Cortical Effects of Noisy Galvanic Vestibular Stimulation Using Functional Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Noisy Galvanic Vestibular Stimulation
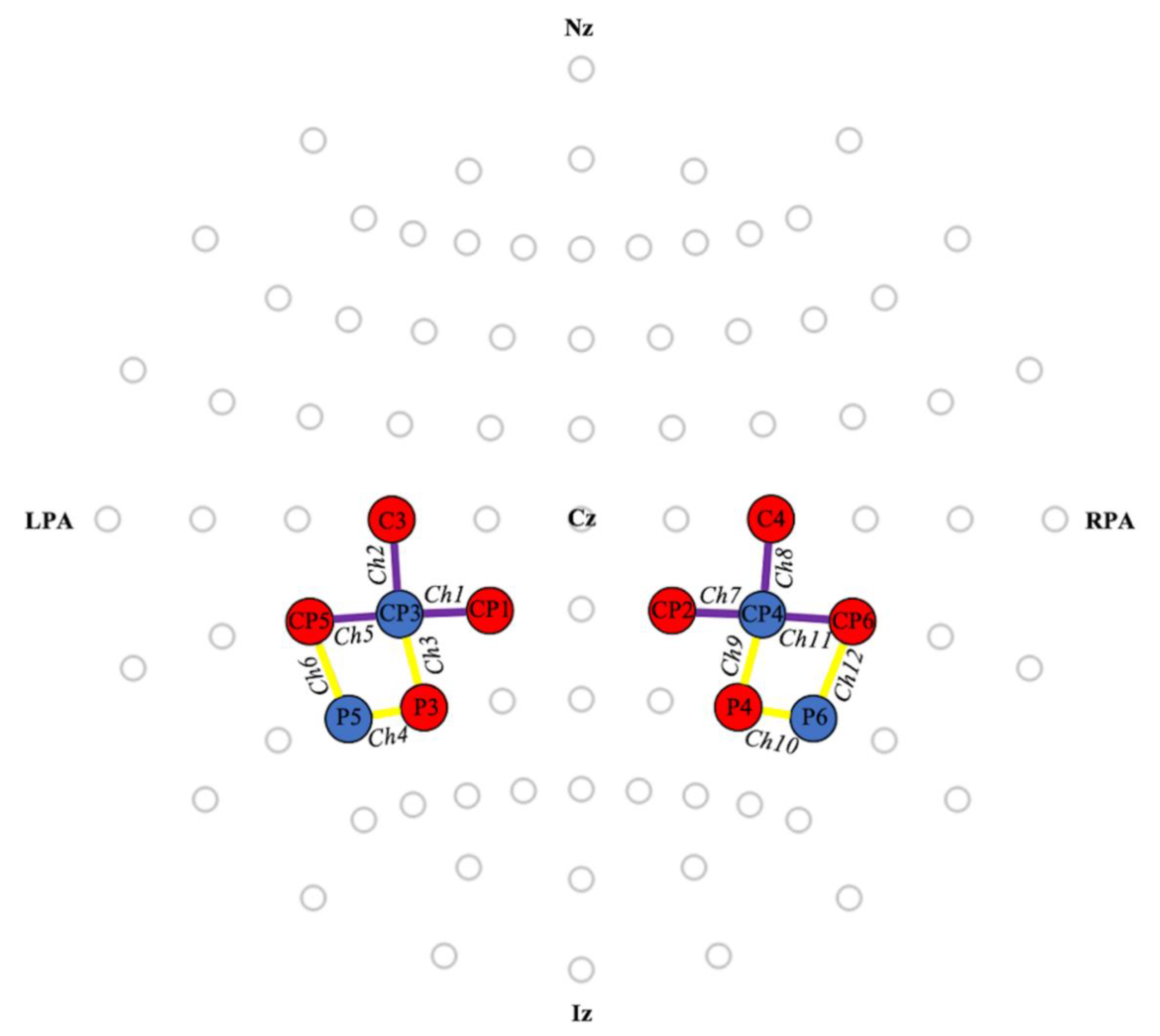
2.3. Functional Near-Infrared Spectroscopy
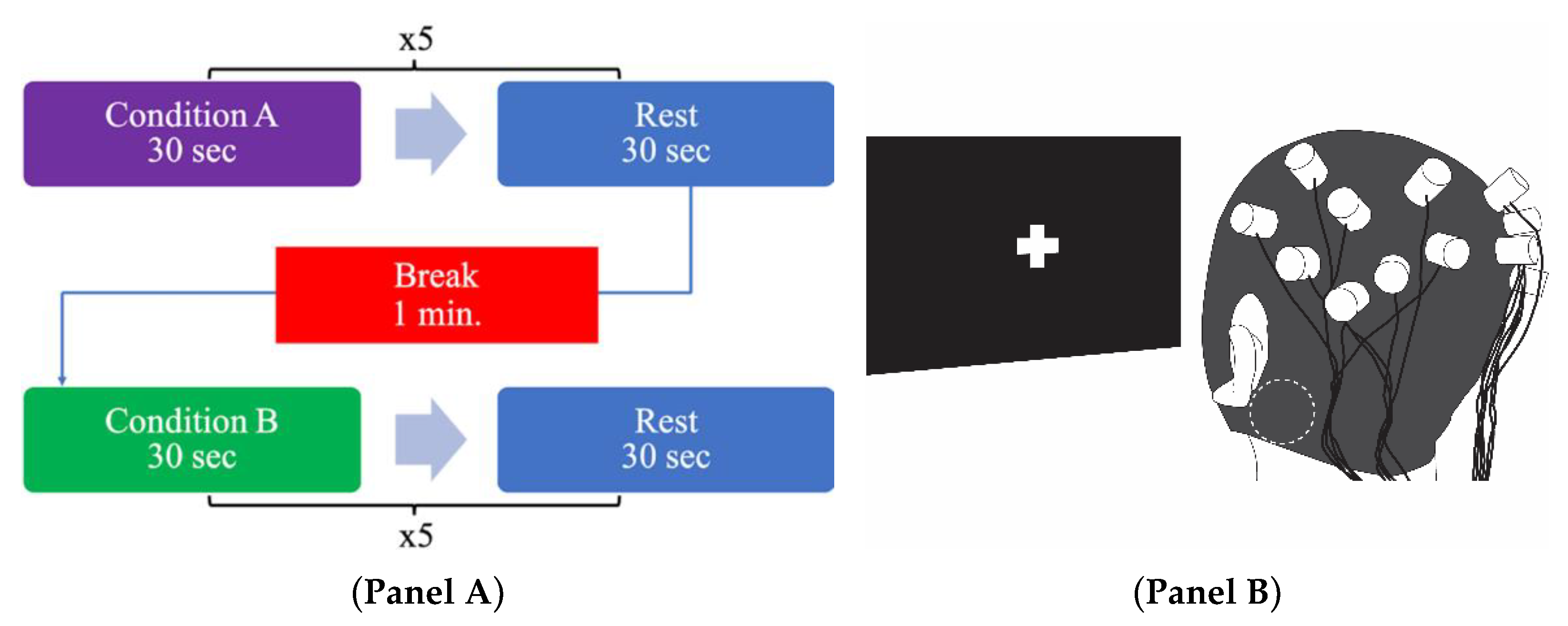
2.4. Experimental Procedure
2.5. Data and Statistical Analysis
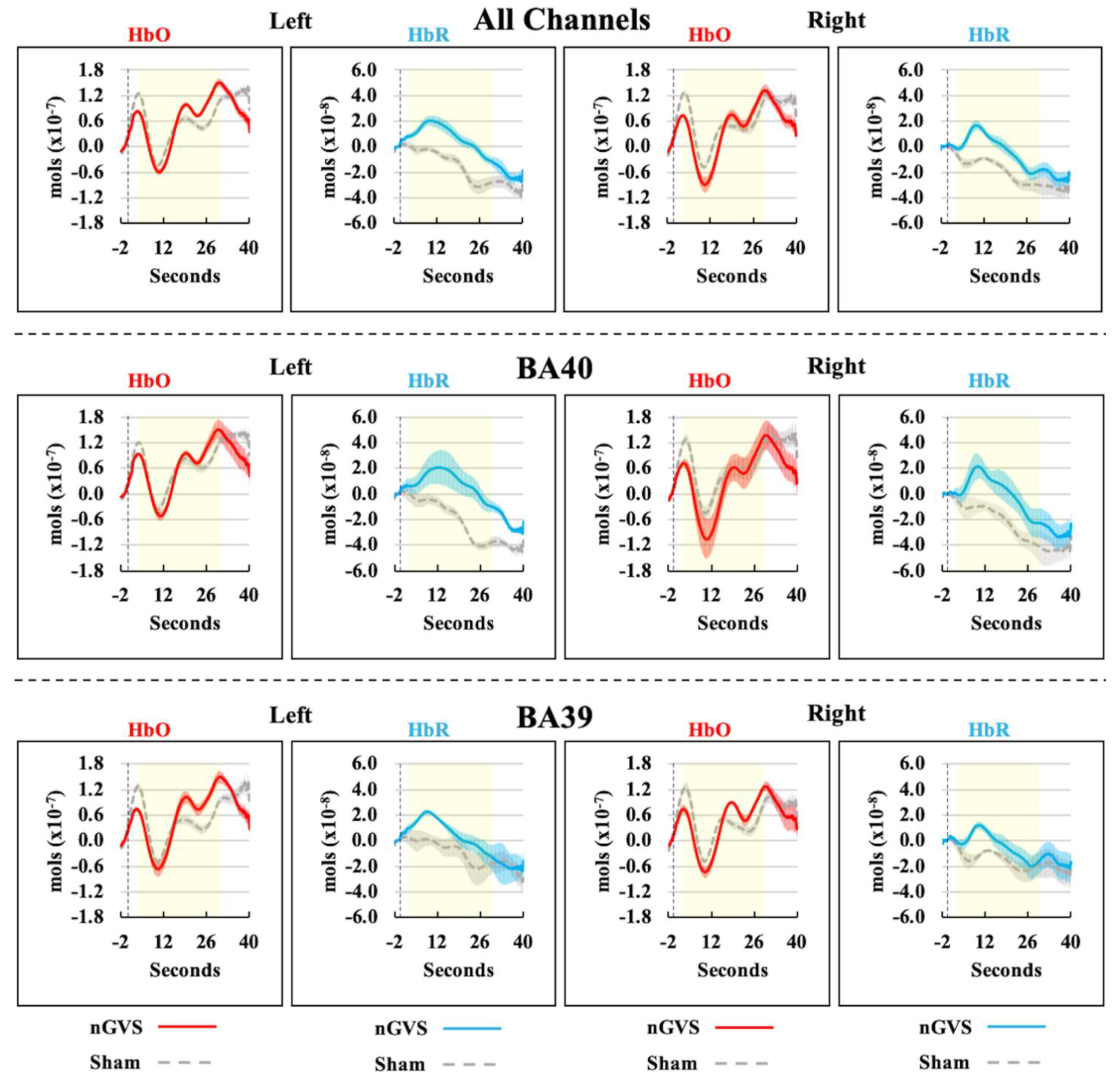
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gensberger, K.D.; Kaufmann, A.K.; Dietrich, H.; Branoner, F.; Banchi, R.; Chagnaud, B.P.; Straka, H. Galvanic vestibular stimulation: Cellular substrates and response patterns of neurons in the vestibulo-ocular network. J. Neurosci. 2016, 36, 9097–9110. [Google Scholar] [CrossRef]
- Kwan, A.; Forbes, P.A.; Mitchell, D.E.; Blouin, J.S.; Cullen, K.E. Neural substrates, dynamics and thresholds of galvanic vestibular stimulation in the behaving primate. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yamamoto, Y.; Togo, F.; Kinoshita, M.; Yoshifuji, Y.; Fujimoto, C.; Yamasoba, T. Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 2014, 82, 969–975. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Fiedler, M.J.; Kofman, I.S.; Wood, S.J.; Serrador, J.M.; Peters, B.; Cohen, H.S.; Reschke, M.F.; Bloomberg, J.J. Improving balance function using vestibular stochastic resonance: Optimizing stimulus characteristics. Exp. Brain Res. 2011, 210, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, C.; Bakkum, A.; Marigold, D.S. Subthreshold stochastic vestibular stimulation affects balance-challenged standing and walking. PLoS ONE 2020, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Fujimoto, C.; Egami, N.; Kinoshita, M.; Togo, F.; Yamamoto, Y.; Yamasoba, T. Noisy vestibular stimulation increases gait speed in normals and in bilateral vestibulopathy. Brain. Stimul. 2018, 11, 709–715. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Kofman, I.S.; De Dios, Y.E.; Miller, C.; Peters, B.T.; Goel, R.; Galvan-Garza, R.; Bloomberg, J.J. Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front. Syst. Neurosci. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Wuehr, M.; Nusser, E.; Krafczyk, S.; Straube, A.; Brandt, T.; Jahn, K.; Schniepp, R. Noise-Enhanced Vestibular Input Improves Dynamic Walking Stability in Healthy Subjects. Brain. Stimul. 2016, 9, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kuatsjah, E.; Khoshnam, M.; Menon, C. Investigation on the effect of noisy galvanic vestibular stimulation on fine motor skills during a visuomotor task in healthy participants. PLoS ONE 2019, 14, 1–23. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.J.; Svenkeson, D.; Parras, G.; Oishi, M.M.K.; McKeown, M.J. Multifaceted effects of noisy galvanic vestibular stimulation on manual tracking behavior in Parkinson’s disease. Front. Syst. Neurosci. 2015, 9, 1–9. [Google Scholar] [CrossRef]
- Iwasaki, S.; Karino, S.; Kamogashira, T.; Togo, F.; Fujimoto, C.; Yamamoto, Y.; Yamasoba, T. Effect of noisy Galvanic vestibular stimulation on ocular vestibular-evoked myogenic potentials to bone-conducted vibration. Front. Neurol. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Serrador, J.M.; Deegan, B.M.; Geraghty, M.C.; Wood, S.J. Enhancing vestibular function in the elderly with imperceptible electrical stimulation. Sci. Rep. 2018, 8, 6–11. [Google Scholar] [CrossRef]
- Galvan-Garza, R.C.; Clark, T.K.; Mulavara, A.P.; Oman, C.M. Exhibition of stochastic resonance in vestibular tilt motion perception. Brain. Stimul. 2018, 11, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Keywan, A.; Wuehr, M.; Pradhan, C.; Jahn, K. Noisy galvanic stimulation improves roll-tilt vestibular perception in healthy subjects. Front. Neurol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Wilkinson, D.; Nicholls, S.; Pattenden, C.; Kilduff, P.; Milberg, W. Galvanic vestibular stimulation speeds visual memory recall. Exp. Brain Res. 2008, 189, 243–248. [Google Scholar] [CrossRef]
- Hilliard, D.; Passow, S.; Thurm, F.; Schuck, N.W.; Garthe, A.; Kempermann, G.; Li, S.C. Noisy galvanic vestibular stimulation modulates spatial memory in young healthy adults. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Nusser, E.; Decker, J.; Krafczyk, S.; Straube, A.; Brandt, T.; Jahn, K.; Schniepp, R. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 2016, 86, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Boerner, J.C.; Pradhan, C.; Decker, J.; Jahn, K.; Brandt, T.; Schniepp, R. Stochastic resonance in the human vestibular system—Noise-induced facilitation of vestibulospinal reflexes. Brain. Stimul. 2018, 11, 261–263. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.D.; Abbott, D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 2009, 5, 1–9. [Google Scholar] [CrossRef]
- Moss, F.; Ward, L.M.; Sannita, W.G. Stochastic resonance and sensory information processing: A tutorial and review of application. Clin. Neurophysiol. 2004, 115, 267–281. [Google Scholar] [CrossRef]
- Bronstein, A.M.; Patel, M.; Arshad, Q. A brief review of the clinical anatomy of the vestibular-ocular connections—How much do we know? Eye 2015, 29, 163–170. [Google Scholar] [CrossRef]
- Dakin, C.J.; Inglis, J.T.; Chua, R.; Blouin, J.S. Muscle-specific modulation of vestibular reflexes with increased locomotor velocity and cadence. J. Neurophysiol. 2013, 110, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nepveu, J.F.; Mikhail, Y.; Pion, C.H.; Gossard, J.P.; Barthélemy, D. Assessment of vestibulocortical interactions during standing in healthy subjects. PLoS ONE 2020, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011, 67, 119–146. [Google Scholar] [CrossRef]
- Bense, S.; Stephan, T.; Yousry, T.A.; Brandt, T.; Dieterich, M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J. Neurophysiol. 2001, 85, 886–899. [Google Scholar] [CrossRef]
- Bucher, S.F.; Dieterich, M.; Wiesmann, M.; Weiss, A.; Zink, R.; Yousry, T.A.; Brandt, T. Cerebral functional magnetic resonance imaging of vestibular, auditory, and nociceptive areas during galvanic stimulation. Ann. Neurol. 1998, 44, 120–125. [Google Scholar] [CrossRef]
- Becker-Bense, S.; Wiloch, F.; Stephan, T.; Brendel, M.; Yakushev, I.; Habs, M.; Ziegler, S.; Herz, M.; Schwaiger, M.; Dieterich, M.; et al. Direct comparison of activation maps during galvanic vestibular stimulation: A hybrid H2[15O] PET-BOLD MRI activation study. PLoS ONE 2020, 15, e0233262. [Google Scholar] [CrossRef]
- Lobel, E.; Kleine, J.F.; Le Bihan, D.; Leroy-Willig, A.; Berthoz, A. Functional MRI of galvanic vestibular stimulation. J. Neurophysiol. 1998, 80, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Stephan, T.; Deutschländer, A.; Nolte, A.; Schneider, E.; Wiesmann, M.; Brandt, T.; Dieterich, M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 2005, 26, 721–732. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata, P.J.; Wolf, U. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Ertl, M.; Boegle, R. Investigating the vestibular system using modern imaging techniques—A review on the available stimulation and imaging methods. J. Neurosci. Methods. 2019, 326, 108363. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2018, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Cheung, B. Detection of cerebral oxyhaemoglobin changes during vestibular Coriolis cross-coupling stimulation using near infrared spectroscopy. Neurosci. Lett. 2006, 394, 83–87. [Google Scholar] [CrossRef]
- De Winkel, K.N.; Nesti, A.; Ayaz, H.; Bülthoff, H.H. Neural correlates of decision making on whole body yaw rotation: An fNIRS study. Neurosci. Lett. 2017, 654, 56–62. [Google Scholar] [CrossRef]
- Karim, H.T.; Fuhrman, S.I.; Furman, J.M.; Huppert, T.J. Neuroimaging to detect cortical projection of vestibular response to caloric stimulation in young and older adults using functional near-infrared spectroscopy (fNIRS). Neuroimage 2013, 76, 1–10. [Google Scholar] [CrossRef]
- Iida, M.; Haida, M.; Igarashi, M. Vertigo and cerebral hemoglobin changes during unilateral caloric stimulation: A near-infrared spectroscopy study. Ann. N. Y. Acad. Sci. 2009, 1164, 386–3869. [Google Scholar] [CrossRef]
- Shida, K.; Amimoto, K.; Sawa, K.; Ikeda, Y.; Fujino, Y.; Takahashi, H.; Makita, S. Changes of cortical activation during and after galvanic vestibular stimulation (GVS)—A functional near-infrared spectroscopy (fNIRS) study. J. Neurol. Sci. 2017, 381, 950–951. [Google Scholar] [CrossRef]
- Valdés, B.A.; Menon, C. Effects of Noisy Galvanic Vestibular Stimulation During a Bimanual Tracking Robotic Task. Front. Neurosci. 2019, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blanke, O.; Mast, F.W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 2012, 212, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. FNIRS Optodes’ Location Decider (fOLD): A toolbox for probe arrangement guided by brain regions-of-interest. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Sealed Envelope Ltd. Create a Blocked Randomisation List. 2019. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 2 December 2019).
- Jahani, S.; Fantana, A.L.; Harper, D.; Ellison, J.M.; Boas, D.A.; Forester, B.P. FNIRS can robustly measure brain activity during memory encoding and retrieval in healthy subjects. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Yang, C.; Lim, S.B.; Peters, S.; Eng, J.J.; Wolf, U. Cortical Activation During Shoulder and Finger Movements in Healthy Adults: A Functional Near-Infrared Spectroscopy (fNIRS) Study. Front. Hum. Neurosci. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Yücel, M.A.; Aasted, C.M.; Petkov, M.P.; Borsook, D.; Boas, D.A.; Becerra, L. Specificity of Hemodynamic Brain Responses to Painful Stimuli: A functional near-infrared spectroscopy study. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Setarehdan, S.K.; Boas, D.A.; Yücel, M.A. Motion artifact detection and correction in functional near-infrared spectroscopy: A new hybrid method based on spline interpolation method and Savitzky–Golay filtering. Neurophotonics 2018, 5, 015003. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, D.; Peng, K.; Lee, A.; Borsook, D.; Picard, R. Pain Detection with fNIRS-Measured Brain Signals: A Personalized Machine Learning Approach Using the Wavelet Transform and Bayesian Hierarchical Modeling with Dirichlet Process Priors. In Proceedings of the 2019 8th International Conference on Affective Computing and Intelligent Interaction Workshops and Demos (ACIIW), Cambridge, UK, 3–6 September 2019; pp. 304–309. [Google Scholar] [CrossRef]
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution and accuracy. NeuroImage 2004, 23, S275–S288. [Google Scholar] [CrossRef] [PubMed]
- Holper, L.; Shalóm, D.E.; Wolf, M.; Sigman, M. Understanding inverse oxygenation responses during motor imagery: A functional near-infrared spectroscopy study. Eur. J. Neurosci. 2011, 33, 2318–2328. [Google Scholar] [CrossRef]
- Maggioni, E.; Molteni, E.; Zucca, C.; Reni, G.; Cerutti, S.; Triulzi, F.M. Investigation of negative BOLD responses in human brain through NIRS technique. A visual stimulation study. Neuroimage 2015, 108, 410–422. [Google Scholar] [CrossRef]
- Sato, H.; Fuchino, Y.; Kiguchi, M.; Katura, T.; Maki, A.; Yoro, T.; Koizumi, H. Intersubject variability of near-infrared spectroscopy signals during sensorimotor cortex activation. J. Biomed. Optics. 2005, 10, 044001. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B. Dynamic models of BOLD contrast. Neuroimage 2012, 62, 953–961. [Google Scholar] [CrossRef]
- Mullinger, K.J.; Mayhew, S.D.; Bagshaw, A.P.; Bowtell, R.; Francis, S.T. Evidence that the negative BOLD response is neuronal in origin: A simultaneous EEG-BOLD-CBF study in humans. Neuroimage 2014, 94, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Shmuel, A.; Yacoub, E.; Pfeuffer, J.; Van de Moortele, P.F.; Adriany, G.; Hu, X. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002, 36, 1195–1210. [Google Scholar] [CrossRef]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Bense, S.; Lutz, S.; Drzezga, A.; Stephan, T.; Bartenstein, P. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb. Cortex. 2003, 13, 994–1007. [Google Scholar] [CrossRef]
- Suzuki, M.; Kitano, H.; Ito, R.; Kitanishi, T.; Yazawa, Y.; Ogawa, T.; Shiino, A.; Kitajima, K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Cogn. Brain Res. 2001, 12, 441–449. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Frank, S.M.; Greenlee, M.W. The parieto-insular vestibular cortex in humans: More than a single area? J. Neurophysiol. 2018, 120, 1438–1450. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 2014, 8, 1–16. [Google Scholar] [CrossRef]
- Wirth, A.M.; Frank, S.M.; Greenlee, M.W.; Beer, A.L. White Matter Connectivity of the Visual–Vestibular Cortex Examined by Diffusion-Weighted Imaging. Brain. Connect. 2018, 8, 235–244. [Google Scholar] [CrossRef]
- Menant, J.C.; Maidan, I.; Alcock, L.; Al-Yahya, E.; Cerasa, A.; Clark, D.J. A consensus guide to using functional near-infrared spectroscopy in posture and gait research. Gait. Posture. 2020, 82, 254–265. [Google Scholar] [CrossRef] [PubMed]
| HbO | |||||
|---|---|---|---|---|---|
| ROI | t | df | p-value | Conc. Diff. (10−8 mol) | 95% CI (10−8 mol) |
| All Ch. L | 0.14 | 11 | 0.887 | 0.75 | −10.64, 12.14 |
| All Ch. R | −0.23 | 11 | 0.822 | −1.28 | −13.56, 11.00 |
| BA40 L | 0.05 | 11 | 0.960 | 0.26 | −10.64, 11.16 |
| BA40 R | −0.54 | 11 | 0.602 | −2.72 | −13.89, 8.44 |
| BA39 L | 0.23 | 11 | 0.825 | 1.24 | −10.84, 13.33 |
| BA39 R | 0.02 | 11 | 0.980 | 0.16 | −13.50, 13.82 |
| HbR | |||||
| ROI | t | df | p-value | Conc. Diff. (10−8 mol) | 95% CI (10−8 mol) |
| All Ch. L | 2.33 | 11 | 0.040 * | 2.16 | 0.12, 4.20 |
| All Ch. R | 2.05 | 11 | 0.065 | 1.70 | −0.12, 3.52 |
| BA40 L | 2.46 | 11 | 0.032 * | 2.84 | 0.30, 5.39 |
| BA40 R | 1.94 | 11 | 0.079 | 2.23 | −0.30, 4.77 |
| BA39 L | 1.69 | 11 | 0.118 | 1.48 | −0.44, 3.40 |
| BA39 R | 1.12 | 11 | 0.285 | 1.16 | −1.12, 3.44 |
| Channel | Hemisphere | t | df | p-Value | Conc. Diff. (10−8 mol) | 95% CI (10−8 mol) |
|---|---|---|---|---|---|---|
| 1 | L | 2.41 | 11 | 0.035 * | 2.08 | 0.18, 3.99 |
| 7 | R | 2.24 | 11 | 0.047 * | 2.31 | 0.04, 4.59 |
| 2 | L | 2.00 | 11 | 0.071 | 4.35 | −0.45, 9.14 |
| 3 | L | 1.99 | 11 | 0.073 | 2.21 | −0.24, 4.66 |
| 10 | R | 1.76 | 11 | 0.106 | 1.43 | −0.35, 3.22 |
| 5 | L | 1.76 | 11 | 0.107 | 2.09 | −0.53, 4.71 |
| 9 | R | 1.73 | 11 | 0.111 | 1.96 | −0.53, 4.44 |
| 8 | R | 1.35 | 11 | 0.204 | 3.18 | −2.00, 8.36 |
| 6 | L | 1.02 | 11 | 0.332 | 1.46 | −1.71, 4.63 |
| 4 | L | 0.90 | 11 | 0.386 | 0.76 | −1.09, 2.60 |
| 11 | R | 0.81 | 11 | 0.436 | 1.21 | −2.08, 4.49 |
| 12 | R | 0.05 | 11 | 0.965 | 0.10 | −4.96, 5.16 |
| Scale: 1 (None)–5 (Very Strong) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Pain | 92% | 8% | 0% | 0% | 0% |
| Tingling | 75% | 25% | 0% | 0% | 0% |
| Itching | 92% | 8% | 0% | 0% | 0% |
| Burning | 75% | 25% | 0% | 0% | 0% |
| Dizziness | 100% | 0% | 0% | 0% | 0% |
| Fatigue | 83% | 17% | 0% | 0% | 0% |
| Nervousness | 92% | 8% | 0% | 0% | 0% |
| Difficulty in concentration | 67% | 33% | 0% | 0% | 0% |
| Headache | 100% | 0% | 0% | 0% | 0% |
| Unpleasantness | 100% | 0% | 0% | 0% | 0% |
| Metallic Taste | 100% | 0% | 0% | 0% | 0% |
| Visual Sensation | 100% | 0% | 0% | 0% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, B.A.; Lajoie, K.; Marigold, D.S.; Menon, C. Cortical Effects of Noisy Galvanic Vestibular Stimulation Using Functional Near-Infrared Spectroscopy. Sensors 2021, 21, 1476. https://doi.org/10.3390/s21041476
Valdés BA, Lajoie K, Marigold DS, Menon C. Cortical Effects of Noisy Galvanic Vestibular Stimulation Using Functional Near-Infrared Spectroscopy. Sensors. 2021; 21(4):1476. https://doi.org/10.3390/s21041476
Chicago/Turabian StyleValdés, Bulmaro A., Kim Lajoie, Daniel S. Marigold, and Carlo Menon. 2021. "Cortical Effects of Noisy Galvanic Vestibular Stimulation Using Functional Near-Infrared Spectroscopy" Sensors 21, no. 4: 1476. https://doi.org/10.3390/s21041476
APA StyleValdés, B. A., Lajoie, K., Marigold, D. S., & Menon, C. (2021). Cortical Effects of Noisy Galvanic Vestibular Stimulation Using Functional Near-Infrared Spectroscopy. Sensors, 21(4), 1476. https://doi.org/10.3390/s21041476







