BPM Support for Patient-Centred Clinical Pathways in Chronic Diseases
Abstract
:1. Introduction
1.1. Changes in the Social and Work Culture of Health Care Personnel
- Remote contact, no need to spend time and costs in reaching the visit/consultation by the patient or medical staff;
- Possibility of remote consultations with specialists unavailable in the patient’s place of residence;
- The ability to monitor the patient’s health online without the need to hospitalize the patient;
- The possibility of collecting and analysing data sets characterizing the patient’s condition in selected periods of time without the need for hospitalization;
- The possibility of using predictive analytics and artificial intelligence to prevent foreseeable acute states;
- Improvement of the quality of services provided thanks to the use of elements of telemedicine and hyperautomation.
1.2. Clinical Pathways as Patterns of Knowledge-Intensive Business Processes
- The intervention was a structured multidisciplinary plan of care;
- The intervention was used to channel the translation of guidelines or evidence into a local organization and its structures;
- The intervention detailed the steps in a course of treatment or care in a plan, pathway, algorithm, guideline, protocol, or another inventory of actions;
- The intervention had time-frames or criteria-based progression (that is, steps were taken if designated criteria were met).
- The patient is treated, not the disease;
- The patients and their caregivers should be involved in the treatment process;
- Different clinical and peri-clinical pathways (management, support, logistics and others) must form a coherent system;
- Knowledge available in the form of clinical pathways should be available and be reflected in IT and telemedicine technology used on a daily basis by all stakeholders of the diagnostic and therapeutic process.
1.3. Chronic Disease Management
- A level III CP developed on the basis of the applicable guidelines (CP level I and II) in a way that individually adjusts the therapy to a specific patient, taking into account the broad clinical background, including parallel diagnostic and therapeutic procedures in the field of comorbidities;
- Clear definition of goals and parameters used to assess the implementation of tasks set at specific stages of the therapeutic process;
- A clear and understandable description of the forms of medical care planned for implementation and the manner of patient participation in them, so that the patient is aware of the conditions and what activities and therapeutic activities should be undertaken, in what situations he or she should consult the attending physician, and what forms of activity should be strictly abandoned or avoided.
2. Materials and Methods
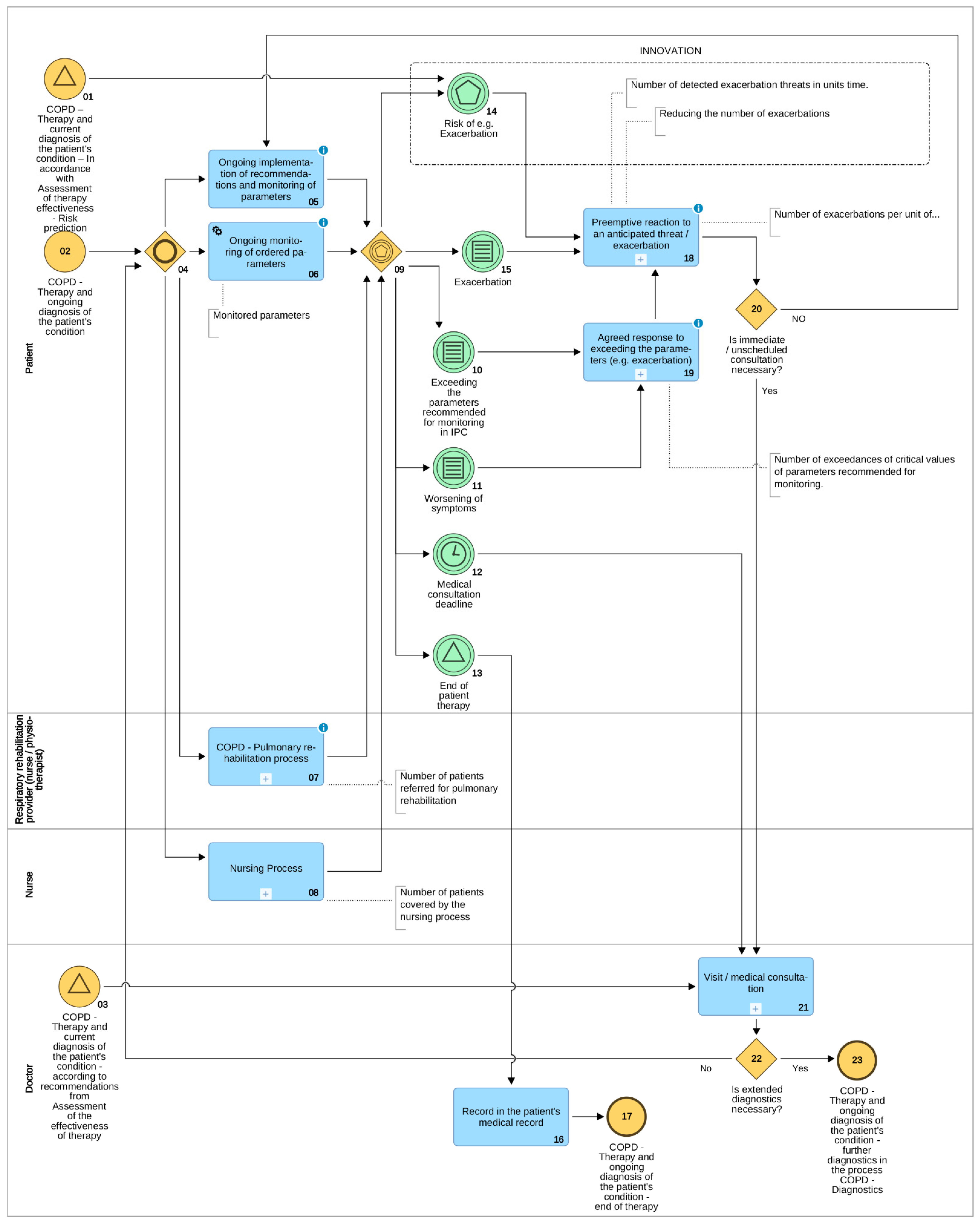
3. Results: Clinical Pathways for Chronic Obstructive Pulmonary Disease
- Raising the feeling of security of patients and their caregivers;
- Lifting the burden off of the healthcare system by eliminating visits/medical consultations which are deemed non-essential according to the adopted IPC;
- On the basis of data collected and analysed on an ongoing basis, the prevention of exacerbations, which usually permanently deteriorate the state of the patient and which may also consist a risk of death;
- An ex post analysis on the basis of collected data of the introduced therapies with a view to verifying existing, as well as creating new fact-driven medical knowledge.
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dumas, M.; La Rosa, M.; Mendling, J.; Reijers, H. Fundamentals of Business Process Management, 2nd ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Becker, J.; Fischer, R.; Janiesch, C. Optimizing U.S. Health Care Processes-A Case Study in Business Process Management. AMCIS 2007 Proceedings. 2007. Available online: https://aisel.aisnet.org/amcis2007/504 (accessed on 31 October 2021).
- De Ramón Fernandez, A.; Ruiz Fernandez, D.; Sabuco García, Y. Business Process Management for optimizing clinical processes: A systematic literature review. Health Inform. J. 2020, 26, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Mans, R.; Aalst, W.; Vanwersch, R. Process Mining in Healthcare Evaluating and Exploiting Operational Healthcare Processes; Springer: Berlin, Germany, 2015. [Google Scholar]
- Andellini, M.; Riesgo, S.F.; Morolli, F.; Ritrovato, M.; Cosoli, P.; Petruzzellis, S.; Rosso, N. Experimental application of Business Process Management technology to manage clinical pathways: A pediatric kidney transplantation follow up case. BMC Med. Inform. Decis. Mak. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- OECD/EU. Health at a Glance: Europe 2016–State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Scaratti, C.; Leonardi, M.; Silvaggi, F.; Ávila, C.; Muñoz-Murillo, A.; Ferraina, S. Mapping European Welfare Models: State of the Art of Strategies for Professional Integration and Reintegration of Persons with Chronic Diseases. Int. J. Environ. Res. Public Health 2018, 15, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, S.; Kumar, G.; Dhaliwal, R.; Paulson, K.; Agrawal, A.; Koul, A.; Dandona, L. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, e1363–e1374. [Google Scholar] [CrossRef] [Green Version]
- Merriam-Webster (n.d.) Telemedicine. Available online: https://www.merriam-webster.com/dictionary/telemedicine (accessed on 17 August 2021).
- Gelburd, R. Telehealth Claim Lines Rise 2938 Percent from November 2019 to November 2020. Am. J. Manag. Care 2021. Available online: https://www.ajmc.com/view/telehealth-claim-lines-rise-2-938-percent-from-november-2019-to-november-2020 (accessed on 12 July 2021).
- de Boer, L. Process Management in Global Healthcare: Innovation in a Crisis. Available online: https://www.signavio.com/post/process-management-in-global-healthcare (accessed on 20 May 2021).
- Amantea, I.; Sulis, E.; Boella, G.; Crespo, A.; Bianca, D.; Brunetti, E.; Marinello, R.; Grosso, M.; Zoels, J.; Visciola, M.; et al. Adopting Technological Devices in Hospital at Home: A Modelling and Simulation Perspective. In Proceedings of the 10th International Conference on Simulation and Modeling Methodologies, Technologies and Applications-SIMULTECH, Paris, France, 8–10 July 2020; pp. 110–119. [Google Scholar]
- COVID-19 Healthcare Coalition. Telehealth Impact Study: 2020. Available online: https://www.ama-assn.org/system/files/2020-10/telehealth-impact-study.pdf (accessed on 20 August 2021).
- Navaz, A.; Serhani, M.; El Kassabi, H.; Al-Qirim, N.; Ismail, H. Trends, Technologies, and Key Challenges in Smart and Connected Healthcare. IEEE Access 2021, 9, 74044–74067. [Google Scholar] [CrossRef]
- Schwab, J. Perspective on mHealth Concepts to Ensure Users’ Empowerment—From Adverse Event Tracking for COVID-19 Vaccinations to Oncological Treatment. IEEE Access 2021, 9, 83863–83875. [Google Scholar] [CrossRef]
- Prodhan, U.K.; Rahman, M.Z.; Jahan, I.; Abid, A.; Bellah, M. Development of a portable telemedicine tool for remote diagnosis of telemedicine application. In Proceedings of the International Conference on Computing, Communication and Automation (ICCCA), Greater Noida, India, 5–6 May 2017; pp. 287–292. [Google Scholar]
- Marrella, A.; Mecella, M.; Sharf, M.; Catarci, T. The TESTMED Project Experience. Process-aware Enactment of Clinical Guidelines through Multimodal Interfaces. arXiv 2019, arXiv:abs/1807.02022. [Google Scholar]
- Szelągowski, M.; Berniak-Woźny, J. A Process-Centered Approach to the Description of Clinical Pathways -Forms and Determinants. Int. J. Environ. Res. Public Health 2019, 16, 2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bleser, L.; Depreitere, R.; De Waele, K.; Vanhaecgt, K.; Vlayen, J.; Sermus, W. Defining pathways. J. Nurs. Manag. 2006, 14, 553–563. [Google Scholar] [CrossRef] [PubMed]
- European Pathway Association (EPA). Care Pathways. 2005. Available online: http://e-p-a.org/care-pathways/ (accessed on 12 May 2021).
- IEEE European Public Policy Committee. ICT for the Prevention of Noncommunicable Diseases and Health Promotion in Europe. 2020. Available online: https://www.ieee.org/content/dam/ieee-org/ieee/web/org/about/european-public-policy/eppc-whitepaper-ict-for-health.pdf (accessed on 18 April 2021).
- Kinsman, L.; Rotter, T.; James, E.; Snow, P.; Willis, J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szelągowski, M. Dynamic Business Process Management in the Knowledge Economy: Creating Value from Intellectual Capital; Lecture Notes in Networks and Systems 71; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Baptista dos Santos França, J.; Manhães Netto, J.; Carvalho, E.S.; Santoro, F.M.; Araujo Baião, F.; Pimentel, M. KIPO: The knowledge-intensive process ontology. Softw. Syst. Modeling 2015, 14, 1127–1157. [Google Scholar] [CrossRef]
- Campbell, H.; Hotchkiss, R.; Bradshaw, N.; Porteous, M. Integrated care pathways. Br. Med. J. (BMJ) 1998, 316, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.; Radnor, Z. An integrative approach to improving patient care pathways. Int. J. Health Care Qual. Assur. 2017, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). About Chronic Diseases. 2021. Available online: https://www.cdc.gov/chronicdisease/about/index.htm (accessed on 3 March 2021).
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 10, CD007228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, S. Telemedicine in the Time of COVID and Beyond. J. Adolesc. Health 2020, 67, 145–146. [Google Scholar] [CrossRef] [PubMed]
- NSW Government. Individual Health Care Planning. 2020. Available online: https://www.education.nsw.gov.au/student-wellbeing/health-and-physical-care/health-care-procedures/individual-planning (accessed on 10 April 2021).
- Fertel, B.S.; Podolsky, S.R.; Mark, J.; McKinsey, R.M.; Ladd, M.E.; Smalley, C.M. Impact of an individual plan of care for frequent and high utilizers in a large healthcare system. Am. J. Emerg. Med. 2019, 37, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.; Bae, J.; Kipnes, J.; Velazquez, M.; Thomas, S.; Setji, N. The highest utilizers of care: Individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J. Hosp. Med. 2015, 10, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2021. Available online: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed on 10 May 2021).
- Schrijvers, G. Integrated Care. Better and Cheaper; Amsterdam Red Business Information: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hayes, R.; Kyer, B.; Weber, E. The Case Study Cookbook; Worcester, Polytechnic Institute: Worcester, MA, USA, 2015. [Google Scholar]
- Davey, L. The Application of Case Study Evaluations; ERIC/TM Digest: Washington, DC, USA, 1991. [Google Scholar]
- Mertens, D. Research and Evaluation in Education and Psychology: Integrating Diversity with Quantitative, Qualitative, and Mixed Methods; Sage Publications: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Yin, R.L. Case Study Research: Design and Methods, 4th ed.; SAGE Publications: Thousand Oaks, CA, USA, 2009. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelągowski, M.; Berniak-Woźny, J.; Lipiński, C. BPM Support for Patient-Centred Clinical Pathways in Chronic Diseases. Sensors 2021, 21, 7383. https://doi.org/10.3390/s21217383
Szelągowski M, Berniak-Woźny J, Lipiński C. BPM Support for Patient-Centred Clinical Pathways in Chronic Diseases. Sensors. 2021; 21(21):7383. https://doi.org/10.3390/s21217383
Chicago/Turabian StyleSzelągowski, Marek, Justyna Berniak-Woźny, and Cezary Lipiński. 2021. "BPM Support for Patient-Centred Clinical Pathways in Chronic Diseases" Sensors 21, no. 21: 7383. https://doi.org/10.3390/s21217383
APA StyleSzelągowski, M., Berniak-Woźny, J., & Lipiński, C. (2021). BPM Support for Patient-Centred Clinical Pathways in Chronic Diseases. Sensors, 21(21), 7383. https://doi.org/10.3390/s21217383






