Automatic Measurement of Morphological Traits of Typical Leaf Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Processing Environment
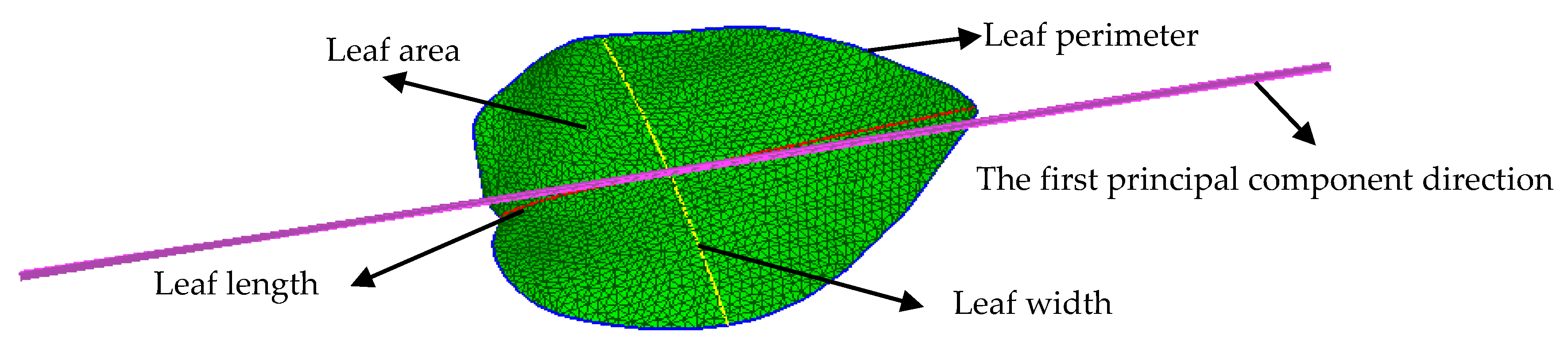
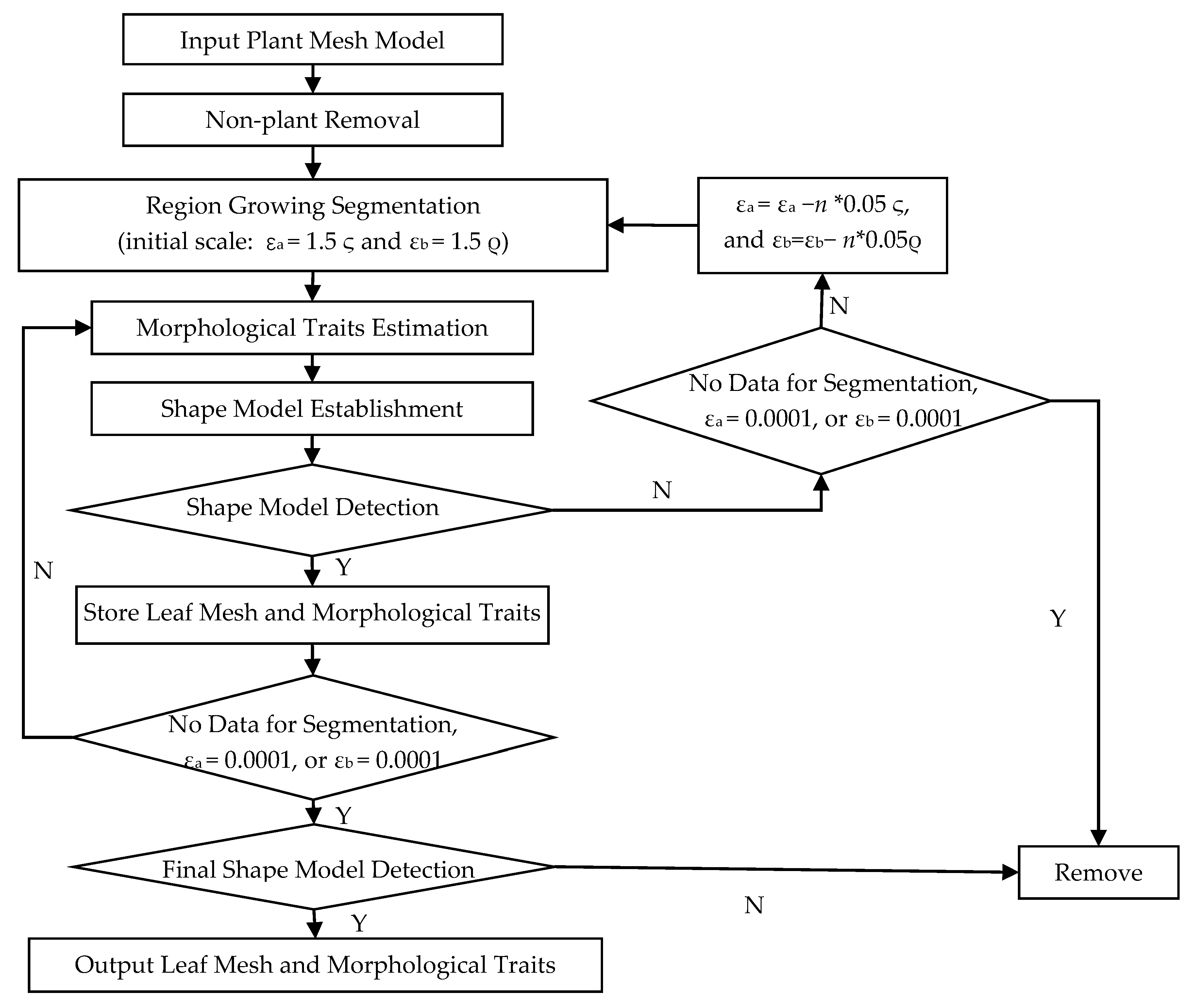
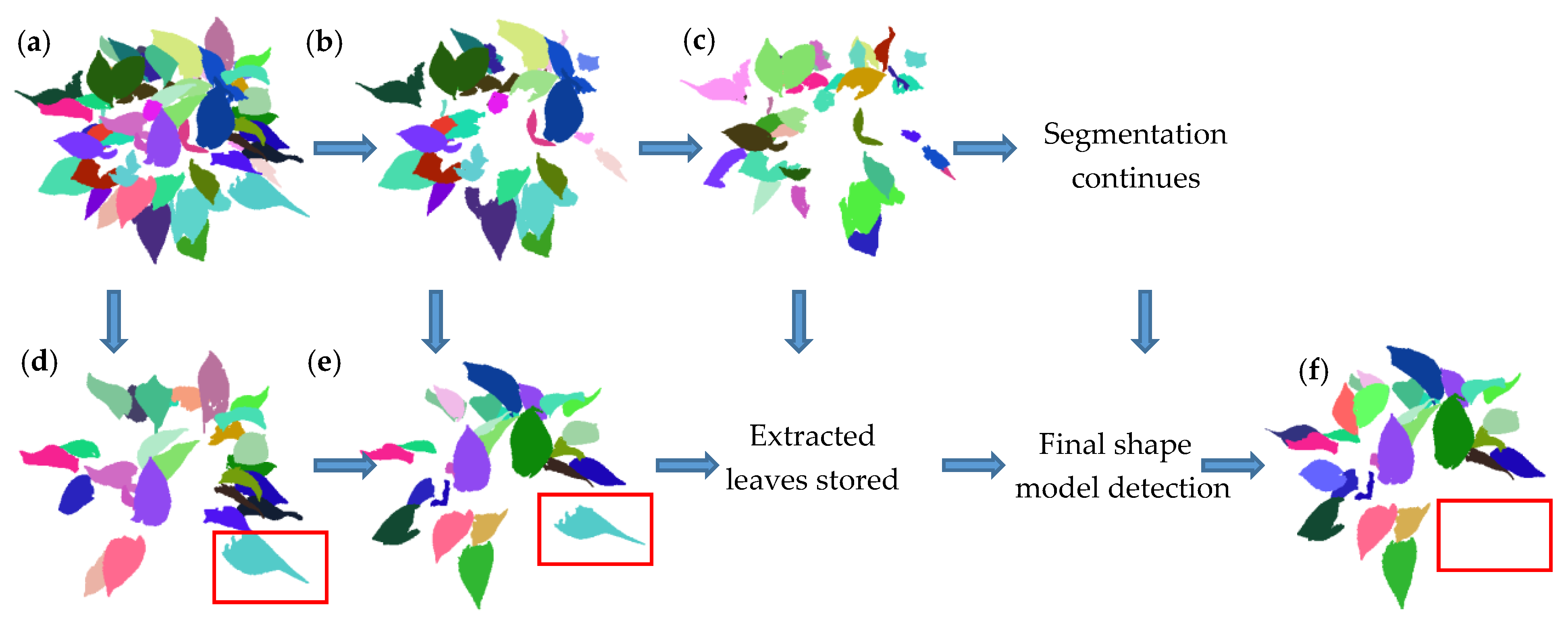
2.2. Typical Leaf Sample Extraction and Morphological Trait Estimation
2.3. Accuracy Analysis
3. Results
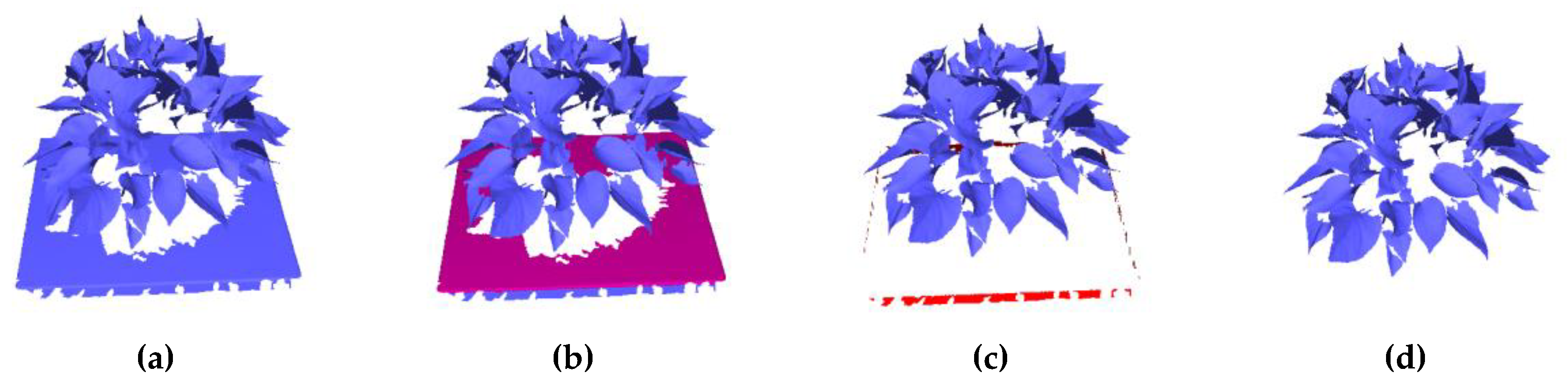
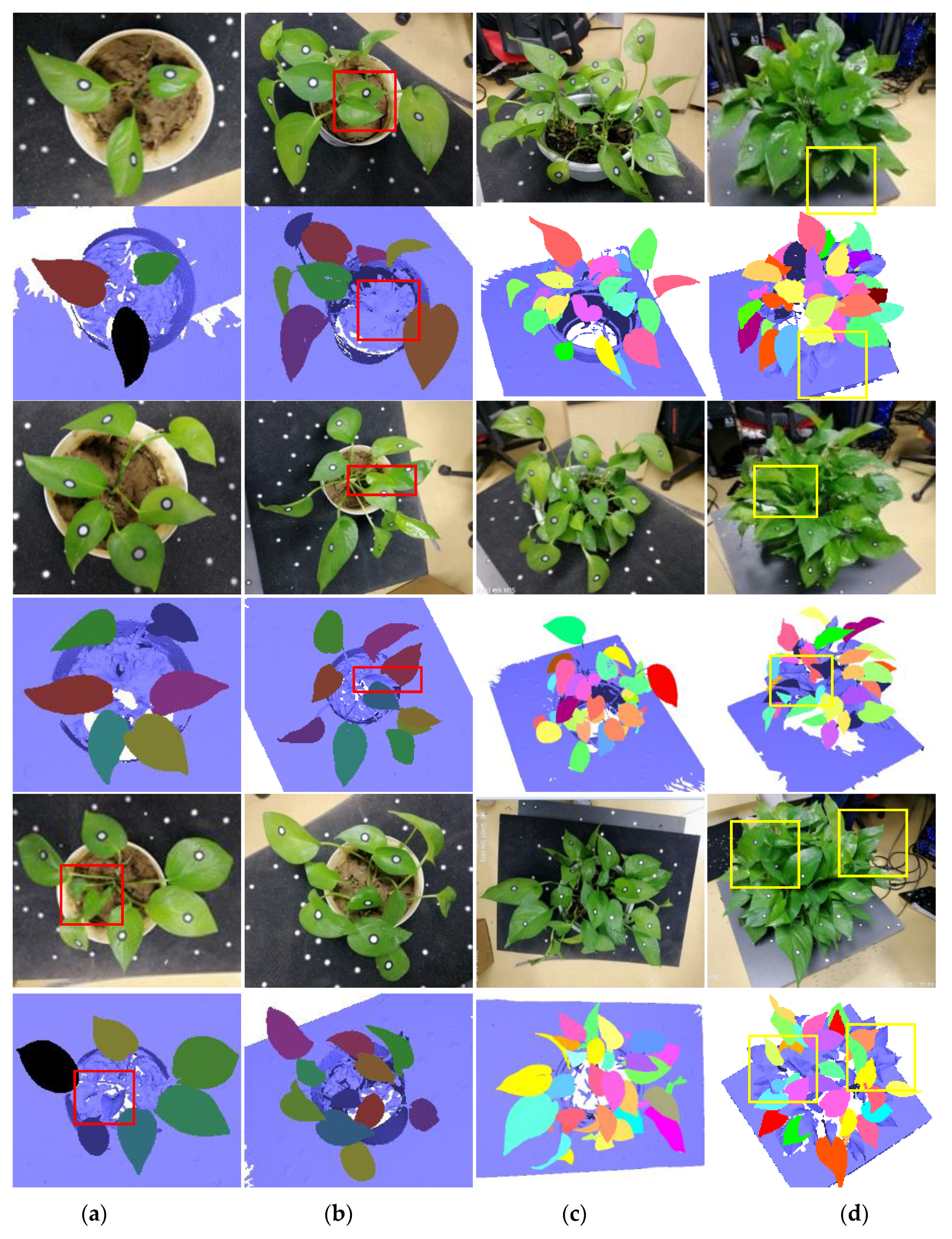
3.1. Results of Data Scanning and Segmentation
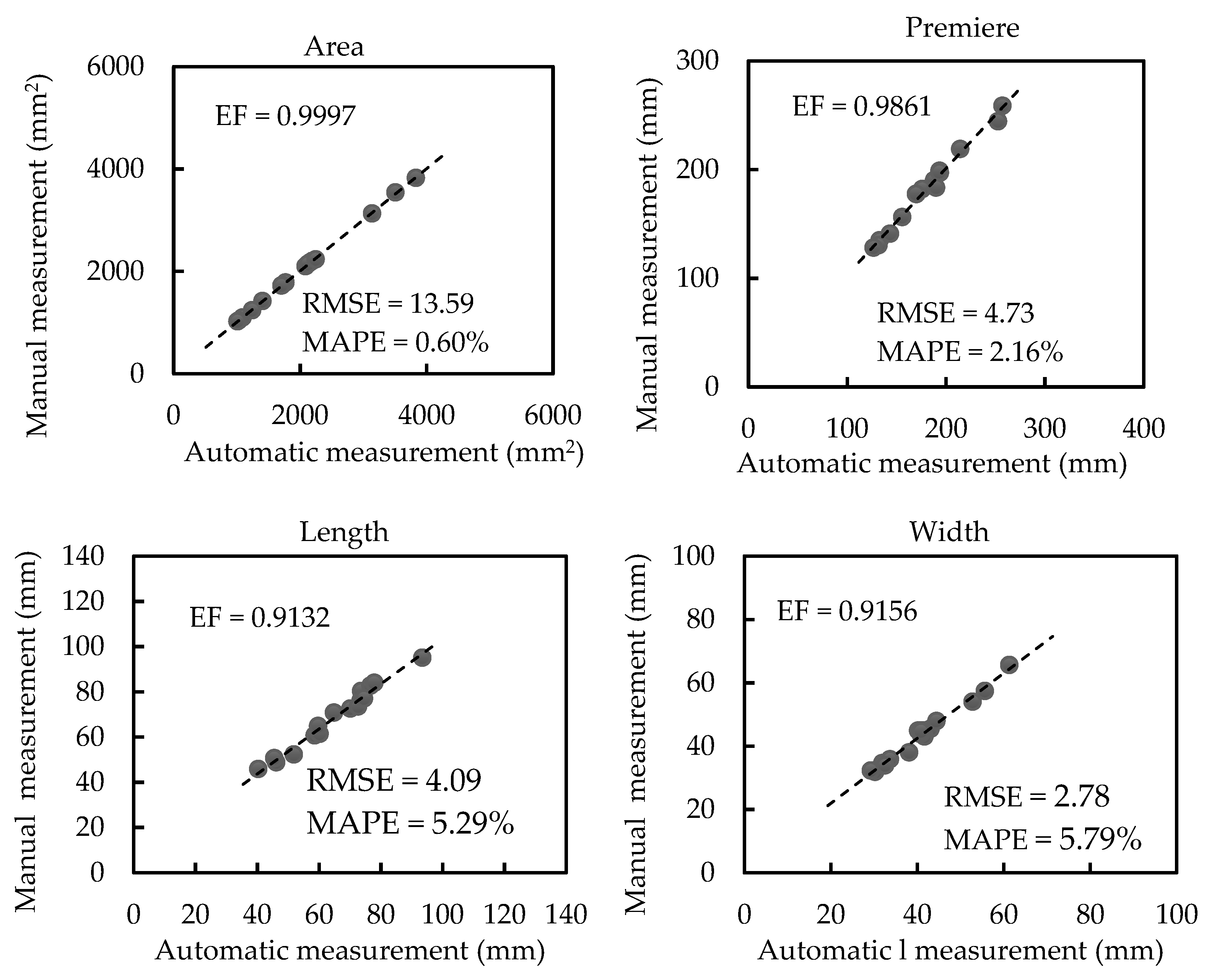
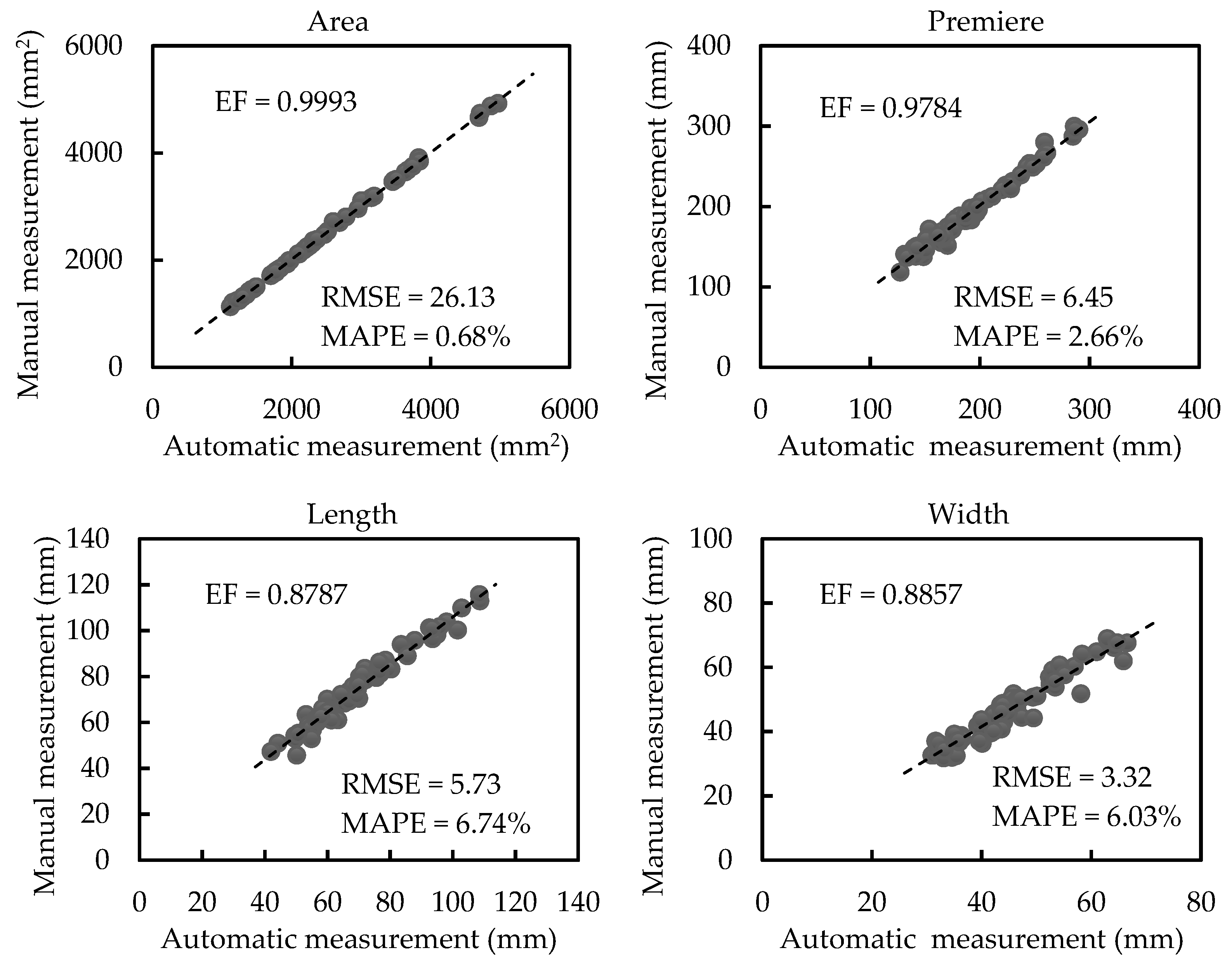
3.2. Results of Morphological Trait Estimation
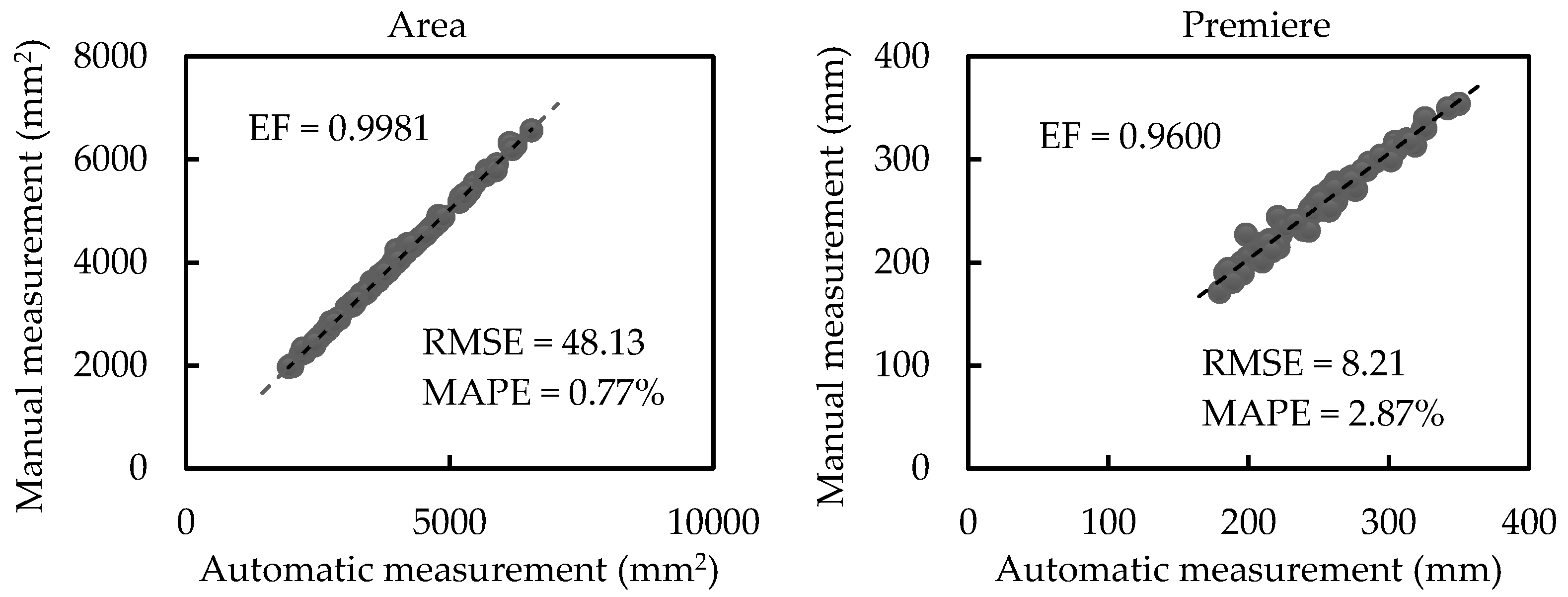
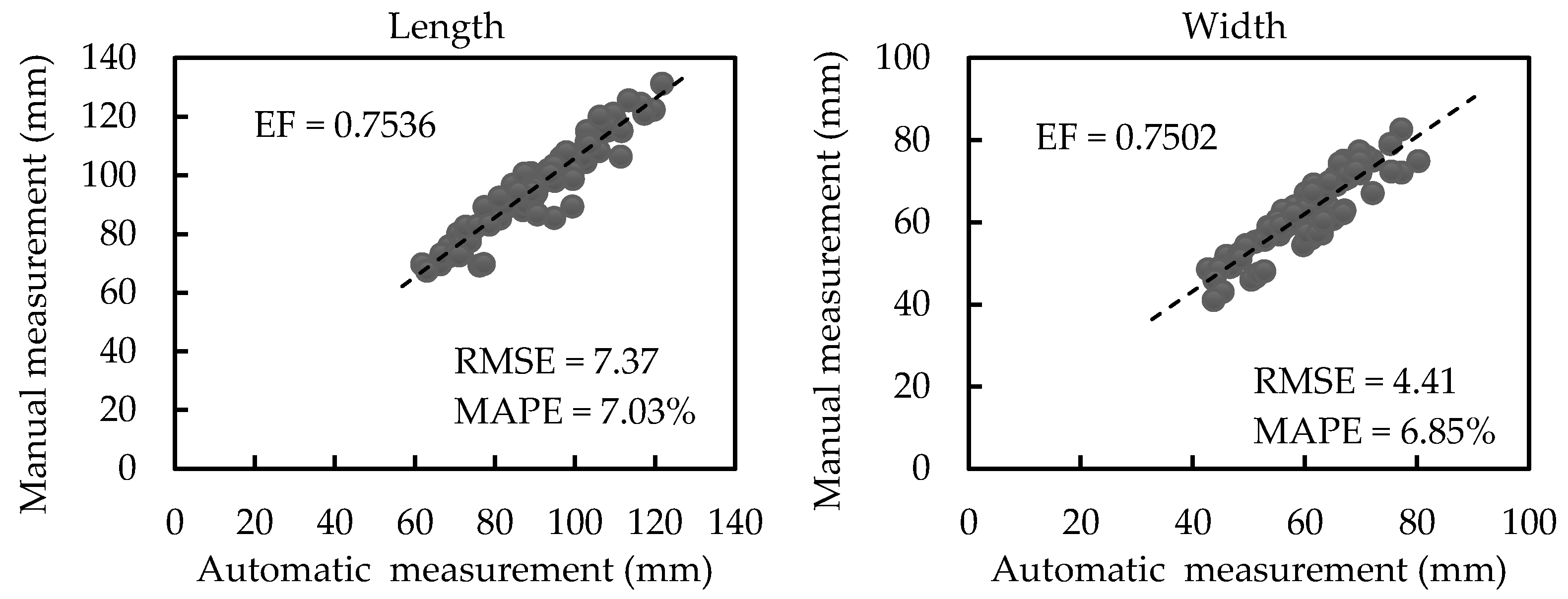
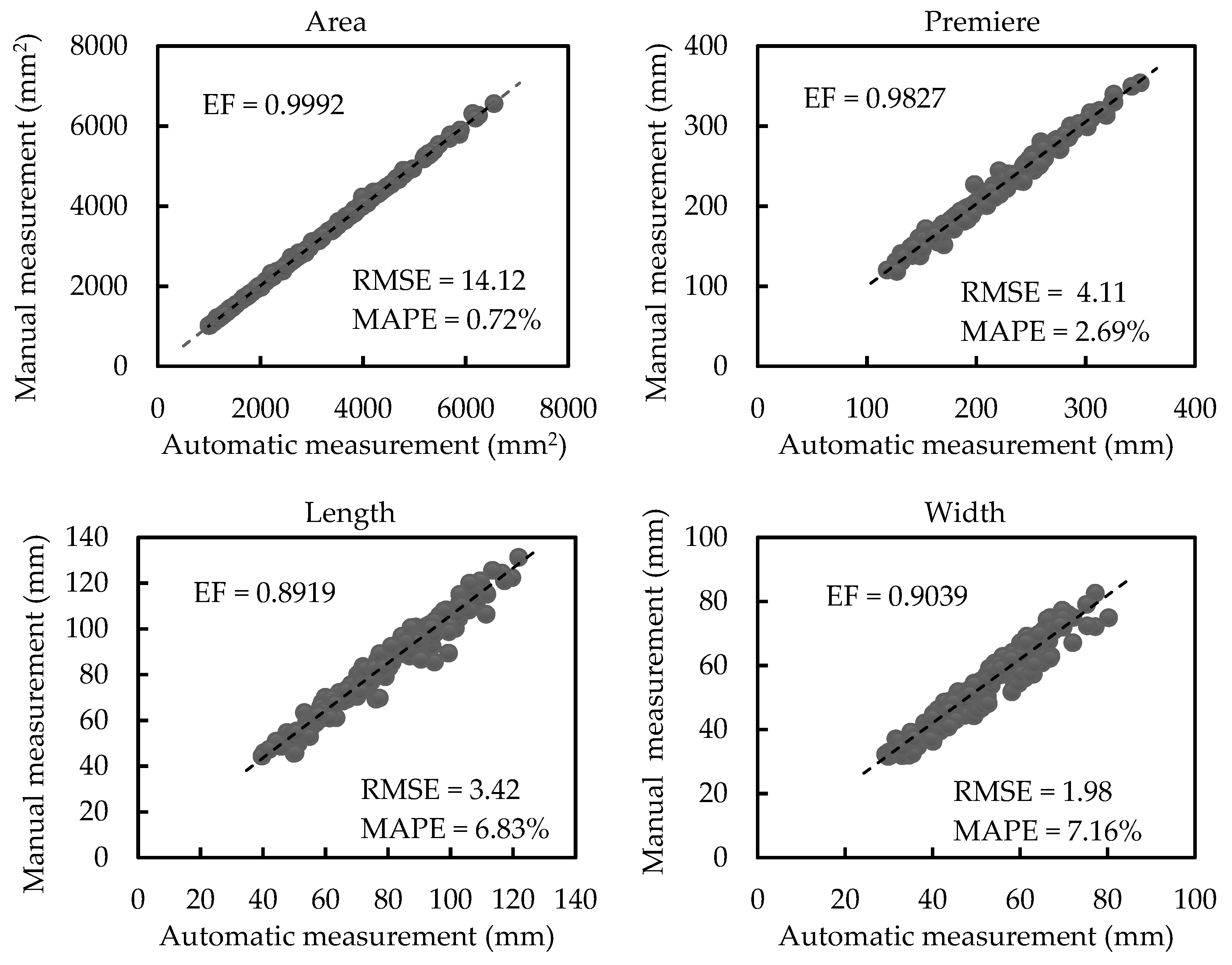
3.2.1. Results of Scale-related Morphological Traits
3.2.2. Results of Scale-invariant Morphological Traits
3.3. Time Cost
4. Discussion
4.1. Measurements on Different Canopy-Occluded Plants
4.2. Comparison of Related Methods
4.3. Advantages, Limitations, Improvements, and Future Works
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Apelt, F.; Breuer, D.; Nikoloski, Z.; Stitt, M.; Kragler, F. Phytotyping(4D): A light-field imaging system for non-invasive and accurate monitoring of spatio-temporal plant growth. Plant J. 2015, 82, 693–706. [Google Scholar] [CrossRef]
- Clark, B.; Bullock, S. Shedding light on plant competition: Modelling the influence of plant morphology on light capture (and vice versa). J. Theor. Biol. 2007, 244, 208–217. [Google Scholar] [CrossRef]
- Watanabe, T.; Hanan, J.S.; Room, P.M.; Hasegawa, T.; Nakagawa, H.; Takahashi, W. Rice morphogenesis and plant architecture: Measurement, specification and the reconstruction of structural development by 3D architectural modelling. Ann. Bot. 2005, 95, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Carolan, M. Publicising food: Big data, precision agriculture, and co-experimental techniques of addition. Sociol. Rural. 2015, 57, 135–154. [Google Scholar] [CrossRef]
- Afonnikov, D.A.; Genaev, M.A.; Doroshkov, A.V.; Komyshev, E.G.; Pshenichnikova, T.A. Methods of high-throughput plant phenotyping for large-scale breeding and genetic experiments. Russ. J. Genet. 2016, 52, 688–701. [Google Scholar] [CrossRef]
- Pantalião, G.F.; Narciso, M.; Guimarães, C.; Castro, A.; Colombari, J.M.; Breseghello, F.; Rodrigues, L.; Vianello, R.P.; Borba, T.O.; Brondani, C. Genome wide association study (GWAS) for grain yield in rice cultivated under water deficit. Genetica 2016, 144, 651–664. [Google Scholar] [CrossRef]
- Perez-Sanz, F.; Navarro, P.J.; Egea-Cortines, M. Plant phenomics: An overview of image acquisition technologies and image data analysis algorithms. Gigascience 2017, 6, gix092. [Google Scholar] [CrossRef]
- Qiu, R.; Wei, S.; Zhang, M.; Li, H.; Sun, H.; Liu, G.; Li, M. Sensors for measuring plant phenotyping: A review. Int. J. Agric. Biol. Eng. 2018, 11, 1–17. [Google Scholar] [CrossRef]
- An, N.; Palmer, C.M.; Baker, R.L.; Markelz, R.J.C.; Ta, J.; Covington, M.F.; Maloof, J.N.; Welch, S.M.; Weinig, C. Plant high-throughput phenotyping using photogrammetry and imaging techniques to measure leaf length and rosette area. Comput. Electron. Agric. 2016, 127, 376–394. [Google Scholar] [CrossRef]
- Dimitrios, F.; Christoph, B.; Johannes, F.M.; Silke, K.; Alexander, P.; Fabio, F.; Andreas, U.; Ulrich, S. Rapid determination of leaf area and plant height by using light curtain arrays in four species with contrasting shoot architecture. Plant Methods 2014, 10, 9–21. [Google Scholar] [CrossRef]
- Pereyrairujo, G.A.; Gasco, E.D.; Peirone, L.S.; Lan, A. GlyPh: A low-cost platform for phenotyping plant growth and water use. Funct. Plant Biol. 2012, 39, 905–913. [Google Scholar] [CrossRef]
- Biskup, B.; Scharr, H.; Schurr, U.; Rascher, U. A stereo imaging system for measuring structural parameters of plant canopies. Plant. Cell Environ. 2007, 30, 1299–1308. [Google Scholar] [CrossRef]
- Gibbs, J.A.; Pound, M.; French, A.P.; Wells, D.M.; Murchie, E.; Pridmore, T. Approaches to three-dimensional reconstruction of plant shoot topology and geometry. Funct. Plant Biol. 2016, 44, 62–75. [Google Scholar] [CrossRef]
- Kjaer, K.H.; Ottosen, C.O. 3D laser triangulation for plant phenotyping in challenging environments. Sensors 2015, 15, 13533–13547. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, L.; Chung, B.K.; Lee, J.M. In situ 3D segmentation of individual plant leaves using a RGB-D camera for agricultural automation. Sensors 2015, 15, 20463–20479. [Google Scholar] [CrossRef]
- Drapikowski, P.; Kazimierczak-Grygiel, E.; Korecki, D.; Wiland-Szymańska, J. Verification of geometric model-based plant phenotyping methods for studies of xerophytic plants. Sensors 2016, 16, 924. [Google Scholar] [CrossRef]
- Martínezguanter, J.; Garridoizard, M.; Valero, C.; Slaughter, D.C.; Pérezruiz, M. Optical sensing to determine tomato plant spacing for precise agrochemical application: Two scenarios. Sensors 2017, 17, 1096. [Google Scholar] [CrossRef]
- Dionisio, A.; Mikel, C.; César, F.Q.; Ángela, R.; José, D. Three-dimensional modeling of weed plants using low-cost photogrammetry. Sensors 2018, 18, 1077. [Google Scholar] [CrossRef]
- Paulus, S. Measuring crops in 3D: Using geometry for plant phenotyping. Plant Methods 2019, 15, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Thyagharajan, K.K.; Raji, I.K. A review of visual descriptors and classification techniques used in leaf species identification. Arch. Comput. Methods Eng. 2018, 1–28. [Google Scholar] [CrossRef]
- Tan, J.W.; Chang, S.W.; Sameem, B.A.K.; Jen, Y.H.; Yong, K.T. Deep learning for plant species classification using leaf vein morphometric. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 17, 82–90. [Google Scholar] [CrossRef]
- Hang, L.; Tang, L.; Steven, W.; Yu, M. A robotic platform for corn seedling morphological traits characterization. Sensors 2017, 17, 2082. [Google Scholar] [CrossRef]
- Chaivivatrakul, S.; Tang, L.; Dailey, M.N.; Nakarmi, A.D. Automatic morphological trait characterization for corn plants via 3D holographic reconstruction. Comput. Electron. Agric. 2014, 109, 109–123. [Google Scholar] [CrossRef]
- Zermas, D.; Morellas, V.; Mulla, D.; Papanikolopoulos, N. 3D model processing for high throughput phenotype extraction—The case of corn. Comput. Electron. Agric. 2019, 172, 105047–105066. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wei, H.; Zhu, X.; Huang, H. A technique system for the measurement, reconstruction and character extraction of rice plant architecture. PLoS ONE 2017, 12, e0177205. [Google Scholar] [CrossRef]
- Fang, W.; Feng, H.; Yang, W.; Duan, L.; Chen, G.; Xiong, L.; Liu, Q. High-throughput volumetric reconstruction for 3D wheat plant architecture studies. J. Innov. Opt. Health Sci. 2016, 9, 1650037–1650050. [Google Scholar] [CrossRef]
- Li, D.; Yan, C.; Tang, X.S.; Yan, S.; Xin, C. Leaf segmentation on dense plant point clouds with facet region growing. Sensors 2018, 18, 3625. [Google Scholar] [CrossRef]
- Kazmi, W.; Foix, S.; Alenya, G.; Andersen, H.J. Indoor and outdoor depth imaging of leaves with time-of-flight and stereo vision sensors: Analysis and comparison. Isprs J. Photogramm. Remote Sens. 2014, 88, 128–146. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y. A low-cost 3D phenotype measurement method of leafy vegetables using video recordings from smartphones. Sensors 2020, 20, 6068. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, Y.; Shi, G.; Cai, X.; Chen, Y.; Wang, S.; Yan, S. An overlapping-free leaf segmentation method for plant point clouds. IEEE Access 2019, 7, 129054–129070. [Google Scholar] [CrossRef]
- Schnabel, R.; Wahl, R.; Klein, R. Efficient RANSAC for point-cloud shape detection. Comput. Graph. Forum 2007, 26, 214–226. [Google Scholar] [CrossRef]
- Mayer, D.G.; Butler, D.G. Statistical validation. Ecol. Model. 1993, 68, 21–32. [Google Scholar] [CrossRef]
- Suresh, T.; Zhu, F.; Harkamal, W.; Yu, H.; Ge, Y. A novel LIDAR-based instrument for high-throughput, 3D measurement of morphological traits in maize and sorghum. Sensors 2018, 18, 1187. [Google Scholar] [CrossRef]
- Ten Harkel, J.; Bartholomeus, H.; Kooistra, L. Biomass and crop height estimation of different crops using UVA-based lidar. Remote Sens. 2019, 12, 17. [Google Scholar] [CrossRef]
- Xiaodan, M.; Kexin, Z.; Haiou, G.; Jiarui, F.; Song, Y. Calculation method for phenotypic traits based on the 3D reconstruction of maize canopies. Sensors 2019, 19, 1201. [Google Scholar] [CrossRef]
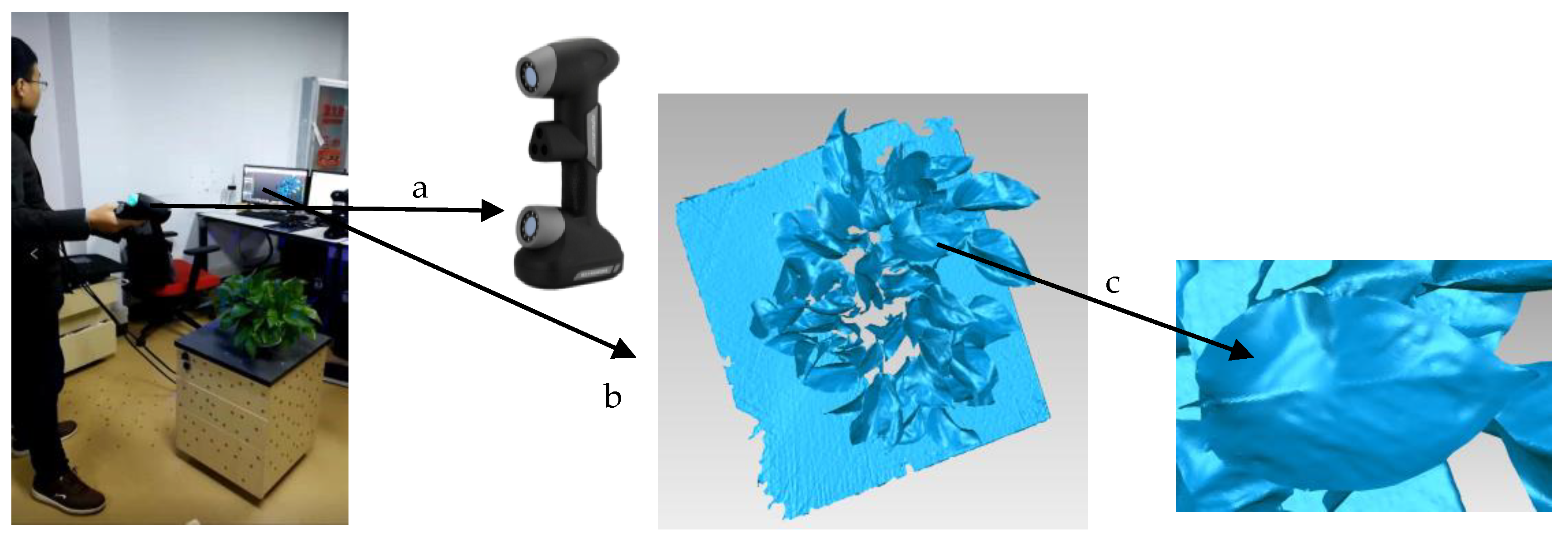


| Types | Parameters | Types | Parameters |
|---|---|---|---|
| Weight | 1.0 kg | Accuracy | 0.03 mm |
| Volume | 310 × 147 × 80 mm | Field depth | 550 mm |
| Scanning area | 275 × 250 mm | Transfer method | USB 3.0 |
| Speed | 480,000 times/s | Work temperatures | −20–40 °C |
| Light | 7 laser crosses (+1) | Work humidity | 10–90% |
| Light security | Ⅱ | Outputs | Point clouds/3D mesh |
| Scale-Related Traits | Sym. | Var. | Scale-Invariant Traits | Sym. | Var. |
|---|---|---|---|---|---|
| Area | s | X01 | Area perimeter ratio | s/c | X11 |
| Perimeter | c | X02 | Area length ratio | s/l | X12 |
| Length | l | X03 | Area width ratio | s/w | X13 |
| Width | w | X04 | Perimeter length ratio | c/l | X14 |
| Perimeter width ratio | c/w | X15 | |||
| Aspect ratio | l/w | X16 |
| Groups | NO. | N0 | N1 | N2 | N3 | R_scan | R_seg1 | R_seg2 | R1 | R2 | R3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (No occlusion) | 1 | 3 | 3 | 3 | 3 | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
| 2 | 6 | 6 | 6 | 6 | 100.00% | 100.00% | 100.00% | ||||
| 3 | 8 | 6 | 6 | 6 | 100.00% | 100.00% | 100.00% | ||||
| 2 (A little occlusion) | 4 | 10 | 8 | 8 | 8 | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
| 5 | 10 | 10 | 10 | 10 | 100.00% | 100.00% | 100.00% | ||||
| 6 | 11 | 11 | 11 | 11 | 100.00% | 100.00% | 100.00% | ||||
| 3 (Medium occlusion) | 7 | 26 | 24 | 23 | 22 | 95.83% | 95.65% | 91.67% | 96.20% | 94.94% | 91.33% |
| 8 | 32 | 25 | 24 | 23 | 96.00% | 95.83% | 92.00% | ||||
| 9 | 34 | 31 | 30 | 28 | 96.77% | 93.33% | 90.32% | ||||
| 4 (Heavy occlusion) | 10 | 45 | 37 | 35 | 32 | 94.59% | 91.43% | 86.49% | 89.96% | 88.80% | 79.95% |
| 11 | 50 | 37 | 33 | 29 | 89.19% | 87.88% | 78.38% | ||||
| 12 | 53 | 36 | 31 | 27 | 86.11% | 87.10% | 75.00% | ||||
| all | 1–12 | 288 | 234 | 220 | 205 | 94.02% | 93.18% | 87.61% | - | - | - |
| Groups | X11 | X12 | X13 | X14 | X15 | X16 |
|---|---|---|---|---|---|---|
| 1 | 0.9857 | 0.9202 | 0.9102 | 0.8728 | 0.8312 | 0.8268 |
| 2 | 0.9883 | 0.9130 | 0.9386 | 0.8513 | 0.8147 | 0.7594 |
| 3 | 0.9692 | 0.8507 | 0.9017 | 0.8472 | 0.7449 | 0.7322 |
| 4 | 0.9579 | 0.8461 | 0.8276 | 0.7775 | 0.6891 | 0.6895 |
| All | 0.9811 | 0.8908 | 0.9162 | 0.8509 | 0.7838 | 0.7434 |
| Groups | RMSE | MAPE (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X11 | X12 | X13 | X14 | X15 | X16 | X11 | X12 | X13 | X14 | X15 | X16 | ||
| 1 | 0.27 | 1.83 | 2.83 | 0.17 | 0.27 | 0.07 | 1.95 | 5.30 | 5.88 | 4.83 | 5.66 | 3.38 | |
| 2 | 0.28 | 2.20 | 2.95 | 0.18 | 0.23 | 0.10 | 2.02 | 6.77 | 5.76 | 5.37 | 4.56 | 4.54 | |
| 3 | 0.38 | 2.39 | 3.44 | 0.23 | 0.34 | 0.14 | 2.65 | 7.08 | 6.21 | 7.07 | 7.04 | 6.19 | |
| 4 | 0.44 | 3.19 | 4.47 | 0.21 | 0.31 | 0.13 | 2.64 | 7.06 | 6.78 | 6.59 | 6.21 | 5.87 | |
| All | 0.23 | 1.46 | 2.09 | 0.14 | 0.20 | 0.08 | 2.50 | 6.89 | 6.34 | 6.44 | 6.21 | 5.52 | |
| Plants | Points | T_scan | T_mesh | T_p | Plants | Points | T_scan | T_mesh | T_p |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 203,343 | 120 | 5.68 | 4.02 | 7 | 434,177 | 185 | 6.08 | 4.25 |
| 2 | 279,350 | 130 | 5.71 | 4.02 | 8 | 400,144 | 191 | 6.00 | 4.27 |
| 3 | 304,232 | 140 | 5.81 | 4.02 | 9 | 420,915 | 199 | 6.05 | 4.29 |
| 4 | 364,758 | 160 | 5.89 | 4.08 | 10 | 449,642 | 265 | 6.12 | 5.13 |
| 5 | 364,299 | 170 | 5.88 | 4.09 | 11 | 456,619 | 247 | 6.13 | 5.04 |
| 6 | 359,396 | 176 | 5.81 | 4.09 | 12 | 493,968 | 250 | 6.24 | 5.06 |
| Methods | Area | Perimeter | Length | Width | |
|---|---|---|---|---|---|
| Method A | EF | 0.8430 | 0.8299 | 0.7872 | 0.7501 |
| RMSE | 54.98 mm2 | 18.56 mm | 11.44 mm | 9.66 mm | |
| MAPE | 5.44% | 9.44% | 16.03% | 18.57% | |
| Method B | EF | 0.9354 | 0.9103 | 0.8546 | 0.8212 |
| RMSE | 23.98 mm2 | 12.13 mm | 7.12 mm | 5.47 mm | |
| MAPE | 2.13% | 6.12% | 9.12% | 11.57% | |
| Our work | EF | 0.9992 | 0.9827 | 0.8919 | 0.9039 |
| RMSE | 14.12 mm2 | 4.11 mm | 3.42 mm | 1.98 mm | |
| MAPE | 0.72% | 2.69% | 6.83% | 7.16% |
| Methods | X11 | X12 | X13 | X14 | X15 | X16 | |
|---|---|---|---|---|---|---|---|
| Method A | EF | 0.8286 | 0.7812 | 0.7513 | 0.7001 | 0.6108 | 0.5603 |
| RMSE | 0.78 | 5.67 | 7.63 | 0.45 | 0.49 | 0.17 | |
| MAPE | 5.17% | 14.39% | 12.68% | 12.99% | 12.68% | 10.34% | |
| Method B | EF | 0.9102 | 0.8512 | 0.8417 | 0.8103 | 0.7029 | 0.6819 |
| RMSE | 0.46 | 3.57 | 4.09 | 0.25 | 0.30 | 0.10 | |
| MAPE | 3.59% | 10.47% | 9.46% | 9.79% | 9.56% | 7.89% | |
| Our work | EF | 0.9811 | 0.8908 | 0.9162 | 0.8509 | 0.7838 | 0.7434 |
| RMSE | 0.23 | 1.46 | 2.09 | 0.14 | 0.20 | 0.08 | |
| MAPE | 2.50% | 6.89% | 6.34% | 6.44% | 6.21% | 5.52% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zheng, S.; Gui, L. Automatic Measurement of Morphological Traits of Typical Leaf Samples. Sensors 2021, 21, 2247. https://doi.org/10.3390/s21062247
Huang X, Zheng S, Gui L. Automatic Measurement of Morphological Traits of Typical Leaf Samples. Sensors. 2021; 21(6):2247. https://doi.org/10.3390/s21062247
Chicago/Turabian StyleHuang, Xia, Shunyi Zheng, and Li Gui. 2021. "Automatic Measurement of Morphological Traits of Typical Leaf Samples" Sensors 21, no. 6: 2247. https://doi.org/10.3390/s21062247
APA StyleHuang, X., Zheng, S., & Gui, L. (2021). Automatic Measurement of Morphological Traits of Typical Leaf Samples. Sensors, 21(6), 2247. https://doi.org/10.3390/s21062247







