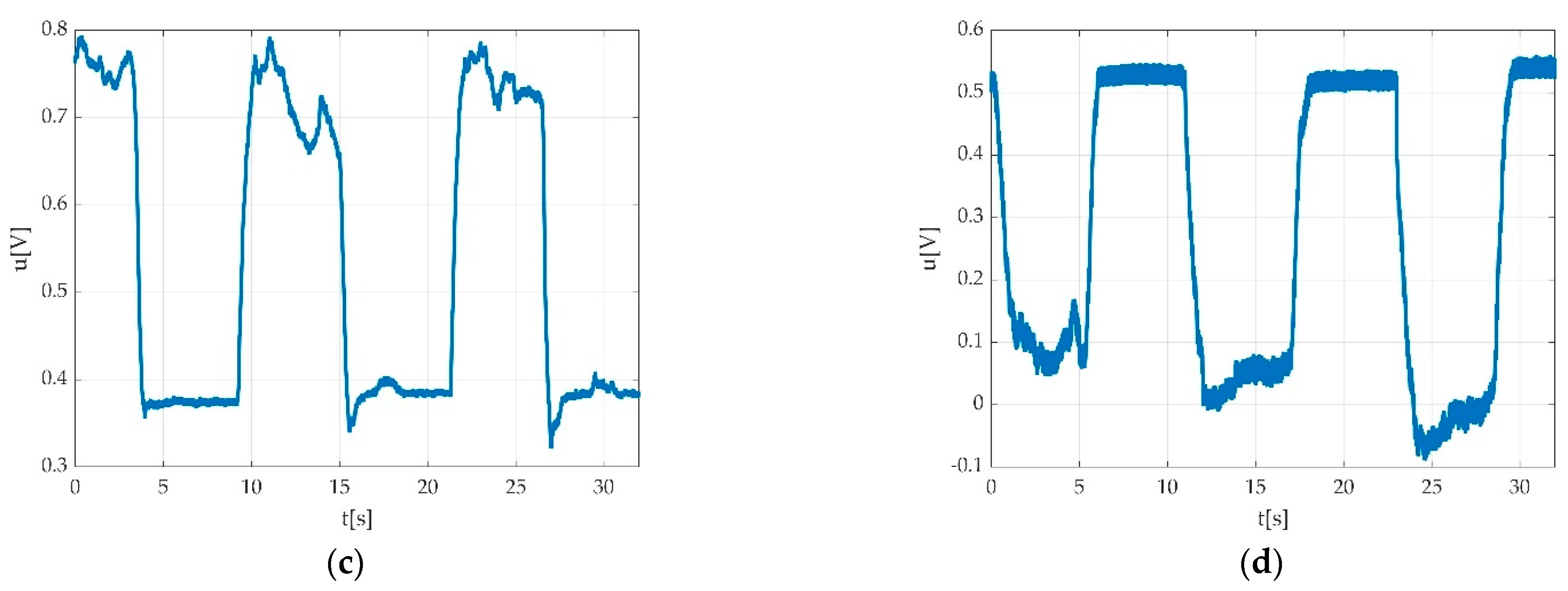



| Keyword | Meaning |
|---|---|
| Sub XX | Identification number of the subject (participants), as digits in the XX place |
| LFT | Measurement is a flexion of the left foot |
| RX | Measurement is a flexion of the right foot |
| PFlex | Measurement is a plantar flexion |
| Dorsi | Measurement is a dorsiflexion |
| −5, | Initial pedal angle is −5° (comma is needed for detection) |
| +5, | Initial pedal angle is +5° (comma is needed for detection) |
| 0, | Initial pedal angle is 0° (comma is needed for detection) |
| BASELINE | First measurement from multiple series |
| 2h | Measurement at 2 h from the baseline in multiple series (similar for other time intervals—4 h, 6 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragoi, I.I.; Petrita, T.; Popescu, F.G.; Alexa, F.; Barac, S.; Bowling, F.L.; Reeves, N.D.; Bondor, C.I.; Ionac, M. A Signal Processing Method for Assessing Ankle Torque with a Custom-Made Electronic Dynamometer in Participants Affected by Diabetic Peripheral Neuropathy. Sensors 2022, 22, 6310. https://doi.org/10.3390/s22166310
Dragoi II, Petrita T, Popescu FG, Alexa F, Barac S, Bowling FL, Reeves ND, Bondor CI, Ionac M. A Signal Processing Method for Assessing Ankle Torque with a Custom-Made Electronic Dynamometer in Participants Affected by Diabetic Peripheral Neuropathy. Sensors. 2022; 22(16):6310. https://doi.org/10.3390/s22166310
Chicago/Turabian StyleDragoi, Iulia Iovanca, Teodor Petrita, Florina Georgeta Popescu, Florin Alexa, Sorin Barac, Frank L. Bowling, Neil D. Reeves, Cosmina Ioana Bondor, and Mihai Ionac. 2022. "A Signal Processing Method for Assessing Ankle Torque with a Custom-Made Electronic Dynamometer in Participants Affected by Diabetic Peripheral Neuropathy" Sensors 22, no. 16: 6310. https://doi.org/10.3390/s22166310
APA StyleDragoi, I. I., Petrita, T., Popescu, F. G., Alexa, F., Barac, S., Bowling, F. L., Reeves, N. D., Bondor, C. I., & Ionac, M. (2022). A Signal Processing Method for Assessing Ankle Torque with a Custom-Made Electronic Dynamometer in Participants Affected by Diabetic Peripheral Neuropathy. Sensors, 22(16), 6310. https://doi.org/10.3390/s22166310






