Detection of Horse Locomotion Modifications Due to Training with Inertial Measurement Units: A Proof-of-Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
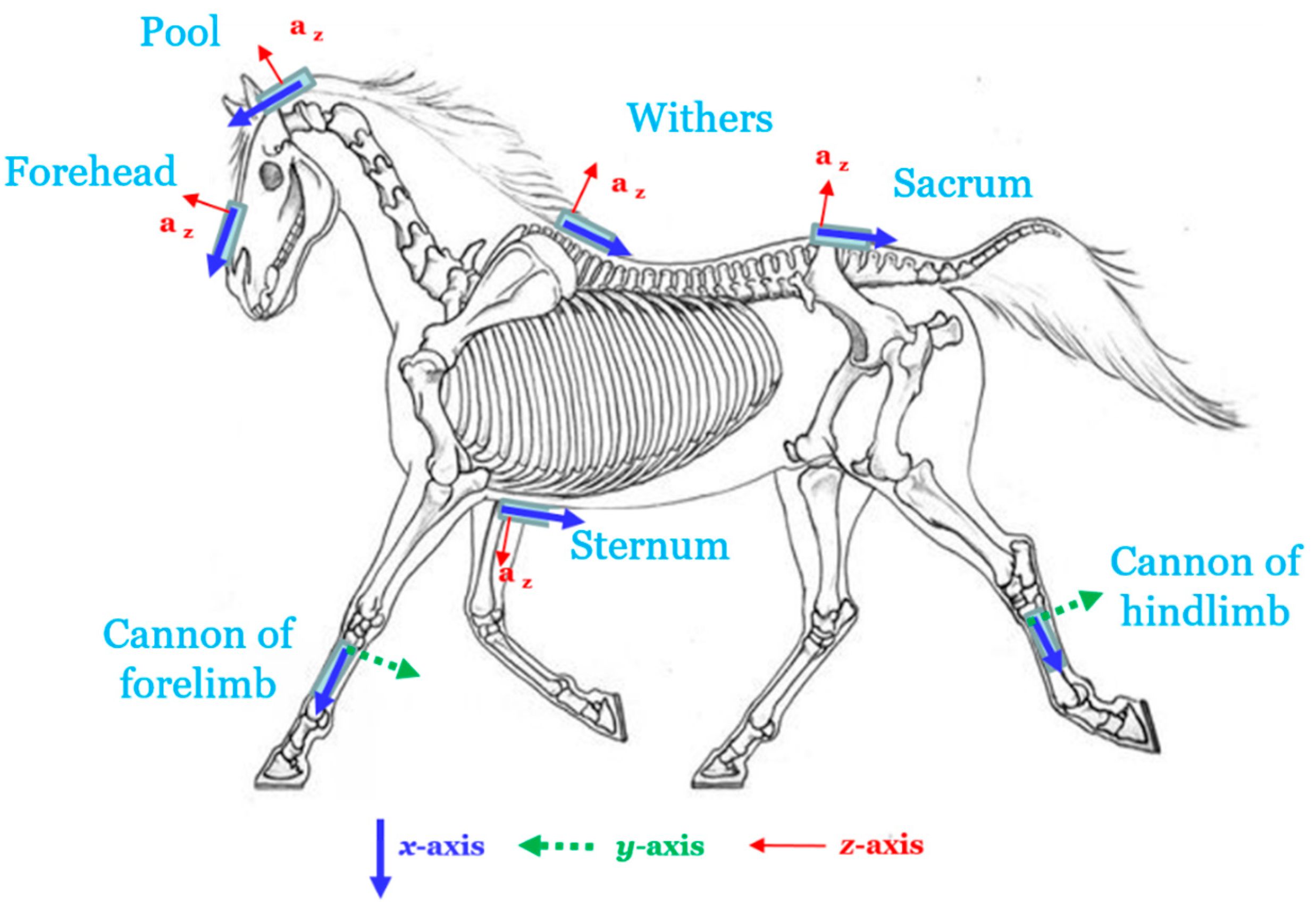
2.2. Material
2.3. Protocol
2.4. Data Processing
2.4.1. Kinematics Data
2.4.2. Stride Segmentation
2.4.3. Stride Kinematics’ Comparison
2.4.4. Smoothness
2.5. Statistical Analysis
3. Results
3.1. Energetic Solicitation during the Training Session
3.2. Kinematics’ Modifications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CANTER | |||||
|---|---|---|---|---|---|
| Variable | Axis | Position | p-Value | Before/Intra | Post/Inter |
| Acceleration | h | FC | 0.027 | 0.28 (0.09) | 0.18 (0.14) |
| h | St | 0.004 | 0.61 (0.14) | 0.5 (0.19) | |
| x | FC | 0.031 | 0.31 (0.11) | 0.1 (0.1) | |
| x | HC | 0.039 | 0.39 (0.08) | 0.26 (0.12) | |
| x | Sa | 0.031 | 0.77 (0.23) | 0.67 (0.21) | |
| x | St | 0.008 | 0.72 (0.09) | 0.59 (0.07) | |
| y | FC | 0.031 | 0.32 (0.07) | 0.16 (0.1) | |
| y | HC | 0.039 | 0.41 (0.07) | 0.29 (0.11) | |
| z | FC | 0.020 | 0.24 (0.07) | 0.12 (0.07) | |
| z | HC | 0.023 | 0.33 (0.1) | 0.23 (0.12) | |
| v | FC | 0.016 | 0.45 (0.12) | 0.28 (0.14) | |
| v | HC | 0.027 | 0.36 (0.08) | 0.21 (0.1) | |
| v | Po | 0.039 | 0.63 (0.26) | 0.56 (0.27) | |
| Angle | Twist | FC | 0.008 | 0.65 (0.09) | 0.15 (0.57) |
| x/v | FC | 0.031 | 0.24 (0.13) | 0.13 (0.07) | |
| z/v | St | 0.012 | 0.74 (0.29) | 0.66 (0.37) | |
| Angular velocity | x | FC | 0.027 | 0.36 (0.08) | 0.25 (0.13) |
| x | HC | 0.020 | 0.38 (0.1) | 0.25 (0.1) | |
| x | FH | 0.016 | 0.27 (0.07) | 0.1 (0.12) | |
| x | St | 0.039 | 0.2 (0.06) | 0.07 (0.13) | |
| y | FC | 0.031 | 0.35 (0.12) | 0.23 (0.11) | |
| y | HC | 0.016 | 0.29 (0.11) | 0.17 (0.1) | |
| z | FH | 0.039 | 0.28 (0.14) | 0.14 (0.24) | |
| TROT | |||||
|---|---|---|---|---|---|
| Variable | Axis | Position | p-Value | Intra | Inter |
| Acceleration | h | FC | 0.02 | 0.62 (0.16) | 0.37 (0.25) |
| h | HC | 0.039 | 0.54 (0.18) | 0.22 (0.4) | |
| h | Sa | 0.031 | 0.53 (0.14) | 0.33 (0.28) | |
| h | St | 0.008 | 0.6 (0.13) | 0.5 (0.15) | |
| h | Po | 0.039 | 0.61 (0.14) | 0.51 (0.19) | |
| x | FC | 0.016 | 0.6 (0.2) | 0.32 (0.34) | |
| x | HC | 0.016 | 0.62 (0.15) | 0.34 (0.33) | |
| x | Sa | 0.031 | 0.76 (0.09) | 0.53 (0.28) | |
| y | FC | 0.039 | 0.62 (0.2) | 0.32 (0.36) | |
| y | HC | 0.004 | 0.62 (0.18) | 0.29 (0.35) | |
| y | FH | 0.016 | 0.55 (0.13) | 0.22 (0.37) | |
| y | St | 0.008 | 0.57 (0.21) | 0.28 (0.37) | |
| y | Po | 0.004 | 0.63 (0.13) | 0.35 (0.39) | |
| z | FC | 0.008 | 0.42 (0.14) | 0.18 (0.18) | |
| z | HC | 0.004 | 0.56 (0.13) | 0.24 (0.29) | |
| z | FH | 0.016 | 0.75 (0.09) | 0.6 (0.21) | |
| v | FC | 0.012 | 0.26 (0.13) | 0.04 (0.21) | |
| v | HC | 0.02 | 0.22 (0.13) | 0.09 (0.14) | |
| Angle | Twist | HC | 0.039 | 0.66 (0.4) | 0.33 (0.67) |
| z/v | Sa | 0.031 | 0.7 (0.19) | 0.55 (0.26) | |
| z/v | St | 0.004 | 0.72 (0.26) | 0.66 (0.28) | |
| Angular velocity | x | FC | 0.004 | 0.7 (0.12) | 0.43 (0.21) |
| x | HC | 0.039 | 0.74 (0.12) | 0.49 (0.38) | |
| x | FH | 0.016 | 0.5 (0.14) | 0.11 (0.34) | |
| x | St | 0.039 | 0.67 (0.22) | 0.27 (0.57) | |
| x | Po | 0.008 | 0.51 (0.17) | 0.22 (0.31) | |
| y | FC | 0.02 | 0.67 (0.23) | 0.26 (0.44) | |
| y | HC | 0.039 | 0.63 (0.17) | 0.35 (0.43) | |
| y | FH | 0.031 | 0.64 (0.13) | 0.48 (0.29) | |
| y | Po | 0.02 | 0.56 (0.18) | 0.4 (0.32) | |
| z | Po | 0.008 | 0.51 (0.16) | 0.13 (0.37) | |
References
- Di Domenico, F.; Raiola, G. Effects of Training Fatigue on Performance. J. Human Sport Exer. 2021, 16, S769–S780. [Google Scholar] [CrossRef]
- Jones, C.M.; Griffiths, P.C.; Mellalieu, S.D. Training Load and Fatigue Marker Associations with Injury and Illness: A Systematic Review of Longitudinal Studies. Sports Med. 2017, 47, 943–974. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.M.; Whitworth, D.J. Overtraining Syndrome in Horses. CEP 2008, 5, 57. [Google Scholar] [CrossRef]
- Kellmann, M. Preventing Overtraining in Athletes in High-Intensity Sports and Stress/Recovery Monitoring: Preventing Overtraining. Scand. J. Med. Sci. Sports 2010, 20, 95–102. [Google Scholar] [CrossRef]
- Lindner, A.; Mosen, H.; Kissenbeck, S.; Fuhrmann, H.; Sallmann, H.P. Effect of Blood Lactate-Guided Conditioning of Horses with Exercises of Differing Durations and Intensities on Heart Rate and Biochemical Blood Variables. J. Anim. Sci. 2009, 87, 3211–3217. [Google Scholar] [CrossRef]
- Mami, S.; Khaje, G.; Shahriari, A.; Gooraninejad, S. Evaluation of Biological Indicators of Fatigue and Muscle Damage in Arabian Horses After Race. J. Equine Veterinary Sci. 2019, 78, 74–78. [Google Scholar] [CrossRef]
- Winter, S.; Gordon, S.; Watt, K. Effects of Fatigue on Kinematics and Kinetics during Overground Running: A Systematic Review. J. Sports Med. Phys. Fitness 2017, 57, 887–899. [Google Scholar] [CrossRef]
- Barbieri, F.A.; dos Santos, P.C.R.; Lirani-Silva, E.; Vitório, R.; Gobbi, L.T.B.; van Diëen, J.H. Systematic Review of the Effects of Fatigue on Spatiotemporal Gait Parameters. BMR 2013, 26, 125–131. [Google Scholar] [CrossRef]
- Johnston, C.; Gottlieb-Vedi, M.; Drevemo, S.; Roepstorff, L. The Kinematics of Loading and Fatigue in the Standardbred Trotter. Equine Veterinary J. 1999, 31, 249–253. [Google Scholar] [CrossRef]
- Wickler, S.J.; Greene, H.M.; Egan, K.; Astudillo, A.; Dutto, D.J.; Hoyt, D.F. Stride Parameters and Hindlimb Length in Horses Fatigued on a Treadmill and at an Endurance Ride. Equine Veterinary J. 2006, 38, 60–64. [Google Scholar] [CrossRef]
- Muñoz, A.; Cuesta, I.; Riber, C.; Gata, J.; Trigo, P.; Castejón, F.M. Trot Asymmetry in Relation to Physical Performance and Metabolism in Equine Endurance Rides. Equine Veterinary J. 2006, 38, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Takahashi, T.; Mukai, K.; Ohmura, H. Effects of Fatigue on Stride Parameters in Thoroughbred Racehorses during Races. J. Equine Veterinary Sci. 2021, 101, 103447. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Mukai, K.; Matsui, A.; Ohmura, H.; Takahashi, T. Electromyographic Changes in Hind Limbs of Thoroughbreds with Fatigue Induced by Treadmill Exercise. Am. J. Veterinary Res. 2018, 79, 828–835. [Google Scholar] [CrossRef]
- Kienapfel, K. The Effect of Three Different Head-Neck Positions on the Average EMG Activity of Three Important Neck Muscles in the Horse. J. Anim. Physiol. Anim. Nutr. 2015, 99, 132–138. [Google Scholar] [CrossRef]
- Pfau, T.; Scott, W.M.; Sternberg Allen, T. Upper Body Movement Symmetry in Reining Quarter Horses during Trot In-Hand, on the Lunge and during Ridden Exercise. Animals 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Sapone, M.; Martin, P.; Ben Mansour, K.; Chateau, H.; Marin, F. The Protraction and Retraction Angles of Horse Limbs: An Estimation during Trotting Using Inertial Sensors. Sensors 2021, 21, 3792. [Google Scholar] [CrossRef]
- Lopes, M.A.F.; Nichols, J.T.; Dearo, A.C.O.; Nelson, S.R. Effects of Forelimb Instrumentation on Lameness Detection in Horses Using a Portable Inertial Sensor-Based System. J. Am. Vet. Med. Assoc. 2021, 259, 892–898. [Google Scholar] [CrossRef]
- Serra Bragança, F.M.; Broomé, S.; Rhodin, M.; Björnsdóttir, S.; Gunnarsson, V.; Voskamp, J.P.; Persson-Sjodin, E.; Back, W.; Lindgren, G.; Novoa-Bravo, M.; et al. Improving Gait Classification in Horses by Using Inertial Measurement Unit (IMU) Generated Data and Machine Learning. Sci. Rep. 2020, 10, 17785. [Google Scholar] [CrossRef]
- Darbandi, H.; Serra Bragança, F.; van der Zwaag, B.J.; Voskamp, J.; Gmel, A.I.; Haraldsdóttir, E.H.; Havinga, P. Using Different Combinations of Body-Mounted IMU Sensors to Estimate Speed of Horses—A Machine Learning Approach. Sensors 2021, 21, 798. [Google Scholar] [CrossRef]
- Walker, V.A.; Tranquillle, C.A.; MacKechnie-Guire, R.; Spear, J.; Newton, R.; Murray, R.C. Effect of Ground and Raised Poles on Kinematics of the Walk. J. Equine Vet. Sci. 2022, 115, 104005. [Google Scholar] [CrossRef]
- Sapone, M.; Martin, P.; Mansour, K.B.; Château, H.; Marin, F. Comparison of Trotting Stance Detection Methods from an Inertial Measurement Unit Mounted on the Horse’s Limb. Sensors 2020, 20, 2983. [Google Scholar] [CrossRef] [PubMed]
- Hatrisse, C.; Macaire, C.; Sapone, M.; Hebert, C.; Hanne-Poujade, S.; De Azevedo, E.; Marin, F.; Martin, P.; Chateau, H. Stance Phase Detection by Inertial Measurement Unit Placed on the Metacarpus of Horses Trotting on Hard and Soft Straight Lines and Circles. Sensors 2022, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, M.; Hernlund, E.; Rhodin, M.; Bosch, S.; Voskamp, J.P.; Nielen, M.; Serra Braganςa, F.M. Automatic Detection of Break-over Phase Onset in Horses Using Hoof-Mounted Inertial Measurement Unit Sensors. PLoS ONE 2020, 15, e0233649. [Google Scholar] [CrossRef]
- Tijssen, M.; Hernlund, E.; Rhodin, M.; Bosch, S.; Voskamp, J.P.; Nielen, M.; Serra Braganςa, F.M. Automatic Hoof-on and -off Detection in Horses Using Hoof-Mounted Inertial Measurement Unit Sensors. PLoS ONE 2020, 15, e0233266. [Google Scholar] [CrossRef]
- Hagen, J.; Jung, F.T.; Brouwer, J.; Bos, R. Detection of Equine Hoof Motion by Using a Hoof-Mounted Inertial Measurement Unit Sensor in Comparison to Examinations with an Optoelectronic Technique—A Pilot Study. J. Equine Vet. Sci. 2021, 101, 103454. [Google Scholar] [CrossRef]
- Bosch, S.; Serra Bragança, F.; Marin-Perianu, M.; Marin-Perianu, R.; van der Zwaag, B.J.; Voskamp, J.; Back, W.; Van Weeren, R.; Havinga, P. Equimoves: A Wireless Networked Inertial Measurement System for Objective Examination of Horse Gait. Sensors 2018, 18, 850. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Melendez-Calderon, A.; Burdet, E. A Robust and Sensitive Metric for Quantifying Movement Smoothness. IEEE Trans. Biomed. Eng. 2012, 59, 2126–2136. [Google Scholar] [CrossRef]
- Harris, C.M.; Wolpert, D.M. Signal-Dependent Noise Determines Motor Planning. Nature 1998, 394, 780–784. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Melendez-Calderon, A.; Roby-Brami, A.; Burdet, E. On the Analysis of Movement Smoothness. J. NeuroEng. Rehabil. 2015, 12, 112. [Google Scholar] [CrossRef]
- Melendez-Calderon, A.; Shirota, C.; Balasubramanian, S. Estimating Movement Smoothness From Inertial Measurement Units. Front. Bioeng. Biotechnol. 2020, 8, 558771. [Google Scholar] [CrossRef]
- Beck, Y.; Herman, T.; Brozgol, M.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. SPARC: A New Approach to Quantifying Gait Smoothness in Patients with Parkinson’s Disease. J. NeuroEng. Rehabil. 2018, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; Clayton, H.M. Equine Locomotion; Elsevier Health Sciences: Amsterdam, Netherlands, 2013; ISBN 978-0-7020-2950-9. [Google Scholar]
- Dobrowolski, P. Swing-Twist Decomposition in Clifford Algebra. arXiv 2015, arXiv:1506.05481. [Google Scholar]
- Gondim, F.J.; Zoppi, C.C.; Pereira-da-Silva, L.; de Macedo, D.V. Determination of the Anaerobic Threshold and Maximal Lactate Steady State Speed in Equines Using the Lactate Minimum Speed Protocol. Compar. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.; von Wittke, P.; Schmald, M.; Kusserow, J.; Sommer, H. Maximal Lactate Concentrations in Horses after Exercise of Different Duration and Intensity. J. Equine Veterinary Sci. 1992, 12, 36–39. [Google Scholar] [CrossRef]
- Galindo-Martínez, A.; López-Valenciano, A.; Albaladejo-García, C.; Vallés-González, J.M.; Elvira, J.L.L. Changes in the Trunk and Lower Extremity Kinematics Due to Fatigue Can Predispose to Chronic Injuries in Cycling. Int. J. Environ. Res. Public Health 2021, 18, 3719. [Google Scholar] [CrossRef]
- Galloux, P.; Richard, N.; Dronka, T.; Léard, M.; Perrot, A.; Jouffroy, J.L.; Cholet, A. Analysis of Equine Gait Using Three-dimensional Accelerometers Fixed on the Saddle. Equine Veterinary J. 1994, 26, 44–47. [Google Scholar] [CrossRef]
- Giandolini, M.; Gimenez, P.; Temesi, J.; Arnal, P.J.; Martin, V.; Rupp, T.; Morin, J.-B.; Samozino, P.; Millet, G.Y. Effect of the Fatigue Induced by a 110-Km Ultramarathon on Tibial Impact Acceleration and Lower Leg Kinematics. PLoS ONE 2016, 11, e0151687. [Google Scholar] [CrossRef]
- Marotta, L.; Scheltinga, B.L.; van Middelaar, R.; Bramer, W.M.; van Beijnum, B.-J.F.; Reenalda, J.; Buurke, J.H. Accelerometer-Based Identification of Fatigue in the Lower Limbs during Cyclical Physical Exercise: A Systematic Review. Sensors 2022, 22, 3008. [Google Scholar] [CrossRef]
- Jiang, Y.; Hernandez, V.; Venture, G.; Kulić, D.; Chen, B.K. A Data-Driven Approach to Predict Fatigue in Exercise Based on Motion Data from Wearable Sensors or Force Plate. Sensors 2021, 21, 1499. [Google Scholar] [CrossRef]
- Strojnik, V.; Komi, P.V. Fatigue after Submaximal Intensive Stretch-Shortening Cycle Exercise. Med. Sci. Sports Exerc. 2000, 32, 1314–1319. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle Fatigue: What, Why and How It Influences Muscle Function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D. Warm Up II: Performance Changes Following Active Warm Up and How to Structure the Warm Up. Sports Med. 2003, 33, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.M. Warming Up Horses When Riding: Why and How. Available online: https://thehorse.com/190044/warming-up-horses-when-riding-why-and-how/ (accessed on 2 May 2022).


| CANTER | TROT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Axis | Position | p-Value | Before/Intra | Post/Inter | Variable | Axis | Position | p-Value | Before/Intra | Post/Inter |
| Acceleration | h | FH | 0.031 | 0.8 (0.06) | 0.76 (0.07) | Acceleration | x | St | 0.008 | 0.83 (0.06) | 0.77 (0.06) |
| x | FH | 0.016 | 0.94 (0.03) | 0.92 (0.04) | x | FH | 0.047 | 0.93 (0.03) | 0.91 (0.03) | ||
| x | Po | 0.008 | 0.92 (0.04) | 0.84(0.11) | x | Po | 0.047 | 0.93 (0.03) | 0.91 (0.03) | ||
| z | FH | 0.016 | 0.88 (0.06) | 0.81 (0.12) | z | Po | 0.004 | 0.94 (0.02) | 0.91 (0.03) | ||
| z | St | 0.039 | 0.92 (0.04) | 0.89 (0.03) | z | Sa | 0.031 | 0.96 (0.03) | 0.93 (0.05) | ||
| z | Po | 0.008 | 0.92 (0.05) | 0.87 (0.09) | v | St | 0.039 | 0.94 (0.04) | 0.93 (0.05) | ||
| v | St | 0.004 | 0.91 (0.04) | 0.87 (0.04) | v | Sa | 0.031 | 0.95 (0.03) | 0.93 (0.05) | ||
| Angle | x/v | Sa | 0.031 | 0.88 (0.25) | 0.86 (0.26) | Angle | x/v | Sa | 0.031 | 0.82 (0.13) | 0.69 (0.24) |
| x/v | St | 0.027 | 0.96 (0.03) | 0.95 (0.04) | x/v | FC | 0.039 | 0.84 (0.17) | 0.51 (0.5) | ||
| Angular Velocity | y | FH | 0.016 | 0.87 (0.09) | 0.83 (0.11) | x/v | St | 0.004 | 0.9 (0.07) | 0.82 (0.15) | |
| y | Sa | 0.031 | 0.82 (0.27) | 0.76 (0.28) | Angular Velocity | y | Sa | 0.031 | 0.84 (0.08) | 0.76 (0.12) | |
| y | St | 0.02 | 0.89 (0.07) | 0.85 (0.08) | y | St | 0.008 | 0.89 (0.07) | 0.83 (0.13) | ||
| y | Po | 0.004 | 0.82 (0.12) | 0.75 (0.2) | |||||||
| CANTER | TROT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Axis | Position | p-Value | Before/Intra | Post/Inter | Variable | Axis | Position | p-Value | Before/Intra | Post/Inter |
| Acceleration | v | Po | 0.02 | −6.8 (1.6) | −6.2 (1.2) | Acceleration | v | FC | 0.039 | −7.5 (1.8) | −8.5 (1.6) |
| Angle | Swing | HC | 0.004 | −2.4 (0) | −2.4 (0) | Angle | Twist | HC | 0.004 | −2.1 (0.1) | −2.4 (0.1) |
| y/v | FH | 0.031 | −2.4 (0) | −2.4 (0) | Angular Velocity | y | Po | 0.008 | −2.7 (0.2) | −2.5 (0.2) | |
| z/v | HC | 0.004 | −2.4 (0) | −2.4 (0) | z | St | 0.012 | −2.5 (0.2) | −2.7 (0.3) | ||
| Angular Velocity | y | Sa | 0.031 | −2.3 (0.2) | −2.4 (0.3) | ||||||
| Norm | FH | 0.016 | −2.3 (0.1) | −2.2 (0.1) | |||||||
| CANTER | TROT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Axis | Position | p-Value | Before/Intra | Post/Inter | Variable | Axis | Position | p-Value | Before/Intra | Post/Inter |
| Acceleration (m/s−2) | h | FC | 0.047 | 24.7 (1.8) | 29.1 (3.9) | Acceleration (m/s−2) | h | FC | 0.016 | 12.8 (2.7) | 17.4 (4) |
| h | HC | 0.02 | 22 (2.3) | 24.7 (2.5) | h | HC | 0.016 | 10.1 (2) | 13.6 (4.6) | ||
| h | FH | 0.031 | 3.5 (0.9) | 4.3 (0.7) | h | FH | 0.016 | 2.7 (0.9) | 3.2 (1) | ||
| h | St | 0.004 | 3.1 (0.4) | 3.7 (0.8) | h | Sa | 0.031 | 2.8 (0.2) | 3.4 (0.3) | ||
| x | FC | 0.031 | 24.4 (3) | 30.3 (5.1) | h | St | 0.008 | 2.7 (0.4) | 3.1 (0.5) | ||
| x | HC | 0.027 | 21.8 (2.3) | 25.5 (4.1) | h | Po | 0.008 | 4.5 (1.1) | 5.1 (1) | ||
| x | FH | 0.016 | 3.2 (0.8) | 3.8 (0.9) | x | FC | 0.02 | 12.6 (3.3) | 17.3 (5.2) | ||
| x | Sa | 0.031 | 3.7 (1.1) | 4.9 (1) | x | HC | 0.016 | 10.9 (2.8) | 15.1 (5.3) | ||
| x | St | 0.008 | 3.5 (0.5) | 4.3 (0.7) | x | FH | 0.016 | 3.1 (0.5) | 3.6 (0.7) | ||
| x | Po | 0.004 | 4.1 (1.2) | 5.4 (1.8) | x | Sa | 0.031 | 2.2 (0.3) | 3 (0.8) | ||
| y | FC | 0.031 | 36.2 (4.2) | 42.6 (4.6) | x | St | 0.008 | 2.7 (0.5) | 3.2 (0.6) | ||
| y | HC | 0.02 | 28.2 (2.7) | 32.9 (4.7) | x | Po | 0.004 | 3.3 (0.5) | 4.2 (0.8) | ||
| y | St | 0.012 | 3.9 (0.5) | 4.4 (0.7) | y | FC | 0.027 | 21.3 (5.1) | 29.6 (9) | ||
| z | FC | 0.039 | 15.2 (3.1) | 16.7 (2.5) | y | HC | 0.039 | 15.2 (4.1) | 21.3 (8.1) | ||
| z | HC | 0.039 | 13.9 (1.3) | 15.4 (1.8) | y | FH | 0.016 | 2.2 (0.6) | 2.8 (1) | ||
| z | FH | 0.016 | 2.3 (1) | 2.8 (1) | y | St | 0.004 | 2.3 (0.5) | 3.1 (1) | ||
| z | St | 0.039 | 2.7 (0.5) | 3.1 (0.3) | y | Po | 0.004 | 2.3 (0.4) | 3 (0.8) | ||
| z | Po | 0.004 | 2.6 (0.8) | 3.5 (1.2) | z | FC | 0.016 | 8.7 (1.4) | 10.5 (1.6) | ||
| v | FC | 0.031 | 29.6 (1.8) | 35.8 (5.2) | z | HC | 0.039 | 8.3 (1.4) | 10.3 (3.2) | ||
| v | HC | 0.039 | 28 (2.6) | 32.7 (4.6) | z | FH | 0.016 | 2 (0.5) | 2.7 (0.8) | ||
| v | St | 0.004 | 2.8 (0.5) | 3.5 (0.5) | z | Sa | 0.031 | 2 (0.5) | 2.7 (0.6) | ||
| v | Po | 0.039 | 6.6 (3) | 7.3 (2.7) | z | St | 0.012 | 2.1 (0.4) | 2.4 (0.5) | ||
| Angle (°) | Swing | FC | 0.031 | 8.1 (4.7) | 10 (5.8) | z | Po | 0.004 | 2.1 (0.4) | 2.7 (0.4) | |
| Twist | FC | 0.008 | 65.6 (7.7) | 149.1 (77.9) | v | FC | 0.031 | 25.7 (3.1) | 29.4 (3.5) | ||
| x/v | FC | 0.016 | 15.3 (6) | 17.5 (6.6) | v | HC | 0.039 | 21.7 (1.4) | 25.2 (3.4) | ||
| x/v | HC | 0.004 | 10.9 (1.6) | 12.8 (2.1) | v | Sa | 0.031 | 2.2 (0.4) | 2.8 (0.5) | ||
| x/v | St | 0.008 | 3.2 (1.2) | 4.7 (1.6) | v | St | 0.02 | 2.2 (0.3) | 2.5 (0.4) | ||
| y/v | HC | 0.039 | 16.1 (2.8) | 17.9 (2.7) | v | Po | 0.02 | 4.4 (2.1) | 5 (2) | ||
| y/v | Po | 0.02 | 4.7 (1.1) | 6 (2) | Angle (°) | Swing | HC | 0.02 | 4.5 (0.9) | 7 (2.9) | |
| z/v | FC | 0.031 | 8.1 (4.7) | 10 (5.8) | Twist | FC | 0.016 | 88.3 (10.7) | 122 (40.7) | ||
| z/v | Sa | 0.031 | 2.9 (0.9) | 3.3 (0.9) | Twist | HC | 0.039 | 51.3 (18.8) | 112.5 (67.3) | ||
| z/v | St | 0.02 | 3.4 (1.2) | 4.5 (1.6) | x/v | FC | 0.016 | 5.6 (2.8) | 16.9 (16.4) | ||
| Angular Velocity (rad/s) | x | FC | 0.031 | 2.5 (0.4) | 3.1 (0.6) | x/v | HC | 0.008 | 5 (2.8) | 9.4 (5.9) | |
| x | HC | 0.02 | 2.2 (0.4) | 2.6 (0.6) | x/v | St | 0.004 | 2.3 (0.5) | 3.7 (1.6) | ||
| x | FH | 0.047 | 0.6 (0.1) | 0.7 (0.1) | y/v | FC | 0.016 | 8.4 (7.7) | 24.6 (21.4) | ||
| x | St | 0.004 | 0.6(0.1) | 0.7(0.1) | y/v | Sa | 0.031 | 4.9 (1.3) | 6 (1.8) | ||
| y | FC | 0.02 | 1.3 (0.2) | 1.5 (0.3) | y/v | Po | 0.02 | 3.4 (0.8) | 4.7 (2.6) | ||
| y | HC | 0.02 | 1.2 (0.3) | 1.4 (0.4) | z/v | FC | 0.012 | 4.9 (2) | 6.8 (2.4) | ||
| y | FH | 0.016 | 0.5 (0.2) | 0.6 (0.2) | z/v | HC | 0.02 | 4.5 (0.9) | 7 (2.9) | ||
| y | Sa | 0.031 | 0.5 (0.3) | 0.6 (0.3) | z/v | FH | 0.031 | 5.5 (1.2) | 8.2 (5) | ||
| y | St | 0.008 | 0.4 (0.1) | 0.4 (0.1) | z/v | Sa | 0.031 | 2.5 (0.7) | 3.7 (1.5) | ||
| y | Po | 0.02 | 0.5 (0.2) | 0.6 (0.2) | z/v | St | 0.004 | 2.6 (0.5) | 4.4 (1.7) | ||
| z | Sa | 0.031 | 0.4 (0.1) | 0.5 (0.1) | z/v | Po | 0.008 | 4.9 (0.9) | 7.7 (3.6) | ||
| Angular Velocity (rad/s) | x | FC | 0.016 | 1.7 (0.4) | 2.4 (0.9) | ||||||
| x | HC | 0.008 | 1.3 (0.4) | 1.9 (0.9) | |||||||
| x | FH | 0.016 | 0.5 (0.1) | 0.6 (0.3) | |||||||
| x | St | 0.004 | 0.4 (0.1) | 0.6 (0.3) | |||||||
| x | Po | 0.004 | 0.4 (0.1) | 0.5 (0.1) | |||||||
| y | FC | 0.031 | 0.8 (0.3) | 1.2 (0.4) | |||||||
| y | HC | 0.023 | 0.7 (0.2) | 0.9 (0.5) | |||||||
| y | FH | 0.031 | 0.4 (0.1) | 0.5 (0.2) | |||||||
| y | Sa | 0.031 | 0.3 (0) | 0.3 (0) | |||||||
| y | St | 0.008 | 0.3 (0.1) | 0.4 (0.1) | |||||||
| z | FC | 0.031 | 1.6 (1) | 3.6 (2.8) | |||||||
| z | HC | 0.02 | 1.4 (0.7) | 2.4 (1.7) | |||||||
| z | FH | 0.016 | 0.3 (0.1) | 0.4 (0.1) | |||||||
| z | St | 0.02 | 0.3 (0.1) | 0.6 (0.4) | |||||||
| z | Po | 0.004 | 0.4 (0.1) | 0.5 (0.2) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquiet, B.; Biau, S.; Trébot, Q.; Debril, J.-F.; Durand, F.; Fradet, L. Detection of Horse Locomotion Modifications Due to Training with Inertial Measurement Units: A Proof-of-Concept. Sensors 2022, 22, 4981. https://doi.org/10.3390/s22134981
Pasquiet B, Biau S, Trébot Q, Debril J-F, Durand F, Fradet L. Detection of Horse Locomotion Modifications Due to Training with Inertial Measurement Units: A Proof-of-Concept. Sensors. 2022; 22(13):4981. https://doi.org/10.3390/s22134981
Chicago/Turabian StylePasquiet, Benoît, Sophie Biau, Quentin Trébot, Jean-François Debril, François Durand, and Laetitia Fradet. 2022. "Detection of Horse Locomotion Modifications Due to Training with Inertial Measurement Units: A Proof-of-Concept" Sensors 22, no. 13: 4981. https://doi.org/10.3390/s22134981
APA StylePasquiet, B., Biau, S., Trébot, Q., Debril, J.-F., Durand, F., & Fradet, L. (2022). Detection of Horse Locomotion Modifications Due to Training with Inertial Measurement Units: A Proof-of-Concept. Sensors, 22(13), 4981. https://doi.org/10.3390/s22134981






