Monitoring Contractile Cardiomyocytes via Impedance Using Multipurpose Thin Film Ruthenium Oxide Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Setups
2.1.1. Well Plate
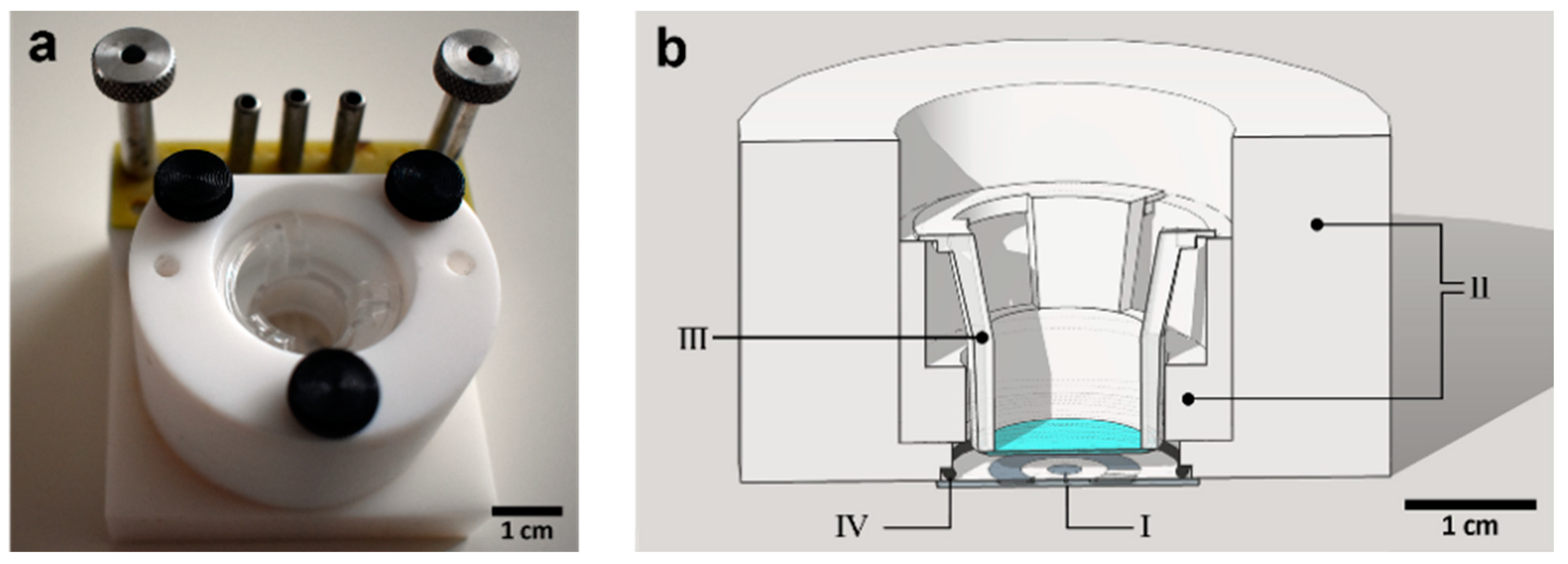
2.1.2. Teflon Chip Holder
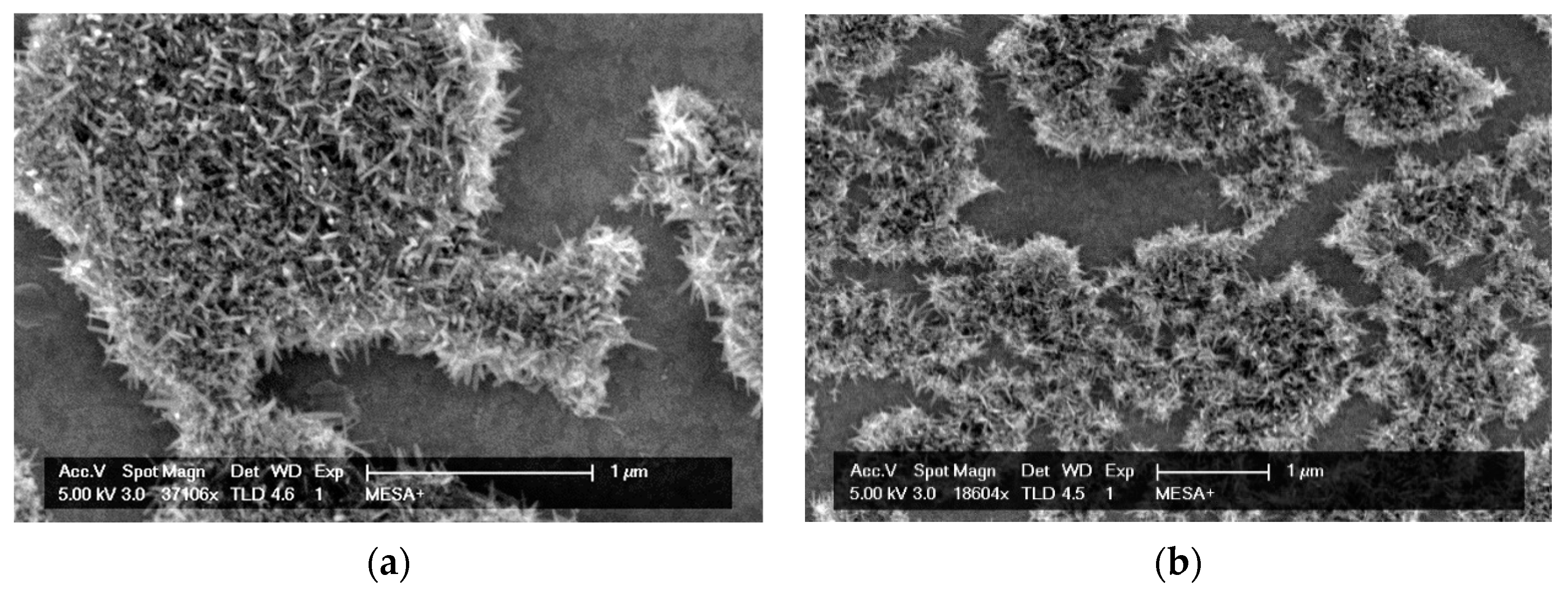
2.1.3. RuOx Electrode
2.2. Cells Seeding and Culture
2.3. Impedance and Visual Recording
3. Results and Discussion
3.1. Measurement Frequency
3.2. Measurement in a Well Plate with Pt Wires
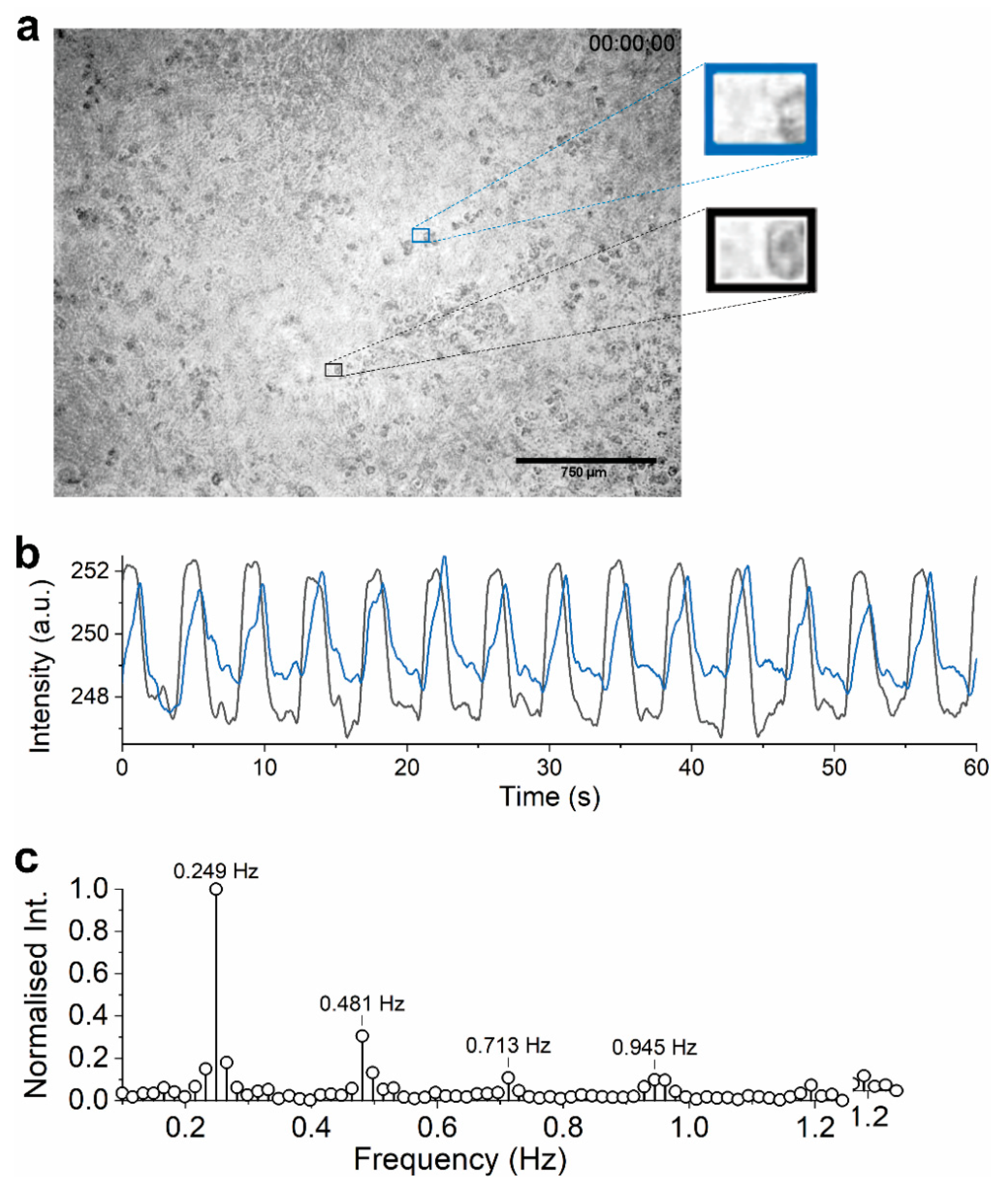
3.2.1. Visual Data
3.2.2. Electrical Data
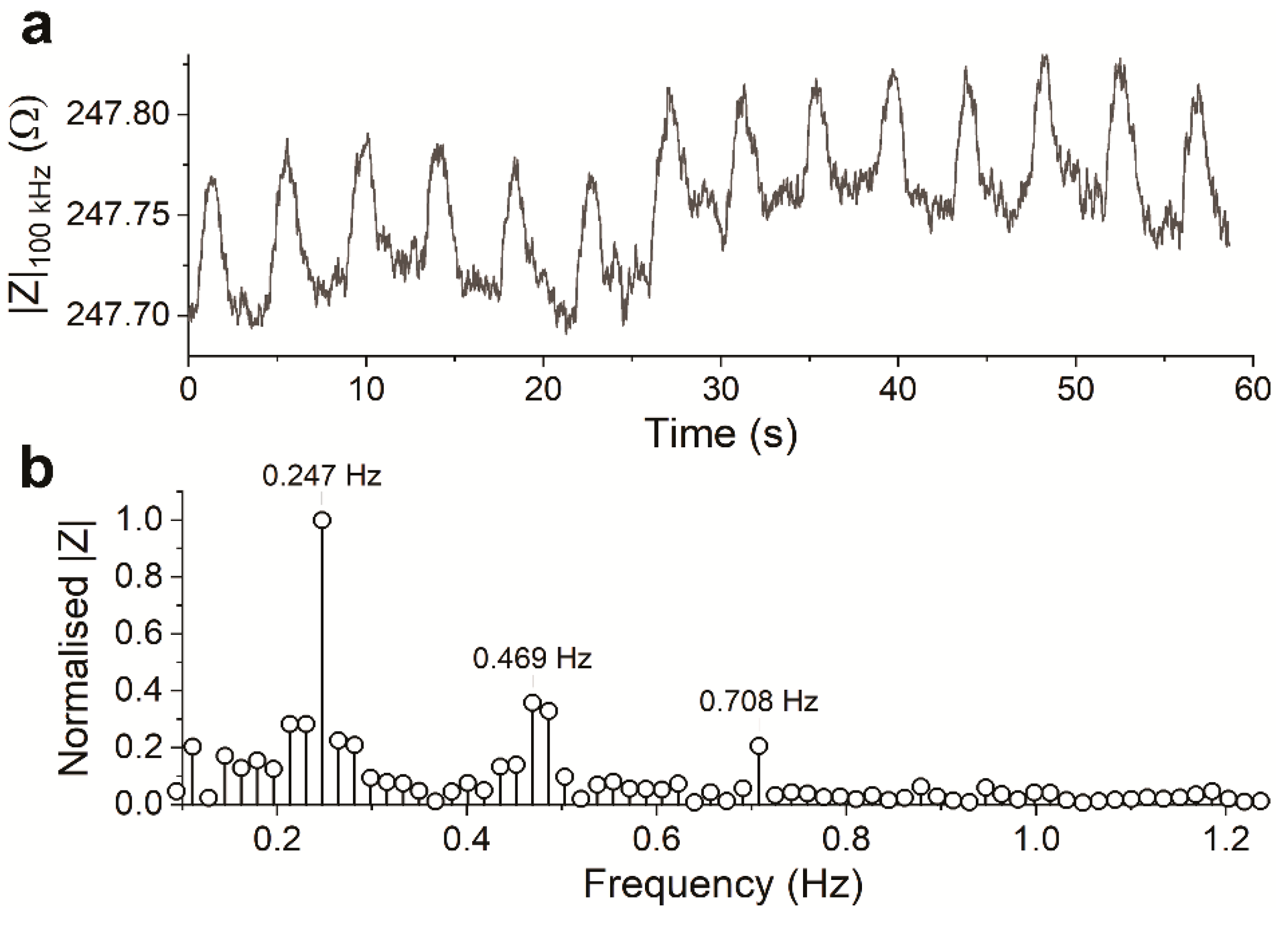
3.3. Measurement in Chip Holder with RuOx Electrode
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Van der Meer, A.D.; Van den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr. Biol. 2012, 4, 461–470. [Google Scholar] [CrossRef]
- Sung, J.H.; Wang, Y.I.; Sriram, N.N.; Jackson, M.; Long, C.; Hickman, J.J.; Shuler, M.L. Recent Advances in Body-on-a-Chip Systems. Anal. Chem. 2019, 91, 330–351. [Google Scholar] [CrossRef]
- Shiku, H. Electrochemical Biosensing System for Single Cells, Cellular Aggregates and Microenvironments. Anal. Sci. 2019, 35, 29–38. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—From 2D culture to organ-on-chip. Lab Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef]
- Tanumihardja, E.; Olthuis, W.; Van den Berg, A. Ruthenium Oxide Nanorods as Potentiometric pH Sensor for Organs-On-Chip Purposes. Sensors 2018, 18, 2901. [Google Scholar] [CrossRef]
- Mingels, R.; Kalsi, S.; Cheong, Y.; Morgan, H. Iridium and Ruthenium oxide miniature pH sensors: Long-term performance. Sens. Actuators B Chem. 2019, 297, 126779. [Google Scholar] [CrossRef]
- Tanumihardja, E.; Slaats, R.H.; Van der Meer, A.D.; Passier, R.; Olthuis, W.; Van den Berg, A. Measuring Both pH and O2 with a Single On-Chip Sensor in Cultures of Human Pluripotent Stem Cell-Derived Cardiomyocytes to Track Induced Changes in Cellular Metabolism. ACS Sens. 2021, 6, 267–274. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jung, H.; Lee, C.; Kim, M.H.; Lee, Y. Biological application of RuO2 nanorods grown on a single carbon fiber for the real-time direct nitric oxide sensing. Sens. Actuators B Chem. 2014, 191, 298–304. [Google Scholar] [CrossRef]
- Tanumihardja, E.; Paradelo Rodríguez, A.; Loessberg-Zahl, J.T.; Mei, B.; Olthuis, W.; Van den Berg, A. On-chip electrocatalytic NO sensing using ruthenium oxide nanorods. Sens. Actuators B Chem. 2021, 334. [Google Scholar] [CrossRef]
- Atmaramani, R.; Chakraborty, B.; Rihani, R.T.; Usoro, J.; Hammack, A.; Abbott, J.; Nnoromele, P.; Black, B.J.; Pancrazio, J.J.; Cogan, S.F. Ruthenium oxide based microelectrode arrays for in vitro and in vivo neural recording and stimulation. Acta Biomater. 2020, 101, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Yu, D.; Lai, Y.-C.; Lin, K.-Y.; Liau, I. Characterization of the Mechanodynamic Response of Cardiomyocytes with Atomic Force Microscopy. Anal. Chem. 2013, 85, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Laurila, E.; Ahola, A.; Hyttinen, J.; Aalto-Setälä, K. Methods for in vitro functional analysis of iPSC derived cardiomyocytes—Special focus on analyzing the mechanical beating behavior. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1863, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.L.; Graham, B.T.; Pabon, L.M.; Han, S.J.; Murry, C.E.; Sniadecki, N.J. Measuring the Contractile Forces of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes with Arrays of Microposts. J. Biomech. Eng. 2014, 136, 051005–05100510. [Google Scholar] [CrossRef]
- Huebsch, N.; Loskill, P.; Mandegar, M.A.; Marks, N.C.; Sheehan, A.S.; Ma, Z.; Mathur, A.; Nguyen, T.N.; Yoo, J.C.; Judge, L.M.; et al. Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng. Part C Methods 2015, 21, 467–479. [Google Scholar] [CrossRef]
- Peters, M.F.; Lamore, S.D.; Guo, L.; Scott, C.W.; Kolaja, K.L. Human Stem Cell-Derived Cardiomyocytes in Cellular Impedance Assays: Bringing Cardiotoxicity Screening to the Front Line. Cardiovasc. Toxicol. 2014, 15, 127–139. [Google Scholar] [CrossRef]
- Liu, J.; Sun, N.; Bruce, M.A.; Wu, J.C.; Butte, M.J. Atomic Force Mechanobiology of Pluripotent Stem Cell-Derived Cardiomyocytes. PLoS ONE 2012, 7, e37559. [Google Scholar] [CrossRef]
- Lehmann, M.; Baumann, W.; Brischwein, M.; Gahle, H.-J.; Freund, I.; Ehret, R.; Drechsler, S.; Palzer, H.; Kleintges, M.; Sieben, U.; et al. Simultaneous measurement of cellular respiration and acidification with a single CMOS ISFET. Biosens. Bioelectron. 2001, 16, 195–203. [Google Scholar] [CrossRef]
- Lindström, S.; Andersson-Svahn, H. Miniaturization of biological assays—Overview on microwell devices for single-cell analyses. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 308–316. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fernández-Garibay, X.; Castaño, A.G.; De Chiara, F.; Hernández-Albors, A.; Balaguer-Trias, J.; Ramón-Azcón, J. Muscle-on-a-chip with an on-site multiplexed biosensing system for in situ monitoring of secreted IL-6 and TNF-α. Lab Chip 2019, 19, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Wark, A.W.; Lee, H.J.; Corn, R.M. Multiplexed Detection Methods for Profiling MicroRNA Expression in Biological Samples. Angew. Chem. Int. Ed. 2008, 47, 644–652. [Google Scholar] [CrossRef]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef]
- Van der Helm, M.W.; Henry, O.Y.F.; Bein, A.; Hamkins-Indik, T.; Cronce, M.J.; Leineweber, W.D.; Odijk, M.; Van Der Meer, A.D.; Eijkel, J.C.T.; Ingber, D.E.; et al. Non-invasive sensing of transepithelial barrier function and tissue differentiation in organs-on-chips using impedance spectroscopy. Lab Chip 2019, 19, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Segerink, L.I.; Sprenkels, A.J.; Ter Braak, P.M.; Vermes, I.; Berg, A.V.D. On-chip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip 2010, 10, 1018. [Google Scholar] [CrossRef]
- De Wagenaar, B.; Dekker, S.; De Boer, H.L.; Bomer, J.G.; Olthuis, W.; Van den Berg, A.; Segerink, L.I. Towards microfluidic sperm refinement: Impedance-based analysis and sorting of sperm cells. Lab Chip 2016, 16, 1514–1522. [Google Scholar] [CrossRef]
- De Bruijn, D.S.; Ter Braak, P.M.; Van de Waal, D.B.; Olthuis, W.; Van den Berg, A. Coccolithophore calcification studied by single-cell impedance cytometry: Towards single-cell PIC:POC measurements. Biosens. Bioelectron. 2021, 173, 112808. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluidics 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Petchakup, C.; Li, K.H.H.; Hou, H.W. Advances in Single Cell Impedance Cytometry for Biomedical Applications. Micromachines 2017, 8, 87. [Google Scholar] [CrossRef]
- Chen, Z.G.; Pei, F.; Pei, Y.T.; De Hosson, J.T.M. A Versatile Route for the Synthesis of Single Crystalline Oxide Nanorods: Growth Behavior and Field Emission Characteristics. Cryst. Growth Des. 2010, 10, 2585–2590. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Slaats, R.H.; Schwach, V.; Rivera-Arbelaez, J.M.; Tertoolen, L.G.; Van Meer, B.J.; Molenaar, R.; Mummery, C.L.; Claessens, M.M.; Passier, R. A cardiomyocyte show of force: A fluorescent alpha-actinin reporter line sheds light on human cardiomyocyte contractility versus substrate stiffness. J. Mol. Cell. Cardiol. 2020, 141, 54–64. [Google Scholar] [CrossRef]
- Birket, M.J.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; Van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.P.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef]
- Brischwein, M.; Grothe, H.; Wiest, J.; Zottmann, M.; Ressler, J.; Wolf, B. Planar ruthenium oxide sensors for cell-on-a-chip metabolic studies. Chem. Anal. 2009, 54, 1193–1201. [Google Scholar]
- Gawad, S.; Cheung, K.; Seger, U.; Bertsch, A.; Renaud, P. Dielectric spectroscopy in a micromachined flow cytometer: Theoretical and practical considerations. Lab Chip 2004, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Tran, D.D.; George, S.C. Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells 2013, 31, 829–837. [Google Scholar] [CrossRef]
- Hayashi, T.; Tokihiro, T.; Kurihara, H.; Yasuda, K. Community effect of cardiomyocytes in beating rhythms is determined by stable cells. Sci. Rep. 2017, 7, 3–8. [Google Scholar] [CrossRef]
- Kim, J.J.; Yang, L.; Lin, B.; Zhu, X.; Sun, B.; Kaplan, A.D.; Bett, G.C.; Rasmusson, R.L.; London, B.; Salama, G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2015, 81, 81–93. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanumihardja, E.; de Bruijn, D.S.; Slaats, R.H.; Olthuis, W.; van den Berg, A. Monitoring Contractile Cardiomyocytes via Impedance Using Multipurpose Thin Film Ruthenium Oxide Electrodes. Sensors 2021, 21, 1433. https://doi.org/10.3390/s21041433
Tanumihardja E, de Bruijn DS, Slaats RH, Olthuis W, van den Berg A. Monitoring Contractile Cardiomyocytes via Impedance Using Multipurpose Thin Film Ruthenium Oxide Electrodes. Sensors. 2021; 21(4):1433. https://doi.org/10.3390/s21041433
Chicago/Turabian StyleTanumihardja, Esther, Douwe S. de Bruijn, Rolf H. Slaats, Wouter Olthuis, and Albert van den Berg. 2021. "Monitoring Contractile Cardiomyocytes via Impedance Using Multipurpose Thin Film Ruthenium Oxide Electrodes" Sensors 21, no. 4: 1433. https://doi.org/10.3390/s21041433
APA StyleTanumihardja, E., de Bruijn, D. S., Slaats, R. H., Olthuis, W., & van den Berg, A. (2021). Monitoring Contractile Cardiomyocytes via Impedance Using Multipurpose Thin Film Ruthenium Oxide Electrodes. Sensors, 21(4), 1433. https://doi.org/10.3390/s21041433






