Solid-Contact Electrode with Composite PVC-Based 3D-Printed Membrane. Optimization of Fabrication and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the PVC/KCl Composite for Membrane 3D Printing
2.3. Preparation of the Flat Solid Contact
2.4. Preparation of the Reference Cocktails
2.5. Apparatus
2.5.1. 3D Printer
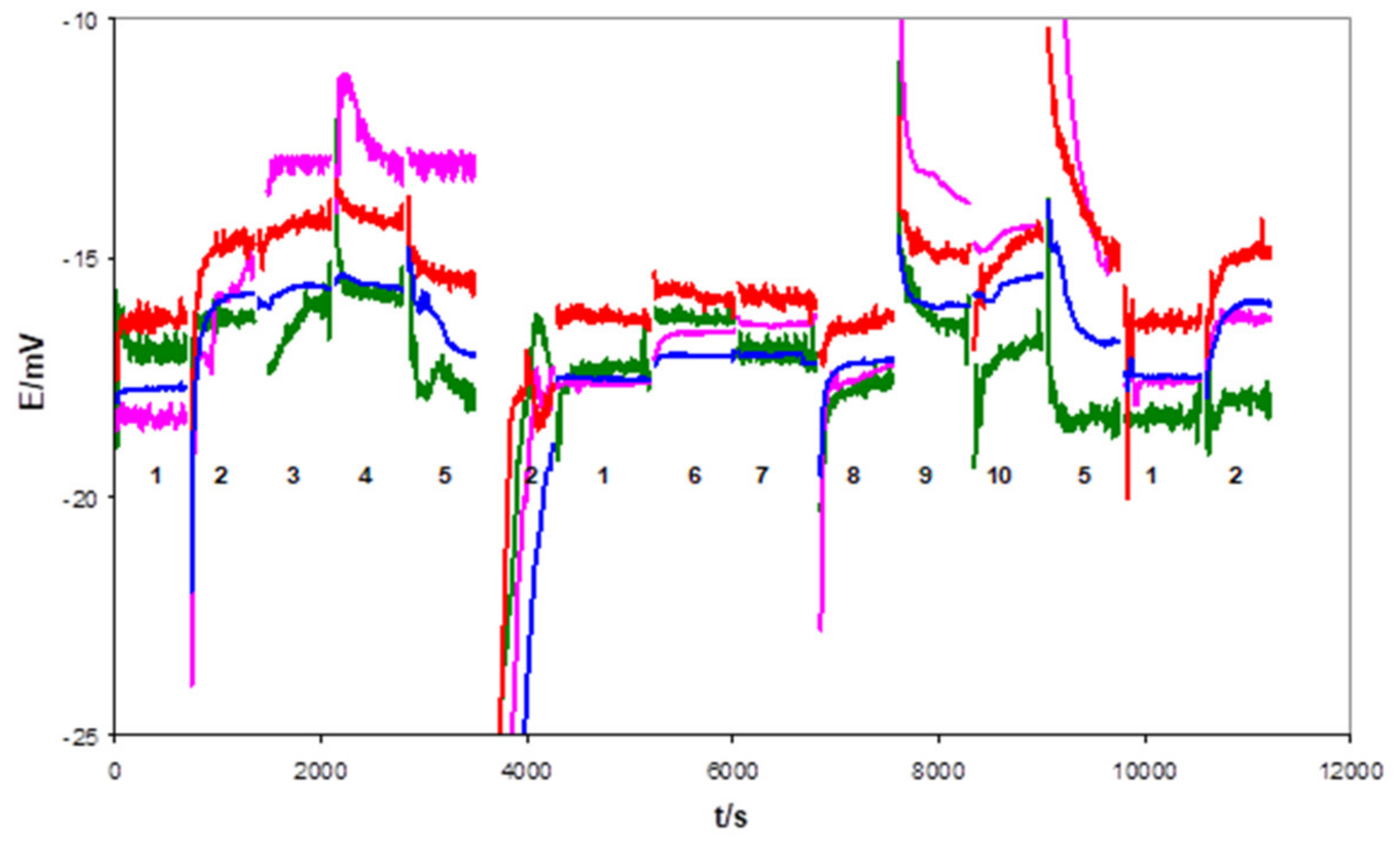
2.5.2. Electrochemical Measurements
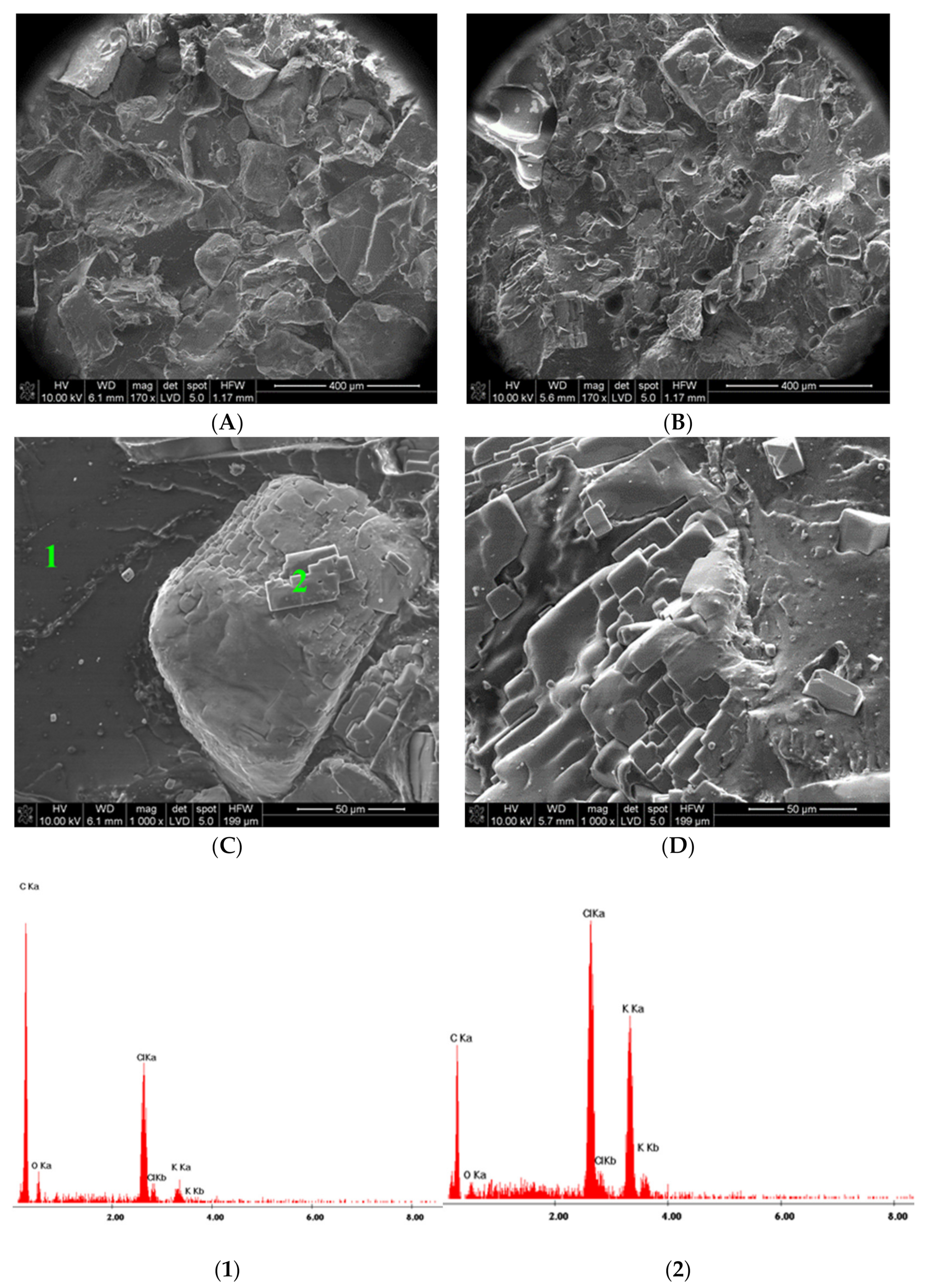
2.5.3. SEM Images
3. Results and Discussion
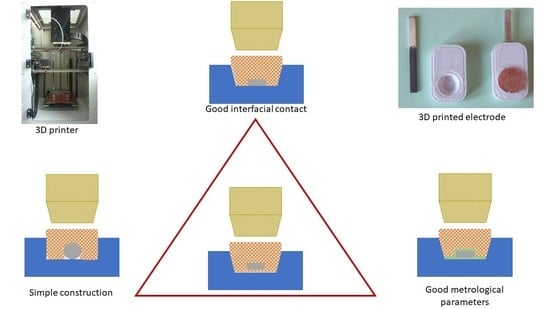
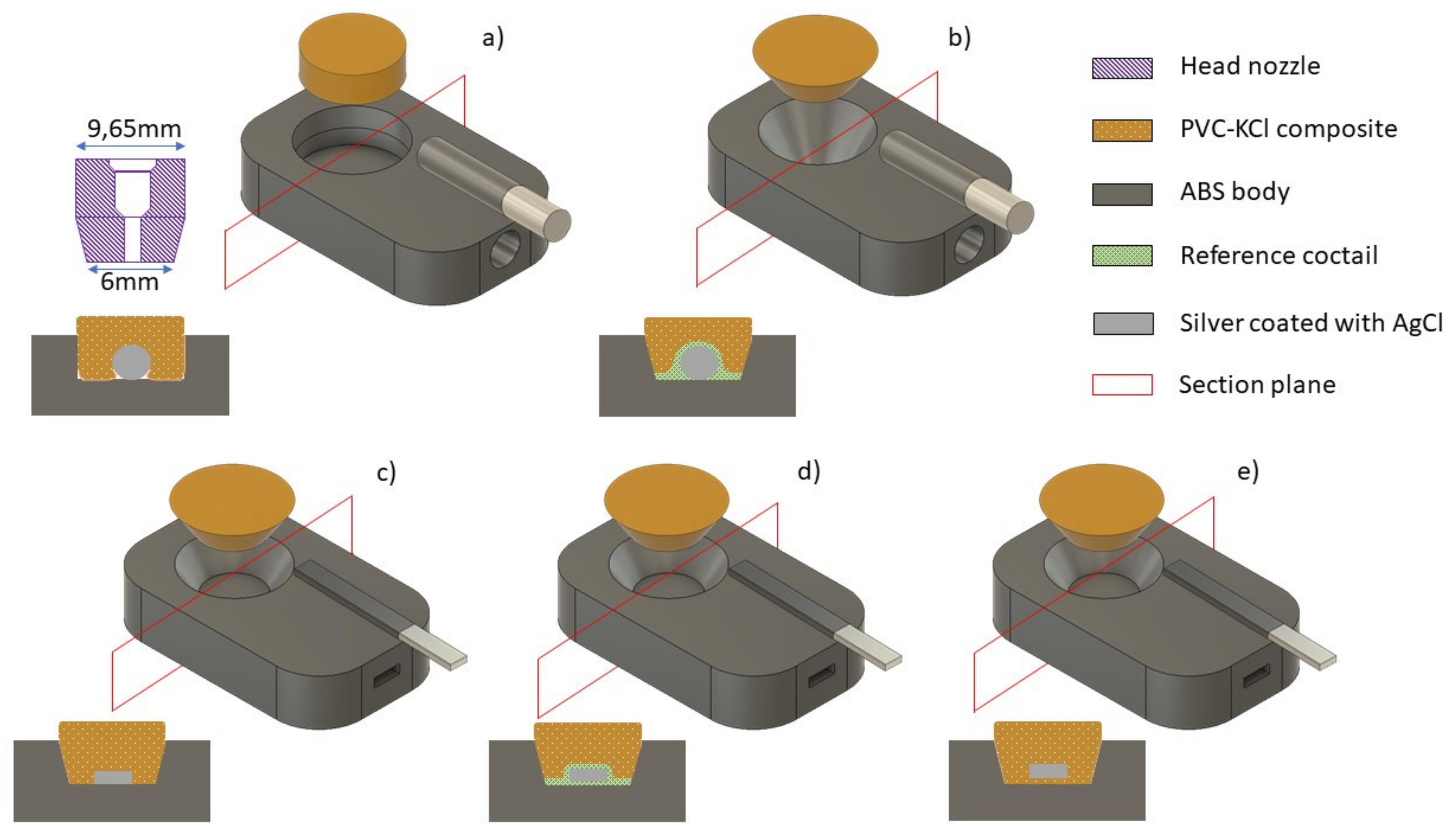
3.1. Sketching the Problems
3.2. Increasing the Adhesion of the Composite to the Embodying Neck
3.3. Increasing Adhesion of the Composite to Solid Contact
3.4. Printing of Optimized SC REF 3D
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Lewenstam, A.; Dołowy, K. Advances in Artificial and Biological Membranes: Mechanisms of Ionic Sensitivity, Ion-Sensor Designs, and Applications for Ion Measurement. Membranes 2020, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Ge, L.; Lisak, G. Highly reproducible solid contact ion selective electrodes: Emerging opportunities for potentiometry—A review. Anal. Chim. Acta 2021, 1162, 338304. [Google Scholar] [CrossRef]
- Rousseau, C.R.; Bühlmann, P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. TrAC Trends Anal. Chem. 2021, 140, 116277. [Google Scholar] [CrossRef]
- Paczosa-Bator, B. All-solid-state selective electrodes using carbon black. Talanta 2012, 93, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A. All-Solid-State Ion Selective and All-Solid-State Reference Electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Inzelt, G.; Lewenstam, A.; Scholz, F. Handbook of Reference Electrodes, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zuliani, C.; Matzeu, G.; Diamond, D. A liquid-junction-free reference electrode based on a PEDOT solid-contact and ionogel capping membrane. Talanta 2014, 125, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.B.; Ho, K.T.; Zou, X.U.; Smyrl, W.H.; Stein, A.; Bühlmann, P. All-solid-state reference electrodes based on colloid-imprinted mesoporous carbon and their application in disposable paper-based potentiometric sensing devices. Anal. Chem. 2015, 87, 2981–2987. [Google Scholar] [CrossRef]
- Bieg, C.; Fuchsberger, K.; Stelzle, M. Introduction to polymer-based solid-contact ion-selective electrodes—basic concepts, practical considerations, and current research topics. Anal. Bioanal. Chem. 2016, 409, 45–61. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Lindner, E.; Guzinski, M.; Khan, T.A.; Pendley, B.D. Reference electrodes with ionic liquid salt bridge: When will these innovative novel reference electrodes gain broad acceptance? ACS Sens. 2019, 4, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Lingenfelter, P.; Bartoszewicz, B.; Migdalski, J.; Sokalski, T.; Bućko, M.M.; Filipek, R.; Lewenstam, A. Reference Electrodes with Polymer-Based Membranes—Comprehensive Performance Characteristics. Membranes 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.S.; Vepsäläinen, M.; Acharya, D.; Wood, C.D.; Wen, D.; Thomson, L.; Peacock, S.; Rodopoulos, T.; Hogan, C.F. An unusually stable solid-state Ag|AgCl reference electrode for long term continuous measurements based on a crosslinked poly(vinyl acetate)/KCl composite. Electrochim. Acta 2021, 368, 137636. [Google Scholar] [CrossRef]
- Sophocleous, M.; Atkinson, J.K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors . Sens. Actuators A Phys. 2017, 267, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Qu, K.; Fang, M.; Zhang, S.; Liu, H.; Zeng, X. A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode. Polymers 2018, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Novotný, F.; Webster, R.D.; Pumera, M. 3D-printed Ag/AgCl pseudo-reference electrodes. Electrochem. Commun. 2019, 103, 104–108. [Google Scholar] [CrossRef]
- Sibug-Torres, S.M.; Go, L.P.; Enriquez, E.P. Fabrication of a 3D-Printed Porous Junction for Ag|AgCl|gel-KCl Reference Electrode. Chemosensors 2020, 8, 130. [Google Scholar] [CrossRef]
- Bananezhad, A.; Jović, M.; Villalobos, F.; Agrawal, K.V.; Ganjali, M.R.; Girault, H.H. Large-scale fabrication of flexible solid-state reference electrodes. J. Electroanal. Chem. 2019, 847, 113241. [Google Scholar] [CrossRef]
- Granholm, K.; Mousavi, Z.; Sokalski, T.; Lewenstam, A. Analytical quality solid-state composite reference electrode manufactured by injection moulding. J. Solid State Electrochem. 2014, 18, 607–612. [Google Scholar] [CrossRef]
- Lewenstam, A.; Blaz, T.; Migdalski, J. All-solid-state reference electrode with heterogeneous membrane. Anal. Chem. 2017, 89, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewicz, B.; Dabrowska, S.; Lewenstam, A.; Migdalski, J. Calibration free solid contact electrodes with two PVC based membranes. Sens. Actuators B Chem. 2018, 274, 268–273. [Google Scholar] [CrossRef]
- Lewenstam, A.; Bartoszewicz, B.; Migdalski, J.; Kochan, A. Solid contact reference electrode with a PVC based composite electroactive element fabricated by 3D printing. Electrochem. Commun. 2019, 109, 106613. [Google Scholar] [CrossRef]
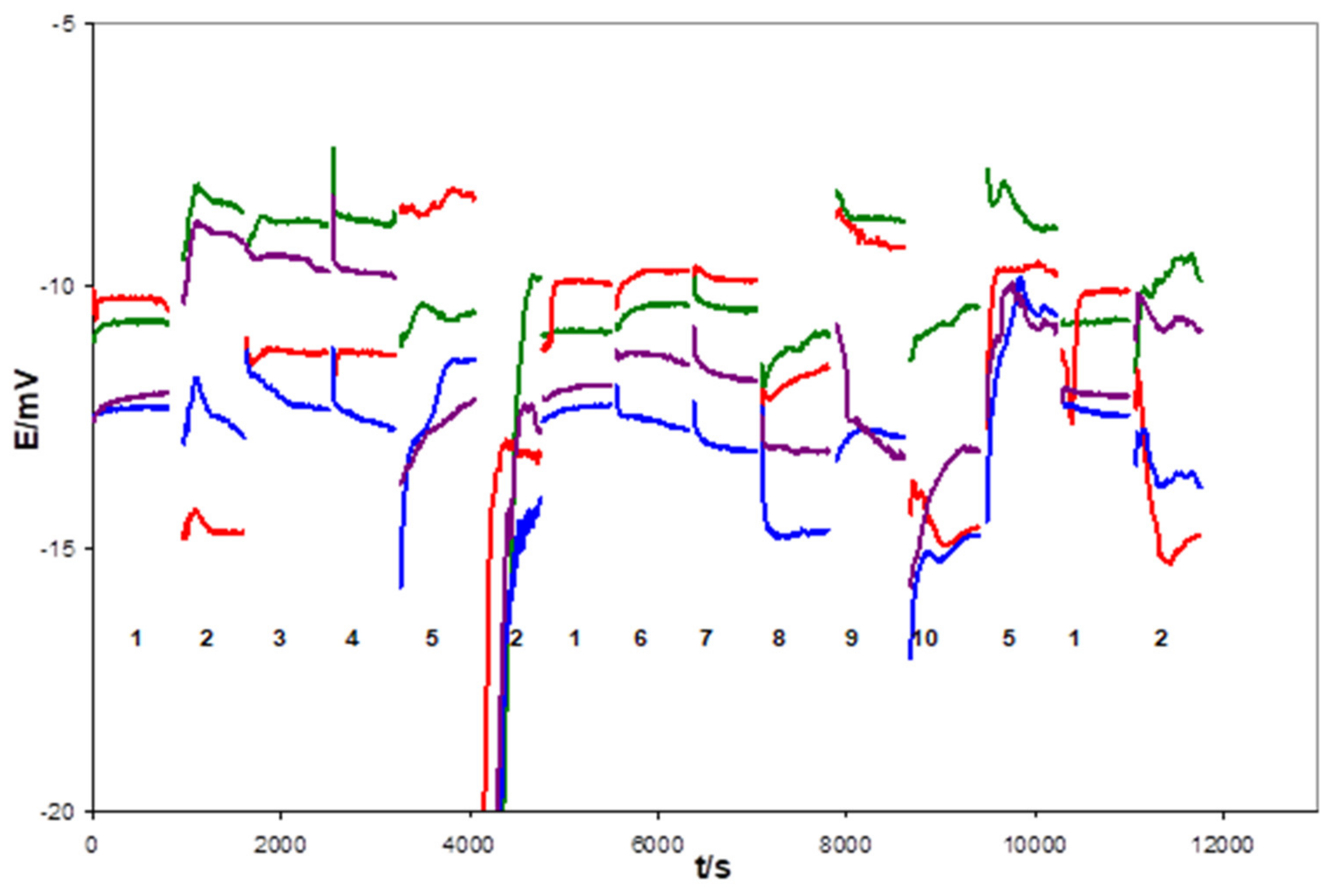
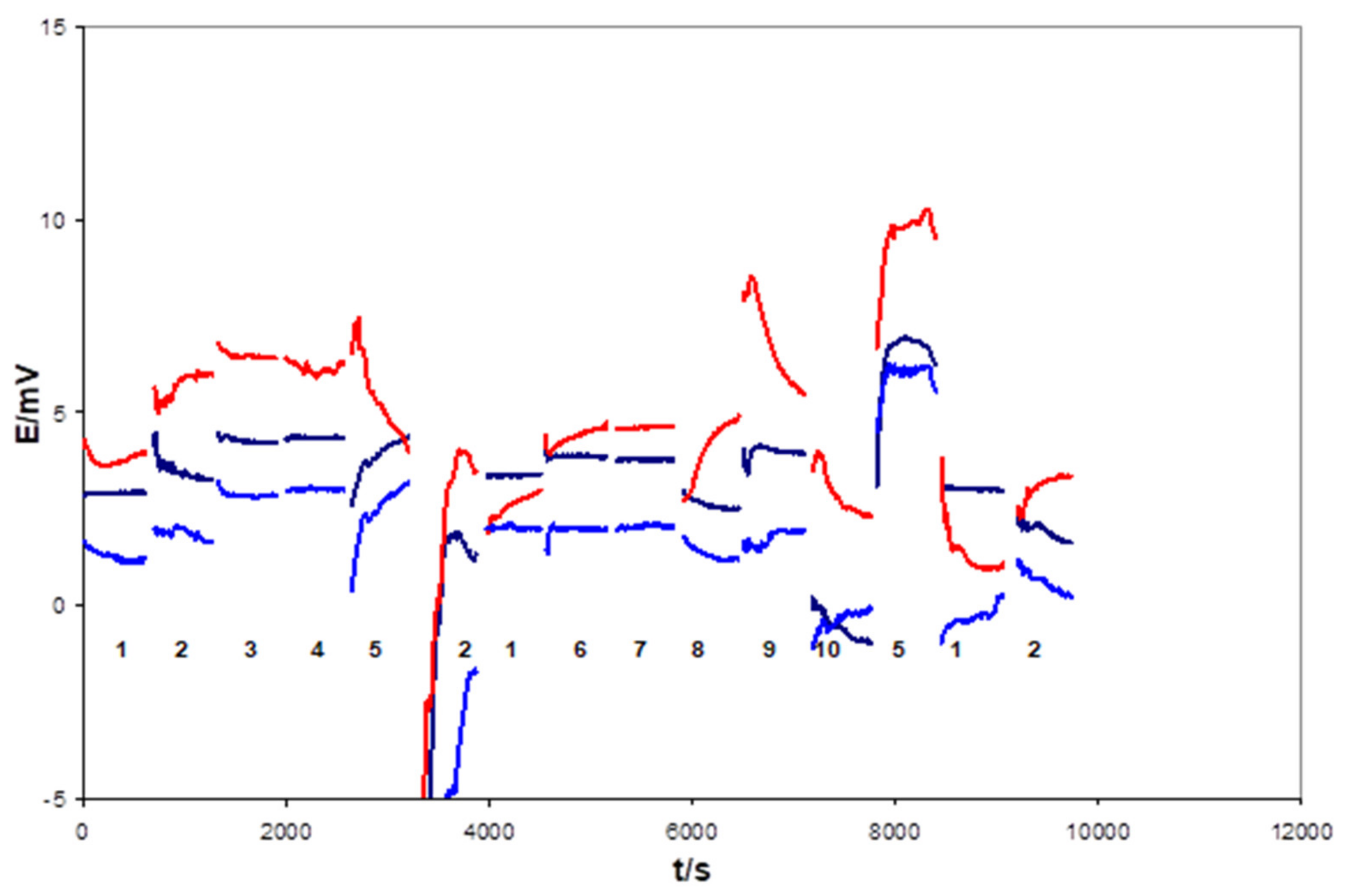
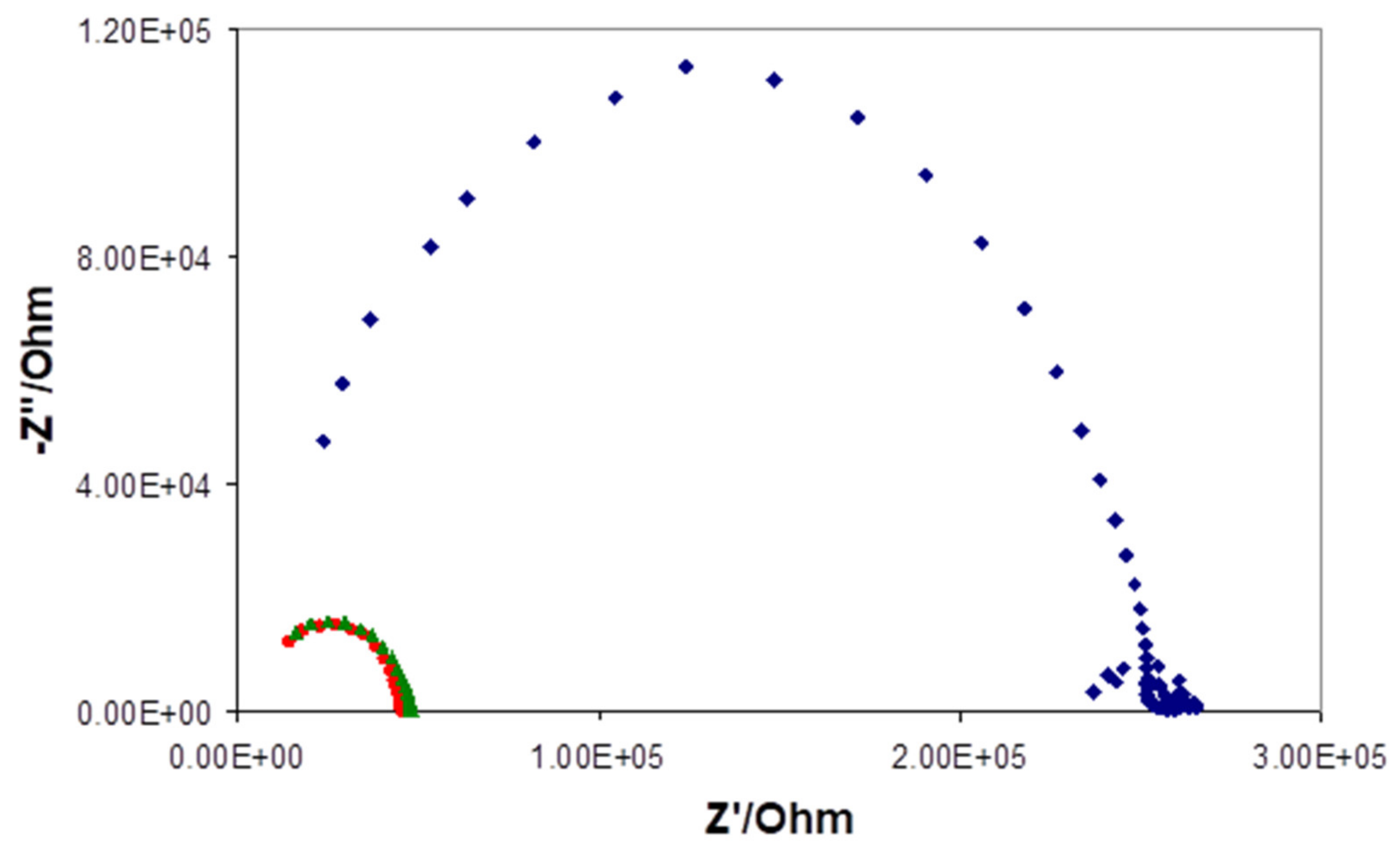
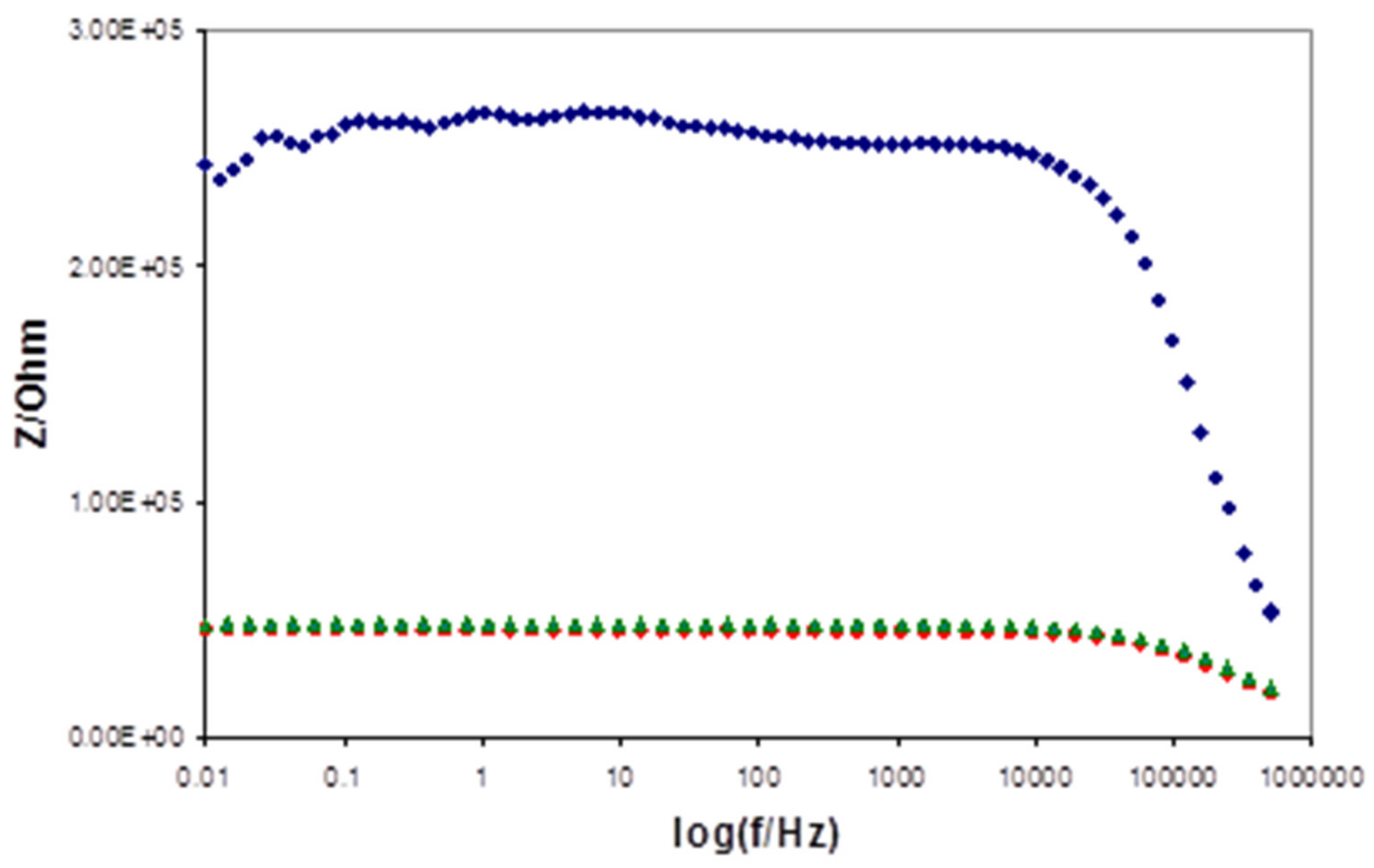

| Days of Soaking | Electrode 1e-1 (mV) | Electrode 1e-2 (mV) | Electrode 1d-1 (mV) | Electrode 1d-2 (mV) | Electrode 1d-3 (mV) |
|---|---|---|---|---|---|
| 1 | −2.4 | 1.7 | −1.4 | −1 | 0.4 |
| 5 | −2.5 | 1.7 | −1.6 | −1.7 | 0.1 |
| 9 | −2.8 | 1.7 | −1.6 | −1.7 | 0.1 |
| 10 | −2.4 | 1.7 | −1.8 | −2.1 | −0.1 |
| 12 | −2.5 | 2.6 | −1.5 | −1.8 | −0.1 |
| 13 | −2.9 | 1.6 | −1.6 | −1.9 | −0.1 |
| 16 | −2.9 | 1.8 | −1.6 | −1.4 | −0.2 |
| 17 | −2.2 | 2.9 | −1.8 | −1.7 | 0.0 |
| 19 | −2.3 | 2.4 | −1.7 | −1.5 | −0.1 |
| 22 | −2.3 | 2.0 | −1.9 | −1.3 | 0.1 |
| 23 | −2.6 | 2.0 | −2.2 | −2.3 | −0.2 |
| 30 | −2.9 | 1.5 | −2.7 | −2.6 | −1.0 |
| 38 | −3.2 | 1.0 | −3.1 | −2.9 | −1.1 |
| 47 | −3.6 | 1.3 | −3.3 | −3.3 | −0.9 |
| 49 | −3.0 | 1.7 | −2.8 | −3.1 | −1.0 |
| 62 | −2.9 | 0.9 | −2.9 | −3.4 | −3.2 |
| mean | −2.71 | 1.78 | −2.09 | −2.11 | −0.46 |
| SD | 0.38 | 0.53 | 0.64 | 0.75 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoszewicz, B.; Lewenstam, A.; Migdalski, J. Solid-Contact Electrode with Composite PVC-Based 3D-Printed Membrane. Optimization of Fabrication and Performance. Sensors 2021, 21, 4909. https://doi.org/10.3390/s21144909
Bartoszewicz B, Lewenstam A, Migdalski J. Solid-Contact Electrode with Composite PVC-Based 3D-Printed Membrane. Optimization of Fabrication and Performance. Sensors. 2021; 21(14):4909. https://doi.org/10.3390/s21144909
Chicago/Turabian StyleBartoszewicz, Bartosz, Andrzej Lewenstam, and Jan Migdalski. 2021. "Solid-Contact Electrode with Composite PVC-Based 3D-Printed Membrane. Optimization of Fabrication and Performance" Sensors 21, no. 14: 4909. https://doi.org/10.3390/s21144909
APA StyleBartoszewicz, B., Lewenstam, A., & Migdalski, J. (2021). Solid-Contact Electrode with Composite PVC-Based 3D-Printed Membrane. Optimization of Fabrication and Performance. Sensors, 21(14), 4909. https://doi.org/10.3390/s21144909