Correlates of Balance and Aerobic Indices in Lower-Limb Prostheses Users on Arm Crank Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Balance
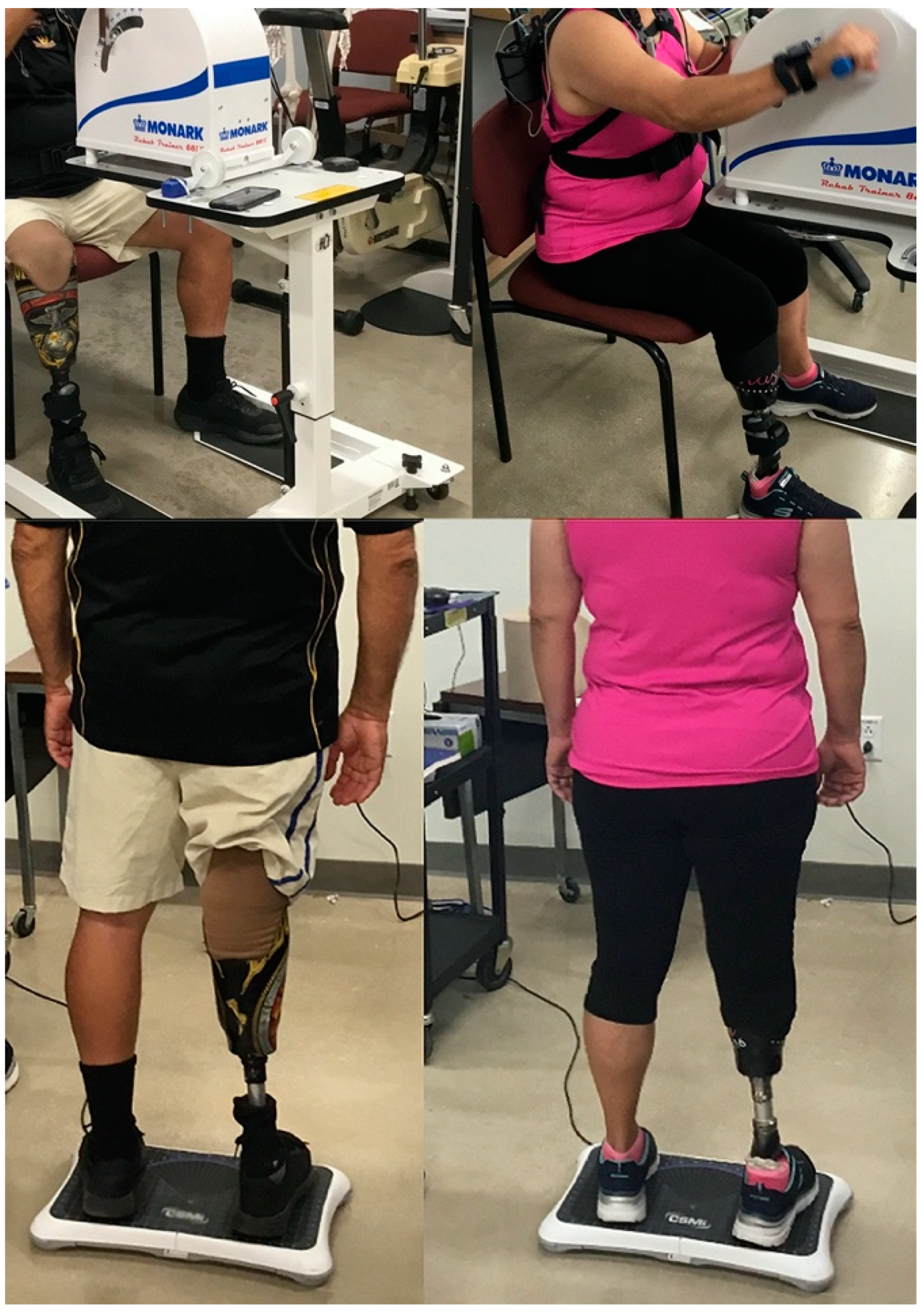
2.2. Arm Crank Ergometer (ACE)
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nederhand, M.J.; Van Asseldonk, E.H.F.; van der Kooij, H.; Rietman, H.S. Dynamic Balance Control (DBC) in lower leg amputee subjects; contribution of the regulatory activity of the prosthesis side. Clin. Biomech. 2012, 27, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Nadollek, H.; Brauer, S.; Isles, R. Outcomes after trans-tibial amputation: The relationship between quiet stance ability, strength of hip abductor muscles and gait. Physiother. Res. Int. 2002, 7, 203–214. [Google Scholar] [CrossRef]
- Curtze, C.; Hof, A.L.; Postema, K.; Otten, B. The relative contributions of the prosthetic and sound limb to balance control in unilateral transtibial amputees. Gait Posture 2012, 36, 276–281. [Google Scholar] [CrossRef]
- Barnett, C.T.; Vanicek, N.; Polman, R.C.J. Postural responses during volitional and perturbed dynamic balance tasks in new lower limb amputees: A longitudinal study. Gait Posture 2013, 37, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Rougier, P.R.; Bergeau, J. Biomechanical analysis of postural control of persons with transtibial or transfemoral amputation. Am. J. Phys. Med. Rehabil. 2009, 88, 896–903. [Google Scholar] [CrossRef]
- Gaunaurd, I.; Gailey, R.; Hafner, B.J.; Gomez-Marin, O.; Kirk-Sanchez, N. Postural asymmetries in transfemoral amputees. Prosthet. Orthot. Int. 2011, 35, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.; Mancini, M.; Peterka, R.; Hayes, T.; Horak, F. Validating and calibrating the nintendo Wii balance board to derive reliable center of pressure measures. Sensors 2014, 14, 18244–18267. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Bryant, A.L.; Pua, Y.; McCrory, P.; Bennell, K.; Hunt, M. Validity and reliability of the Nintendo Wii Balance Board for assessment of standing balance. Gait Posture 2010, 31, 307–310. [Google Scholar] [CrossRef]
- Clark, R.A.; Mentiplay, B.F.; Pua, Y.-H.; Bower, K.J. Reliability and validity of the Wii Balance Board for assessment of standing balance: A systematic review. Gait Posture 2018, 61, 40–54. [Google Scholar] [CrossRef]
- Genin, J.J.; Bastien, G.J.; Franck, B.; Detrembleur, C.; Willems, P.A. Effect of speed on the energy cost of walking in unilateral traumatic lower limb amputees. Eur. J. Appl. Physiol. 2008, 103, 655–663. [Google Scholar] [CrossRef]
- Starholm, I.M.; Mirtaheri, P.; Kapetanovic, N.; Versto, T.; Skyttemyr, G.; Westby, F.T.; Gjovaag, T. Energy expenditure of transfemoral amputees during floor and treadmill walking with different speeds. Prosthet. Orthot. Int. 2016, 40, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, R.; Perry, J.; Antonelli, D.; Hislop, H. Energy cost of walking of amputees. J. Bone Jt. Surg. 1976, 58, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Göktepe, A.S.; Cakir, B.; Yilmaz, B.; Yazicioglu, K. Energy expenditure of walking with prostheses: Comparison of three amputation levels. Prosthet. Orthot. Int. 2010, 34, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, K.; Hansen, J.; Sue, D.; Stringer, W.; Sietsema, K.; Sun, X.; Whipp, B. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Davidoff, G.N.; Lampman, R.M.; Westbury, L.; Deron, J.; Finestone, H.M.; Islam, S. Exercise testing and training of persons with dysvascular amputation: Safety and efficacy of arm ergometry. Arch. Phys. Med. Rehabil. 1992, 73, 334–338. [Google Scholar] [CrossRef]
- Vestering, M.M.; Schoppen, T.; Dekker, R.; Wempe, J.; Geertzen, J.H.B. Development of an exercise testing protocol for patients with a lower limb amputation: Results of a pilot study. Int. J. Rehabil. Res. 2005, 28, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenka, P.; Tiberwala, D.N. Effect of Stump Length on Postural Steadiness During Quiet Stance in Unilateral Trans-Tibial Amputee. Al Ameen J. Med. Sci. 2010, 3, 50–57. [Google Scholar]
- Hermodsson, Y.; Ekdahl, C.; Persson, B.M.; Roxendal, G. Standing balance in trans-tibial amputees following vascular disease or trauma. Prosthetics Orthot. Int. 1994, 18, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Quai, T.M.; Brauer, S.G.; Nitz, J.C. Somatosensation, circulation and stance balance in elderly dysvascular transtibial amputees. Clin. Rehabil. 2005, 19, 668–676. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Erjavec, T.; Vidmar, G.; Burger, H. Exercise testing as a screening measure for ability to walk with aprosthesis after transfemoral amputation due to peripheral vascular disease. Disabil. Rehabil. 2014, 36, 1148–1155. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport Sci. 1992, 17, 338–345. [Google Scholar]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription 10th Edition, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Hubbard, B.; Pothier, D.; Hughes, C.; Rutka, J. A portable, low-cost system for posturography: A platform for longitudinal balance telemetry. J. Otolaryngol. Head Neck Surg. 2012, 41, S31–S35. [Google Scholar] [PubMed]
- Bartlett, H.L.; Ting, L.H.; Bingham, J.T. Accuracy of force and center of pressure measures of the Wii Balance Board. Gait Posture 2014, 39, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koltermann, J.; Gerber, M.; Beck, H.; Beck, M. Validation of the HUMAC Balance System in Comparison with Conventional Force Plates. Technologies 2017, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Mayer, Á.; Tihanyi, J.; Bretz, K.; Csende, Z.; Bretz, É.; Horváth, M. Adaptation to altered balance conditions in unilateral amputees due to atherosclerosis: A randomized controlled study. BMC Musculoskelet. Disord. 2011, 12, 118. [Google Scholar] [CrossRef] [Green Version]
- Howard, C.L.; Perry, B.; Chow, J.W.; Wallace, C.; Stokic, D.S. Increased alertness, better than posture prioritization, explains dual-task performance in prosthesis users and controls under increasing postural and cognitive challenge. Exp. Brain Res. 2017, 235, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-S.; Lee, G. Validity and reliability of balance assessment software using the Nintendo Wii balance board: Usability and validation. J. Neuroeng. Rehabil. 2014, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duclos, C.; Roll, R.; Kavounoudias, A.; Roll, J.-P.; Forget, R. Vibration-induced post-effects: A means to improve postural asymmetry in lower leg amputees? Gait Posture 2007, 26, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; McConville, K.M.V.; Masani, K. Arm movement improves performance in clinical balance and mobility tests. Gait Posture 2011, 33, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Wing, A.; Morris, N. Oxygen uptake and heart rate kinetics during heavy exercise: A comparison between arm cranking and leg cycling. Eur. J. Appl. Physiol. 2002, 88, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.L.; Williamson, P.; Anderson, W.; Ross, R.; McCafferty, S.; Fettes, P. Cardiopulmonary exercise testing: Arm crank vs cycle ergometry. Anaesthesia 2013, 68, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Barnes, M.J.; Hodges, L.D. Comparison study of treadmill versus arm ergometry. Clin. Physiol. Funct. Imaging 2011, 31, 326–331. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Gumber, A.; Crank, H.; Klonizakis, M. Validation of an arm crank ergometer test for use in sedentary adults. J. Sports Sci. Med. 2017, 16, 558–564. [Google Scholar]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Oyabu, H.; Nagakura, Y.; Ojima, I.; Otsuka, H.; Nakagawa, A. Physical fitness of lower limb amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Sawamura, S.; Fujita, H.; Nakajima, S.; Ojima, I.; Oyabu, H.; Nagakura, Y.; Otsuka, H.; Nakagawa, A. Effect of endurance training program based on anaerobic threshold (AT) for lower limb amputees. J. Rehabil. Res. Dev. 2001, 38, 7–11. [Google Scholar]
- Schoppen, T.; Boonstra, A.; Groothoff, J.W.; de Vries, J.; Göeken, L.N.; Eisma, W.H. Physical, mental, and social predictors of functional outcome in unilateral lower-limb amputees. Arch. Phys. Med. Rehabil. 2003, 84, 803–811. [Google Scholar] [CrossRef]
- Imam, B.; Miller, W.C.; McLaren, L.; Chapman, P.; Finlayson, H. Feasibility of the Nintendo WiiFitTM for improving walking in individuals with a lower limb amputation. SAGE Open Med. 2013, 1, 205031211349794. [Google Scholar] [CrossRef] [Green Version]
- Guerra, G.; Srithamboon, S.; Smith, J.D.; Charatrungolan, T.; Aekwatanphol, P.; Boonyawiwat, S.; Wilkins, J.T.; Pluksataporn, T.; Sulakkhana, A. Outcomes of a personalized structured exercise program for transtibial prosthesis wearers: Pilot study. Arch. Phys. Med. Rehabil. 2018, 99, e95–e96. [Google Scholar] [CrossRef]
- Geurts, A.C.; Mulder, T.W.; Nienhuis, B.; Rijken, R.A. Postural reorganization following lower limb amputation. Possible motor and sensory determinants of recovery. Scand. J. Rehabil. Med. 1992, 24, 83–90. [Google Scholar] [PubMed]

| Total (n = 35) | Male (n = 19) | Female (n = 16) | |
|---|---|---|---|
| Age (y) | 48.5 ± 14.8 | 50.2 ± 14.6 | 46.5 ± 15.1 |
| Height (cm) | 171.0 ± 8.5 | 176.5 ± 5.6 | 164.4 ± 6.2 |
| Mass (kg) | 86.0 ± 24.6 | 99.1 ± 21.8 | 70.5 ± 18.0 |
| BMI (kg/m2) | 29.2 ± 7.2 | 31.8 ± 6.9 | 26.1 ± 6.2 |
| Duration of Amputation (y) | 9.7 ± 8.7 | 7.4 ± 5.5 | 12.7 ± 11.2 |
| Classification | |||
| Right Transtibial | 15 | 11 | 4 |
| Left Transtibial | 8 | 3 | 5 |
| Right Transfemoral | 2 | 1 | 1 |
| Left Transfemoral | 7 | 4 | 3 |
| Bilateral Transtibial | 3 | 0 | 3 |
| Balance Task | |||||
|---|---|---|---|---|---|
| N | Mean | SD | Skewness | Kurtosis | |
| EOS (%) | 35 | 88.9 | 4.7 | −2.58 * | 0.34 |
| EOD (cm) | 35 | 41.5 | 26.3 | 5.91 * | 8.64 * |
| EOV (cm/s) | 35 | 1.38 | 0.87 | 5.90 * | 8.62 * |
| ECS (%) | 35 | 83.3 | 10.2 | −5.29 * | 6.13 * |
| ECD (cm) | 35 | 86.7 | 65.4 | 4.21 * | 2.78 * |
| ECV (cm/s) | 35 | 2.81 | 2.07 | 4.60 * | 4.00 * |
| Arm Crank Exercise | |||||
| N | Mean | SD | Skewness | Kurtosis | |
| TTE (s) | 35 | 422 | 158 | 1.46 | −0.31 |
| Powerpeak (W) | 35 | 59.1 | 20.6 | 1.44 | −0.67 |
| HRpeak (b/min) | 29 | 143 | 30 | −0.44 | −0.62 |
| VO2peak (mL/kg/min) | 34 | 15.7 | 7.6 | 2.13 * | 0.21 |
| VEpeak (L/min) | 34 | 52.5 | 25.5 | 2.21 * | 0.77 |
| RERpeak (VCO2/VO2) | 34 | 1.19 | 0.12 | −0.97 | −0.18 |
| RPEpeak | 26 | 15.2 | 2.5 | −0.19 | 0.50 |
| Balance Tasks | |||||
|---|---|---|---|---|---|
| Lowest Performers (n = 12) | Highest Performers (n = 11) | ||||
| Absolute Value (Mean Rank) | Absolute Value (Mean Rank) | U | Z-Value | p-Value | |
| EOS | 89.3 ± 4.9 (13.1) | 88.3 ± 4.3 (10.7) | 52.5 | −0.84 | 0.402 |
| EOD | 16.4 ± 8.9 (14.7) | 16.1 ± 11.9 (9.1) | 34.0 | −1.96 | 0.049 * |
| EOV | 0.54 ± 0.29 (15.6) | 0.53 ± 0.39 (9.2) | 35.0 | −1.91 | 0.056 |
| ECS | 82.2 ± 10.4 (10.1) | 84.4 ± 10.0 (14.1) | 43.5 | −1.39 | 0.164 |
| ECD | 36.3 ± 25.8 (14.5) | 31.8 ± 26.3 (9.3) | 36.0 | −1.84 | 0.065 |
| ECV | 1.2 ± 0.86 (14.7) | 0.99 ± 0.78 (9.0) | 33.0 | −2.03 | 0.042 * |
| Physiological Responses | |||||
| TTE | 303 ± 70 (6.5) | 548 ± 12 (18.0) | 0.00 | −4.06 | 0.001 * |
| Powerpeak | 43.5 ± 9.1 (6.5) | 75.5 ± 16.1 (18.0) | 0.00 | −4.15 | 0.001 * |
| HRpeak | 136 ± 34 (7.6) | 150 ± 23 (11.9) | 21.0 | −1.69 | 0.091 |
| VO2peak | 12.0 ± 5.2 (7.6) | 19.8 ± 7.8 (16.2) | 13.0 | −3.09 | 0.002 * |
| VEpeak | 36.7 ± 12.7 (6.6) | 70.1 ± 21.8 (17.4) | 1.00 | −3.89 | 0.001 * |
| RERpeak | 1.1 ± 0.1 (8.2) | 1.2 ± 0.1 (15.4) | 20.5 | −2.07 | 0.009 * |
| RPEpeak | 15.3 ± 2.9 (7.9) | 15.0 ± 2.0 (10.0) | 27.0 | −0.88 | 0.378 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, G.; Smith, J.D. Correlates of Balance and Aerobic Indices in Lower-Limb Prostheses Users on Arm Crank Exercise. Sensors 2021, 21, 6917. https://doi.org/10.3390/s21206917
Guerra G, Smith JD. Correlates of Balance and Aerobic Indices in Lower-Limb Prostheses Users on Arm Crank Exercise. Sensors. 2021; 21(20):6917. https://doi.org/10.3390/s21206917
Chicago/Turabian StyleGuerra, Gary, and John D. Smith. 2021. "Correlates of Balance and Aerobic Indices in Lower-Limb Prostheses Users on Arm Crank Exercise" Sensors 21, no. 20: 6917. https://doi.org/10.3390/s21206917
APA StyleGuerra, G., & Smith, J. D. (2021). Correlates of Balance and Aerobic Indices in Lower-Limb Prostheses Users on Arm Crank Exercise. Sensors, 21(20), 6917. https://doi.org/10.3390/s21206917







