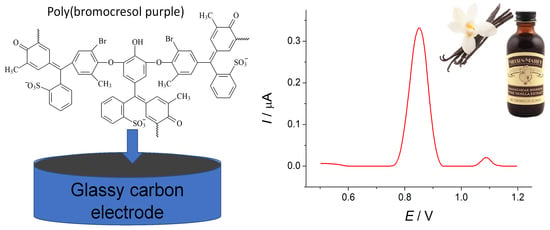
Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Procedures
2.3.1. Sensor Preparation
2.3.2. Electrochemical Measurements
2.3.3. Vanilla Planifolia Extracts Analysis
2.3.4. Statistical Analysis
3. Results and Discussion
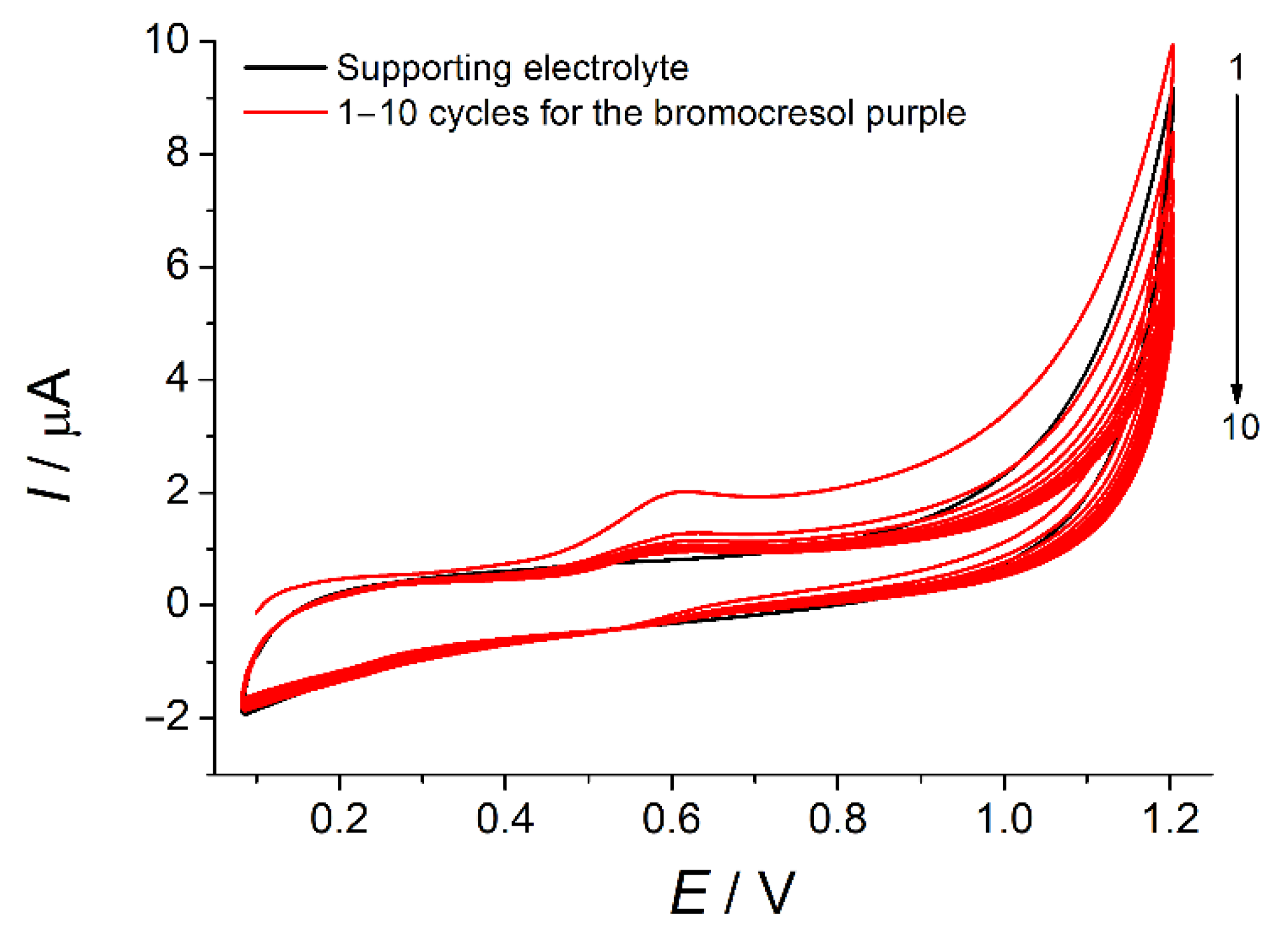
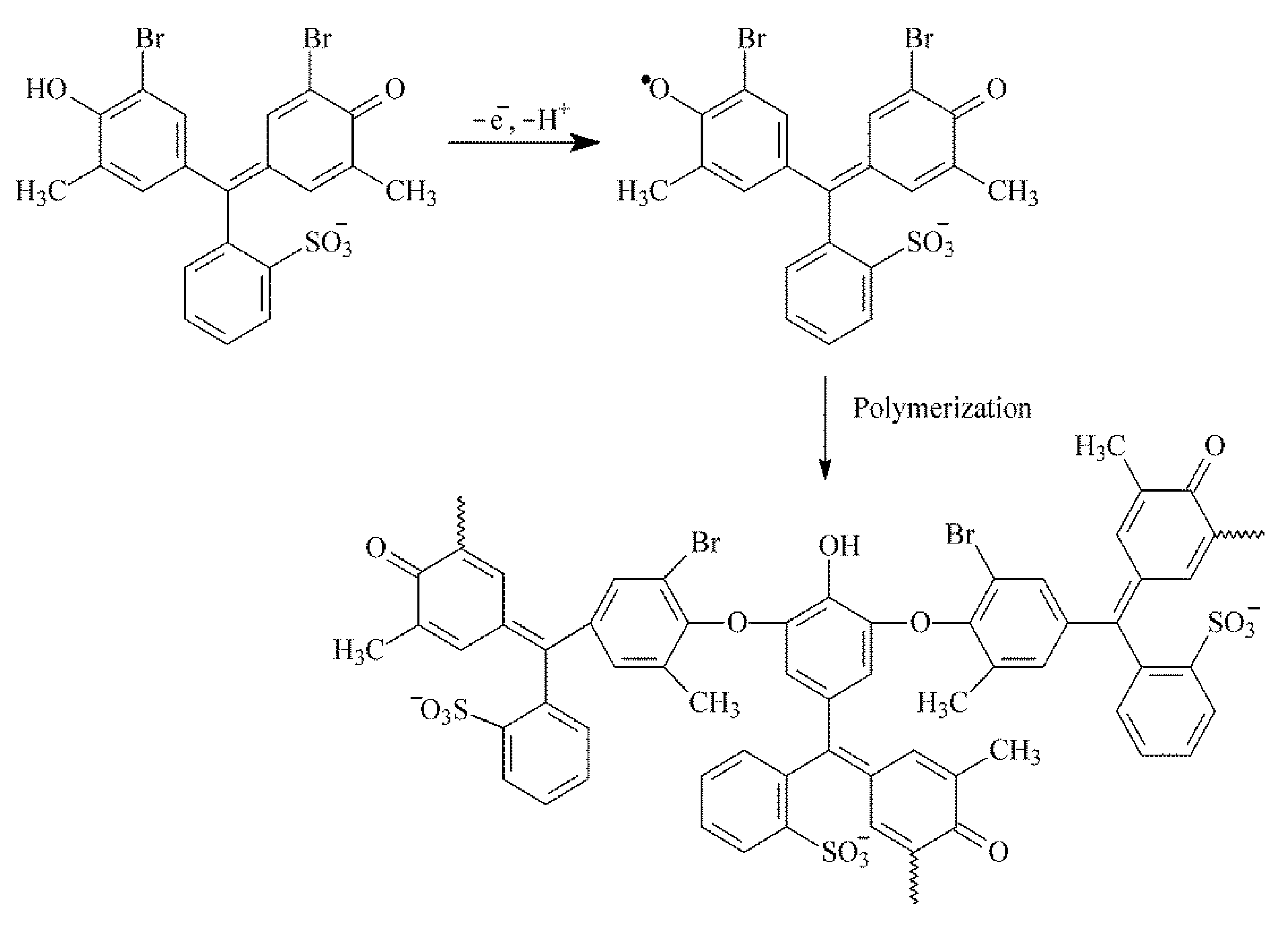
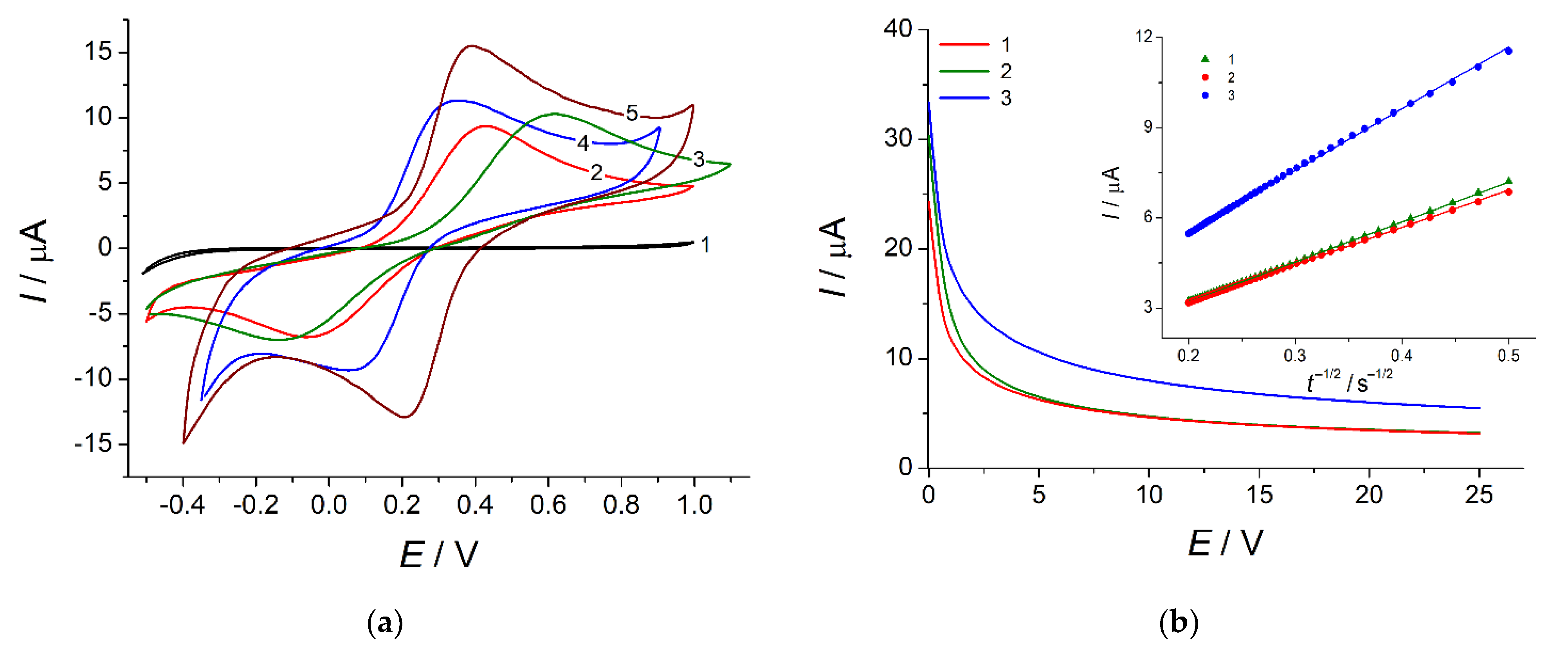
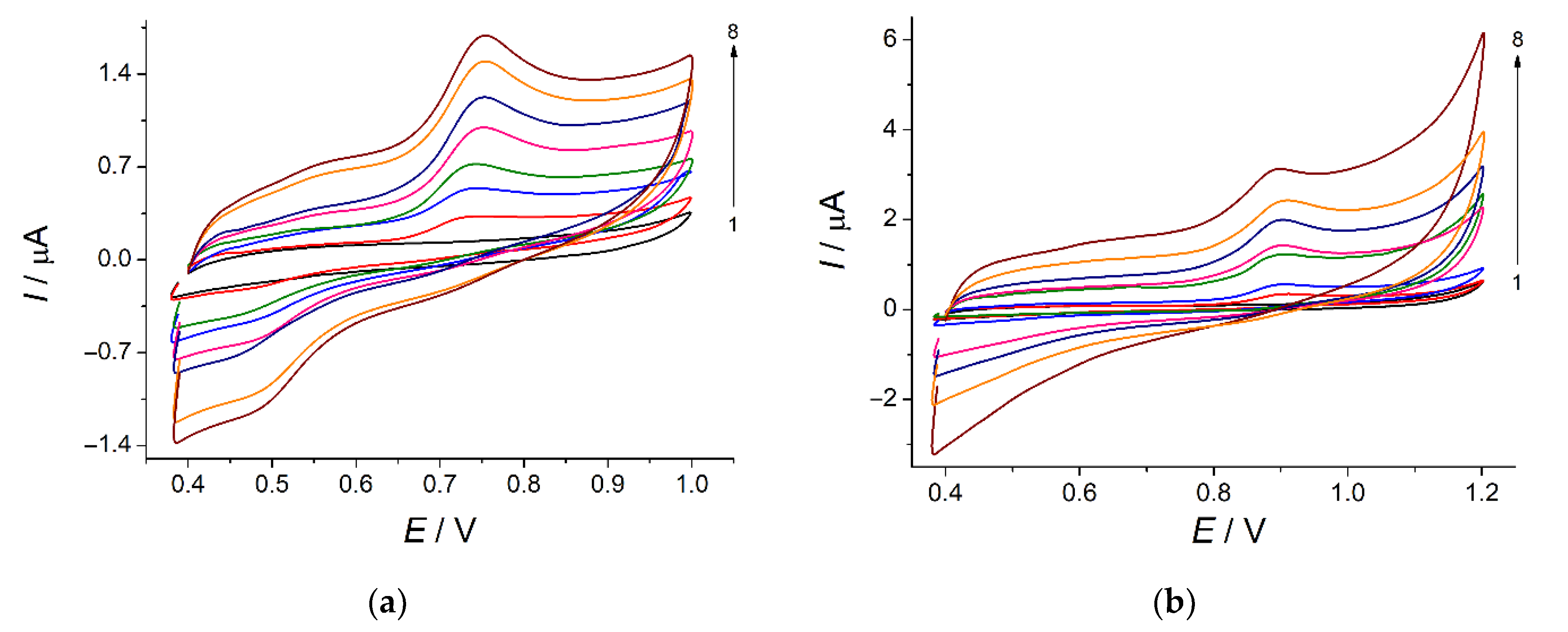
3.1. Electropolymerization of Bromocresol Purple and Its Optimization
3.2. Characterization of the Electrodes
3.2.1. Electrode Surface Morphology
3.2.2. Effective Surface Area of the Electrodes
3.2.3. Electron Transfer Properties
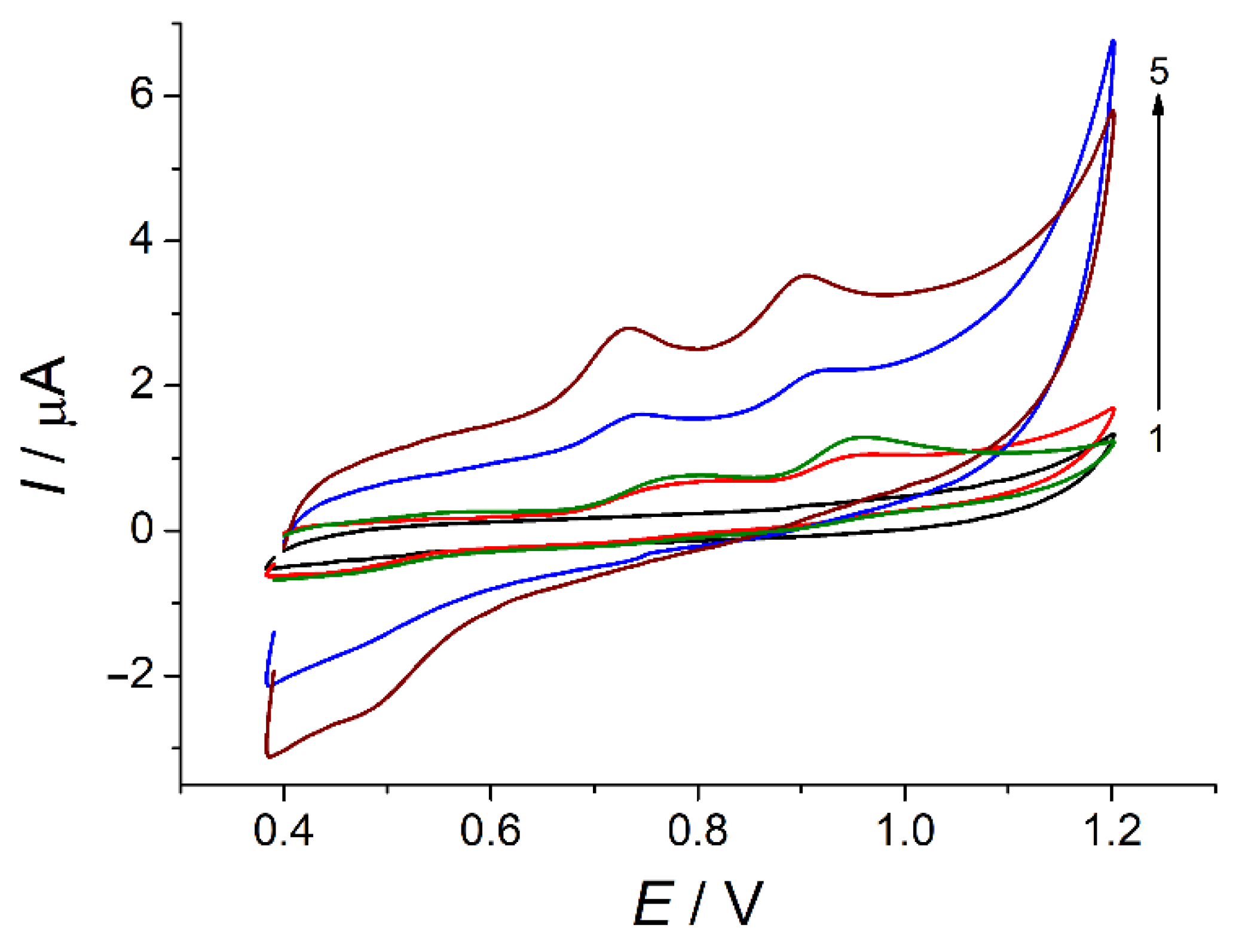
3.3. Cyclic Voltammetry of Ferulic Acid and Vanillin on Bare and Modified Electrodes
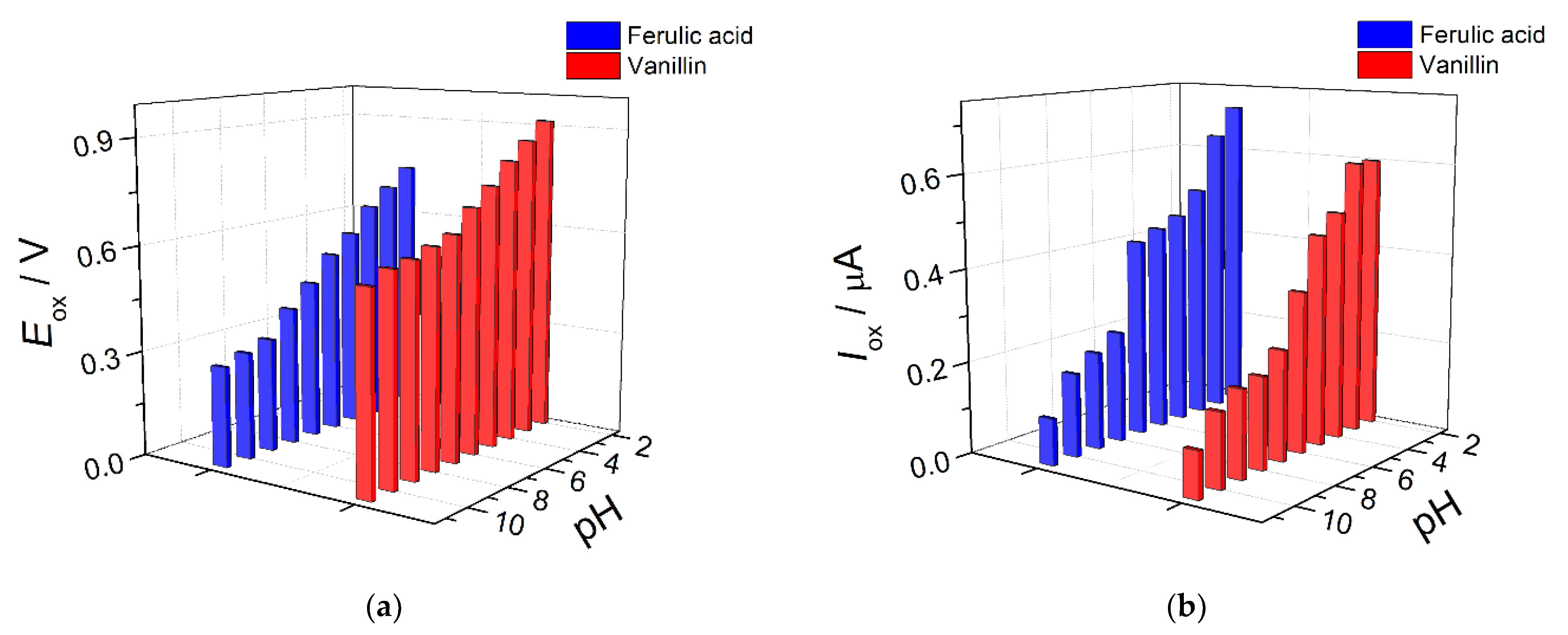
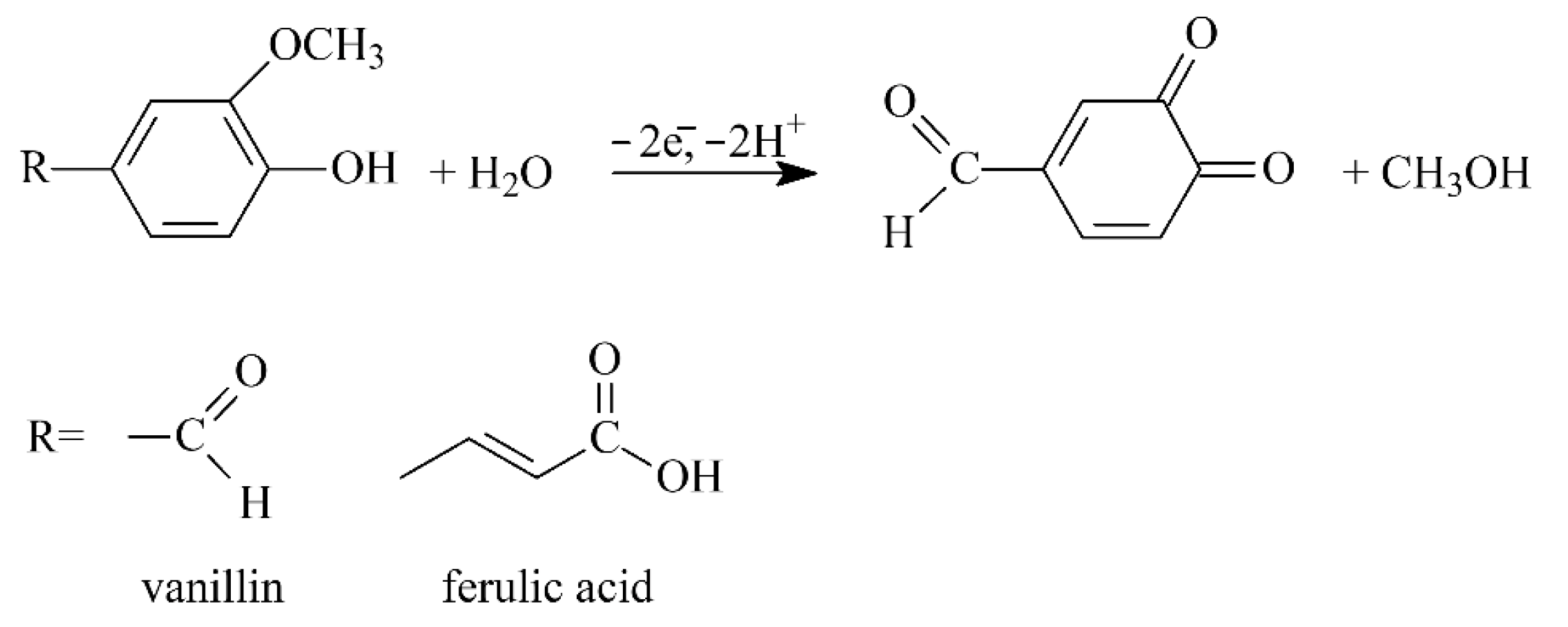
3.4. Electrooxidation of Ferulic Acid and Vanillin on Polymer-Based Electrode
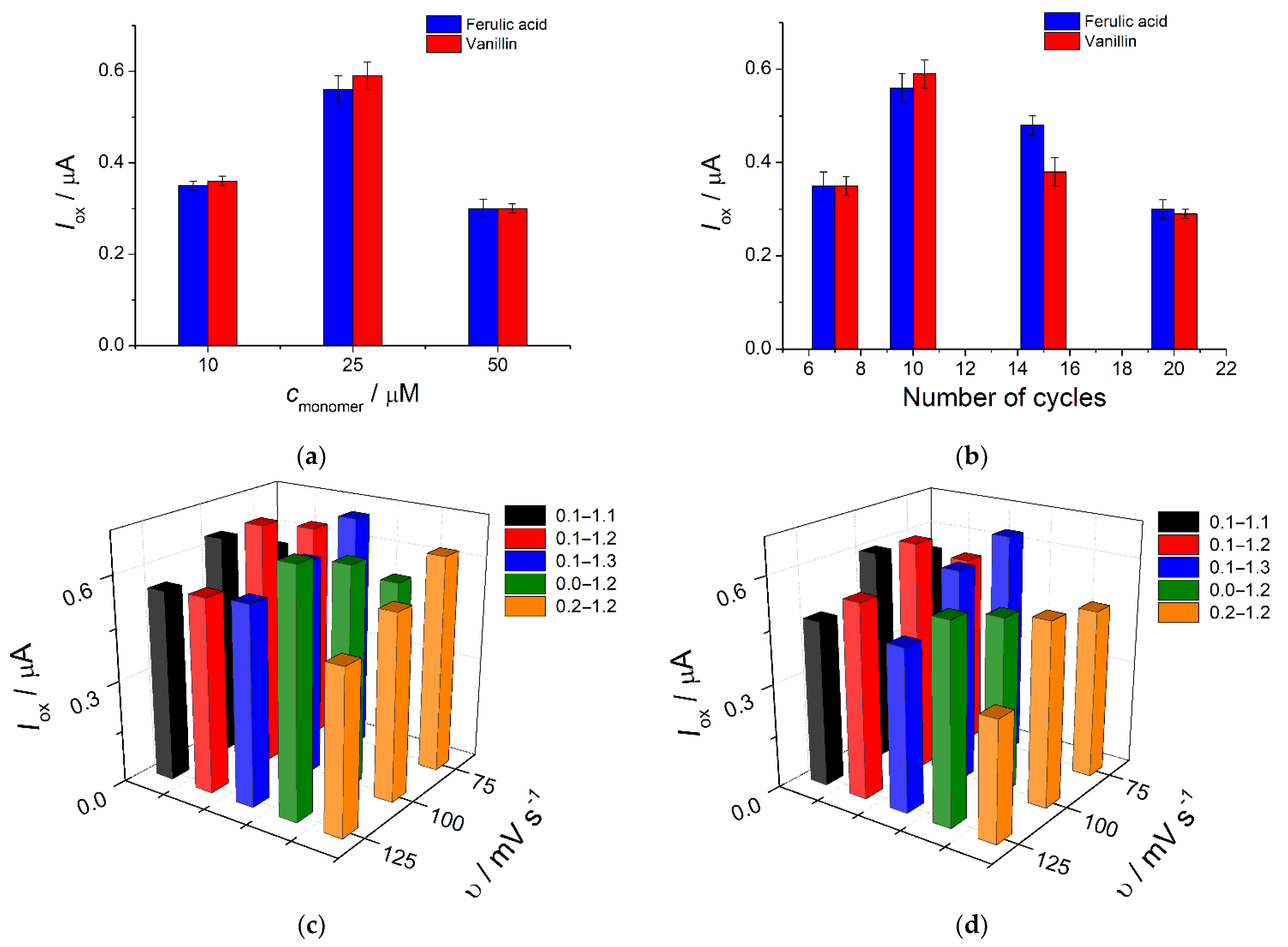
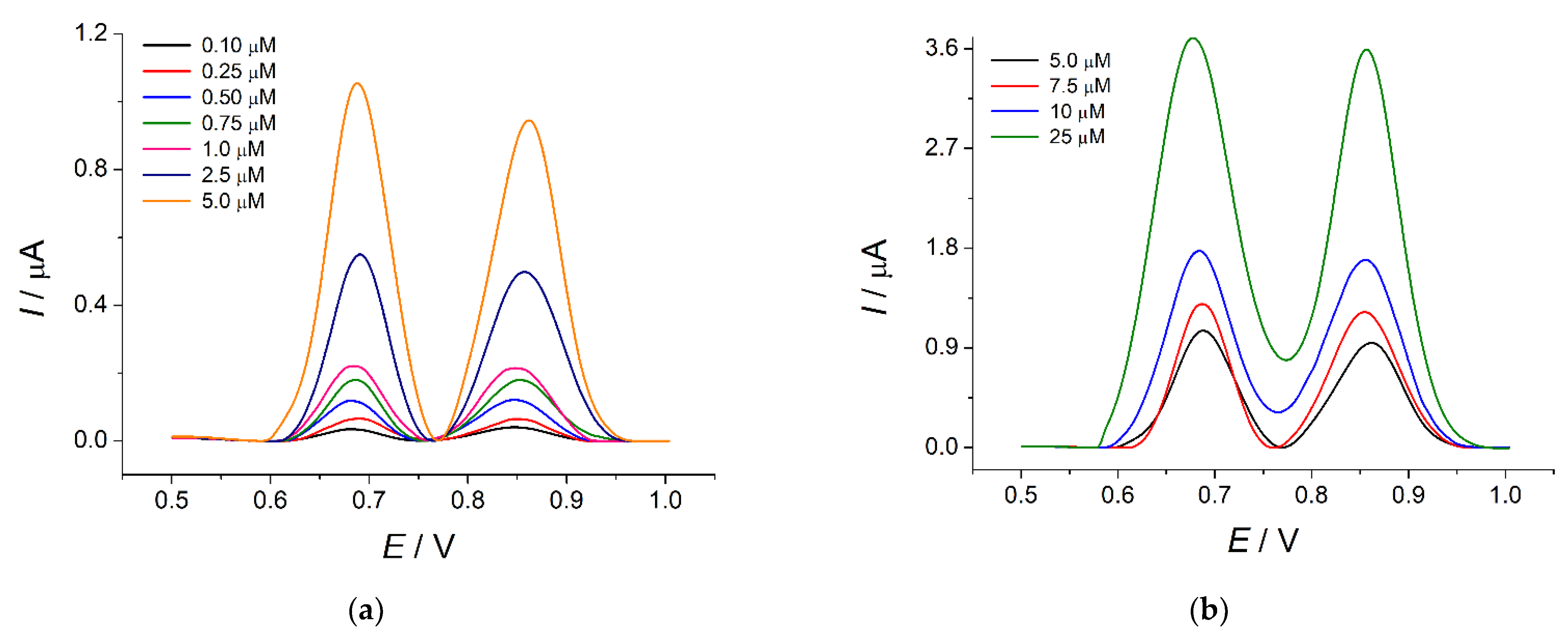
3.5. Analytical Characterization of Poly(Bromocresol Purple)-Based Sensor
3.5.1. Repeatability, Reproducibility and Robustness of Sensor Response
3.5.2. Interference Study
3.6. Application of the Sensor to Vanilla Planifolia Extract Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health Bene55fits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste, 2nd ed.; Vuong, Q.V., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 2; pp. 27–59. [Google Scholar]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [Green Version]
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of essential oils from medicinal plants and their utilization as food antioxidants. Curr. Pharm. Des. 2020, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Budnikov, H.C. Spice antioxidants as objects of analytical chemistry. J. Anal. Chem. 2018, 73, 946–965. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Kosar, M.; Öz, A.T.; Mitchell, A.E. Advanced analytical methods for phenolics in fruits. J. Food Qual. 2018, 2018, 3836064. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Samanidou, V.F. Advances in the optimization of chromatographic conditions for the separation of antioxidants in functional foods. Rev. Sep. Sci. 2019, 1, 17–33. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Huck, C.W.; Stecher, G.; Scherz, H.; Bonn, G. Analysis of drugs, natural and bioactive compounds containing phenolic groups by capillary electrophoresis coupled to mass spectrometry. Electrophoresis 2005, 26, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Česla, P.; Fischer, J.; Jandera, P. Separation of phenolic acids and flavone natural antioxidants by two-dimensional method combining liquid chromatography and micellar electrokinetic capillary chromatography. Electrophoresis 2010, 31, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; Gil, E.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Budnikov, H. Poly(gallic acid)/MWNT-modified electrode for the selective and sensitive voltammetric determination of quercetin in medicinal herbs. J. Electroanal. Chem. 2018, 821, 73–81. [Google Scholar] [CrossRef]
- Kumar, D.R.; Sayed, M.S.; Baynosa, M.L.; Shim, J.-J. 5-Amino-2-mercapto-1,3,4-thiadiazole coated nitrogen-doped-carbon sphere composite for the determination of phenolic compounds. Microchem. J. 2020, 157, 105023. [Google Scholar] [CrossRef]
- Selvi, B.; Sadikoglu, M.; Soylu, U.I.; Yılmaz, S.; Onal, A.; Eser, F. Sensitive determination of quercetin in onion peel by voltammetry using a poly(4-aminobenzene sulfonic acid) modified glassy carbon electrode. Anal. Bioanal. Electrochem. 2017, 9, 574–585. [Google Scholar]
- Ziyatdinova, G.K.; Guss, E.V.; Morozova, E.V.; Budnikov, H.C. An electrode based on electropolymerized sunset yellow for the simultaneous voltammetric determination of chlorogenic and ferulic acids. J. Anal. Chem. 2021, 76, 371–380. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Morozova, E.; Budnikov, H.; Davletshin, R.; Vorobev, V.; Osin, Y. Simultaneous voltammetric determination of gallic and ellagic acids in cognac and brandy using electrode modified with functionalized SWNT and poly(pyrocatechol violet). Food Anal. Methods 2019, 12, 2250–2261. [Google Scholar] [CrossRef]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Guss, E.V.; Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Voltammetric determination of quercetin and rutin in their simultaneous presence on an electrode modified with polythymolphthalein. J. Anal. Chem. 2020, 75, 526–535. [Google Scholar] [CrossRef]
- Gallage, N.J.; Hansen, E.H.; Kannangara, R.; Olsen, C.E.; Motawia, M.S.; Jørgensen, K.; Holme, I.; Hebelstrup, K.; Grisoni, M.; Møller, B.L. Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 2014, 5, 4037. [Google Scholar] [CrossRef] [Green Version]
- Negishi, O.; Sugiura, K.; Negishi, Y. Biosynthesis of vanillin via ferulic acid in Vanilla planifolia. J. Agric. Food Chem. 2009, 57, 9956–9961. [Google Scholar] [CrossRef] [PubMed]
- Ranadive, A.S. Vanillin and related flavor compounds in vanilla extracts made from beans of various global origins. J. Agric. Food Chem. 1992, 40, 1922–1924. [Google Scholar] [CrossRef]
- Sinha, A.K.; Verma, S.C.; Sharma, U.K. Development and validation of an RP-HPLC method for quantitative determination of vanillin and related phenolic compounds in Vanilla planifolia. J. Sep. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hingse, S.S.; Digole, S.B.; Annapure, U.S. Method development for simultaneous detection of ferulic acid and vanillin using high-performance thin layer chromatography. J. Anal. Sci. Technol. 2014, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; 367p. [Google Scholar]
- Saritha, D.; Reddy, A.V.B.; Venu, M.; Madhuri, C.; Kiranmai, S.; Anitha, K.; Madhavi, G. Fabrication, characterization and development of a modified poly(bromocresol purple/multiwalled carbon nanotubes) carbon paste electrode for the determination of sulfanilic acid. Anal. Bioanal. Electrochem. 2019, 11, 123–136. [Google Scholar]
- Yang, G.-J.; Qu, X.-L.; Zhu, A.-P.; Wang, C.-Y.; Qu, Q.-S.; Hu, X.-Y. Characterization, growth mechanism and application of network poly(bromophenol blue). J. Electroanal. Chem. 2007, 604, 48–56. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; 864p. [Google Scholar]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Selective electrochemical sensor based on the electropolymerized p-coumaric acid for the direct determination of L-cysteine. Electrochimica Acta 2018, 270, 369–377. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef]
- Monnappa, A.B.; Manjunatha, J.G.G.; Bhatt, A.S.; Nagarajappa, H.. Sensitive and selective electrochemical detection of vanillin at graphene based poly (methyl orange) modified electrode. J. Sci.: Adv. Mater. Devices 2021, 6, 415–424. [Google Scholar]
- Calam, T.T. Voltammetric determination and electrochemical behavior of vanillin based on 1H-1,2,4-triazole-3-thiol polymer film modified gold electrode. Food Chem. 2020, 328, 127098. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Aytuganova, I.; Nizamova, A.; Budnikov, H. Differential pulse voltammetric assay of coffee antioxidant capacity with MWNT-modified electrode. Food Anal. Methods 2013, 6, 1629–1638. [Google Scholar] [CrossRef]
- Trabelsi, S.K.; Tahar, N.B.; Trabelsi, B.; Abdelhedi, R. Electrochemical oxidation of ferulic acid in aqueous solutions at gold oxide and lead dioxide electrodes. J. Appl. Electrochem. 2005, 35, 967–973. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Tang, Z.; Wang, H.; Deng, P.; Tang, Y. Facile Fabrication of CeO2/Electrochemically Reduced Graphene Oxide Nanocomposites for Vanillin Detection in Commercial Food Products. Nanomaterials 2020, 10, 1356. [Google Scholar] [CrossRef]
- Nie, X.; Deng, P.; Zhang, R.; Tang, Z.; Wang, H.; Tang, Y. Nickel nanoparticles supported on nitrogen-doped carbon for vanillin detection. ACS Appl. Nano Mater. 2020, 3, 11791–11800. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Ziganshina, E.; Budnikov, H. Surfactant/carbon nanofibers-modified electrode for the determination of vanillin. Monatsh. Chem. 2016, 147, 191–200. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Antonova, T.S.; Mubarakova, L.R.; Budnikov, H. An amperometric sensor based on tin dioxide and cetylpyridinium bromide nanoparticles for the determination of vanillin. J. Anal. Chem. 2018, 73, 801–808. [Google Scholar] [CrossRef]
- Velasco, J.G. Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 1997, 9, 880–882. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Voltammetric determination of ferulic acid using polypyrrole-multiwalled carbon nanotubes modified electrode with sample application. Nanomaterials 2015, 5, 1704–1715. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. A molecularly imprinted polymer on reduced graphene oxide-gold nanoparticles modified screen-printed electrode for selective determination of ferulic acid in orange peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Calam, T.T.; Uzun, D. Rapid and selective determination of vanillin in the presence of caffeine, its electrochemical behavior on an Au electrode electropolymerized with 3-Amino-1,2,4-triazole-5-thiol. Electroanalysis 2019, 31, 2347–2358. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Mümin, Y. Simultaneous electrochemical determination of caffeine and vanillin by using poly(alizarin red S) modified glassy carbon electrode. Food Anal. Methods 2017, 10, 31–40. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Tigari, G.; Fattepur, S. Poly(niacin) based carbon nanotube sensor for the sensitive and selective voltammetric detection of vanillin with caffeine. Anal. Bioanal. Electrochem. 2020, 12, 553–568. [Google Scholar]
- Hareesha, N.; Manjunatha, J.G.; Amrutha, B.M.; Sreeharsha, N.; Asdaq, S.M.B.; Anwer, M.K. A fast and selective electrochemical detection of vanillin in food samples on the surface of poly(glutamic acid) functionalized multiwalled carbon nanotubes and graphite composite paste sensor. Colloids Surf. A Physicochem. 2021, 626, 127042. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J.G.; D’Souza, E.S.; Sreeharsha, N. Surfactant and polymer composite modified electrode for the sensitive determination of vanillin in food sample. ChemistrySelect 2021, 6, 2700–2708. [Google Scholar] [CrossRef]
- Wu, W.; Yang, L.; Zhao, F.; Zeng, B. A vanillin electrochemical sensor based on molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus)−multi-walled carbon nanotubes@polydopamine–carboxyl single-walled carbon nanotubes composite. Sens. Actuat. B 2017, 239, 481–487. [Google Scholar] [CrossRef]
- Reichenbächer, M.; Einax, J.W. Challenges in Analytical Quality Assurance; Springer: Berlin/Heidelberg, Germany, 2011; 375p. [Google Scholar]



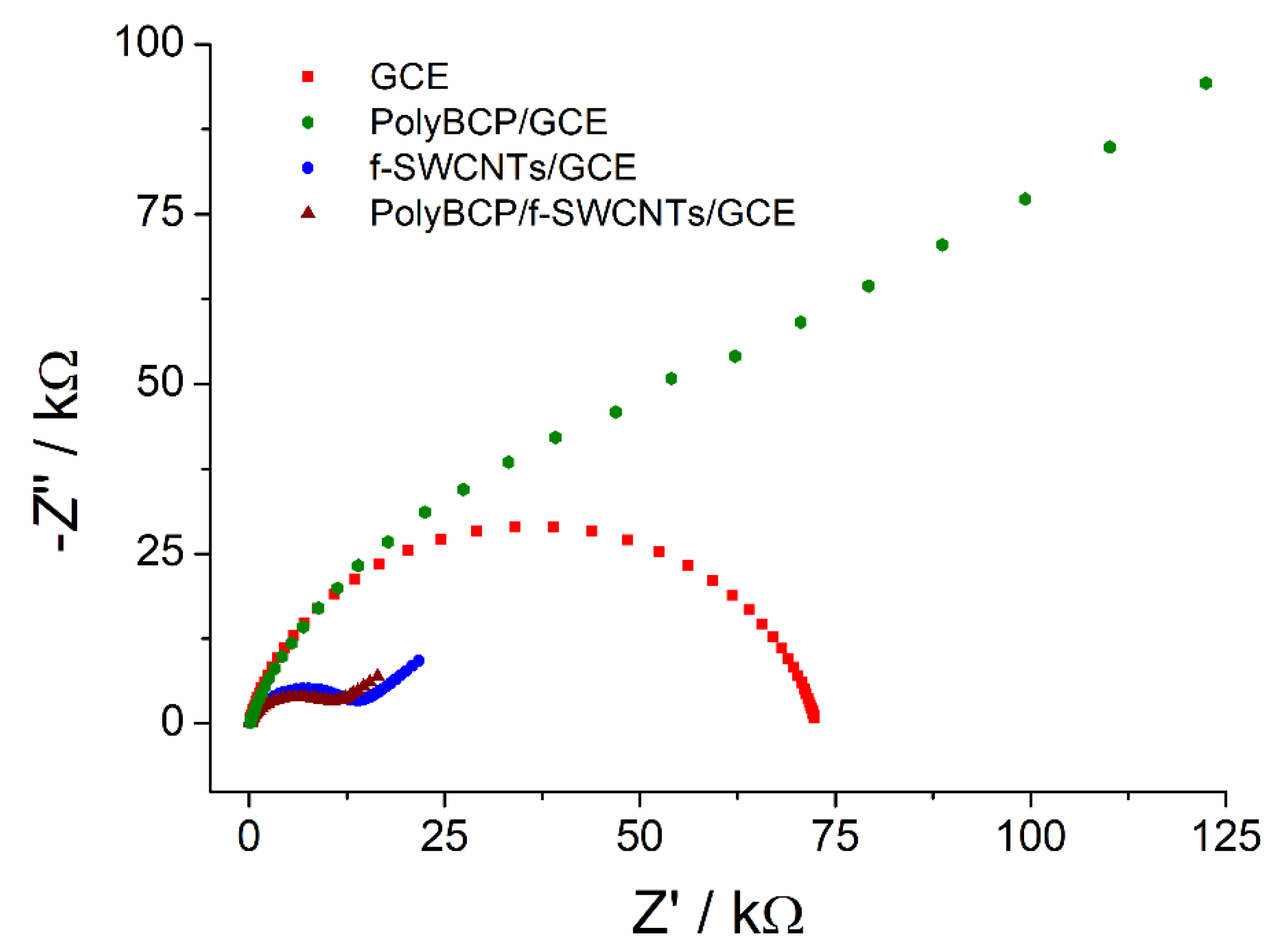


| Electrode | Rs/Ω | Rct/kΩ | Q/µΩ−1 | n | W/µΩ−1 | Χ2 |
|---|---|---|---|---|---|---|
| GCE | 101 ± 6 | 72.5 ± 0.9 | 0.45 ± 0.05 | 0.860 | - | 0.025 |
| PolyBCP/GCE | 105 ± 3 | 84 ± 1 | 0.73 ± 0.06 | 0.822 | 5.3 ± 0.1 | 0.027 |
| f-SWCNTs/GCE | 109 ± 2 | 12.4 ± 0.5 | 2.20 ± 0.05 | 0.819 | 152 ± 4 | 0.033 |
| PolyBCP/f-SWCNTs/GCE | 104 ± 1 | 10.1 ± 0.2 | 5.4 ± 0.1 | 0.811 | 199 ± 1 | 0.021 |
| Parameter | Ferulic Acid | Vanillin |
|---|---|---|
| I (µA) = a + bυ1/2 (mV s−1) | ||
| a ± SD | −0.08 ± 0.01 | −0.06 ± 0.02 |
| b ± SD | 0.058 ± 0.002 | 0.060 ± 0.002 |
| R2 | 0.9950 | 0.9901 |
| lnI (µA) = a + blnυ (V s−1) | ||
| a ± SD | 0.80 ± 0.08 | 0.71 ± 0.08 |
| b ± SD | 0.66 ± 0.03 | 0.58 ± 0.03 |
| R2 | 0.9904 | 0.9886 |
| Sensor | Detection Mode | Limit of Detection/µM | Linear Dynamic Range/µM | Refs. |
|---|---|---|---|---|
| Ferulic acid | ||||
| Poly(sunset yellow)/Multi-walled carbon nanotubes/GCE | DPV 1 | 0.098 | 0.5–4.0 | [16] |
| Polypyrrole-multi-walled carbon nanotubes/GCE | AdASWV 2 | 1.17 | 3.32–25.9 | [40] |
| Molecularly imprinted poly(phenol) film/Au nanoparticles/Reduced graphene oxide/SPE 3 | DPV | 0.0031 | 0.010–1.0 | [41] |
| Vanillin | ||||
| Poly(methyl orange)/Graphene paste electrode | DPV | 0.0735 | 10–35 and 40–100 | [31] |
| Poly(1H-1,2,4-triazole-3-thiol)/Gold electrode | DPV | 0.04 | 0.1–11.3 | [32] |
| Poly(3-amino-1,2,4-triazole-5-thiol/ Gold electrode | DPV | 0.19 | 1.1–76.5 | [42] |
| Poly(Alizarin Red S)/GCE | SWV 4 | 0.06 | 0.5–20 and 20–250 | [43] |
| Polyniacin/Multi-walled carbon nanotubes paste electrode | CV 5 | 1.01 | 2–35 and 40–80 | [44] |
| Poly(glutamic acid)/Multi-walled carbon nanotubes and graphite paste electrode | DPV | 0.0199 | 0.50–18.0 | [45] |
| Octoxynol-9/Poly(titan yellow)/Carbon nanotubes paste electrode | DPV | 0.049 | 2.0–40 | [46] |
| Molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus)−multi-walled carbon nanotubes@polydopamine–carboxylated single-walled carbon nanotubes/ | DPV | 0.1 | 0.2–10 | [47] |
| Added/µM | Found Ferulic Acid/µM | RSD/% | R/% | Found Vanillin/µM | RSD/% | R/% |
|---|---|---|---|---|---|---|
| 0.10 | 0.099 ± 0.006 | 4.7 | 99.0 | 0.099 ± 0.005 | 4.3 | 99.0 |
| 0.75 | 0.75 ± 0.04 | 4.6 | 100 | 0.75 ± 0.03 | 3.0 | 100 |
| 2.5 | 2.50 ± 0.04 | 1.7 | 100 | 2.49 ± 0.05 | 1.5 | 99.6 |
| 7.5 | 7.5 ± 0.3 | 2.7 | 100 | 7.48 ± 0.09 | 0.98 | 99.7 |
| 25 | 25.0 ± 0.5 | 0.83 | 100 | 25.0 ± 0.2 | 0.38 | 100 |
| Sample | Spiked/mg L−1 | Ferulic Acid | Vanillin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Voltammetry/ mg L−1 | RSD/% | HPLC-UV/ mg L−1 | Voltammetry/ mg L−1 | RSD/% | HPLC-UV/mg L−1 | RSD/% | t-Test 1 | F-Test 2 | ||
| 1 (x2) | 0 | 0 | - | 0 | 317 ± 6 | 1.6 | 321 ± 17 | 2.1 | 0.909 | 1.778 |
| 24.3 | 24.3 ± 0.6 | 2.1 | ||||||||
| 48.5 | 48.4 ± 0.7 | 1.5 | ||||||||
| 2 (x3) | 0 | 0 | - | 0 | 519 ± 4 | 0.78 | 516 ± 4 | 0.32 | 1.444 | 5.995 |
| 12.1 | 12.1 ± 0.4 | 1.3 | ||||||||
| 48.5 | 48.5 ± 0.6 | 0.77 | ||||||||
| 3 (x3) | 0 | 0 | - | 0 | 700 ± 9 | 1.0 | 703 ± 18 | 1.0 | 0.423 | 1.019 |
| 12.1 | 12.1 ± 0.4 | 1.3 | ||||||||
| 24.3 | 24.3 ± 0.6 | 1.9 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziyatdinova, G.; Zhupanova, A.; Davletshin, R. Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple. Sensors 2022, 22, 288. https://doi.org/10.3390/s22010288
Ziyatdinova G, Zhupanova A, Davletshin R. Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple. Sensors. 2022; 22(1):288. https://doi.org/10.3390/s22010288
Chicago/Turabian StyleZiyatdinova, Guzel, Anastasiya Zhupanova, and Rustam Davletshin. 2022. "Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple" Sensors 22, no. 1: 288. https://doi.org/10.3390/s22010288
APA StyleZiyatdinova, G., Zhupanova, A., & Davletshin, R. (2022). Simultaneous Determination of Ferulic Acid and Vanillin in Vanilla Extracts Using Voltammetric Sensor Based on Electropolymerized Bromocresol Purple. Sensors, 22(1), 288. https://doi.org/10.3390/s22010288