Detection of Muscle Activation during Resistance Training Using Infrared Thermal Imaging
Abstract
1. Introduction
2. Materials and Methods
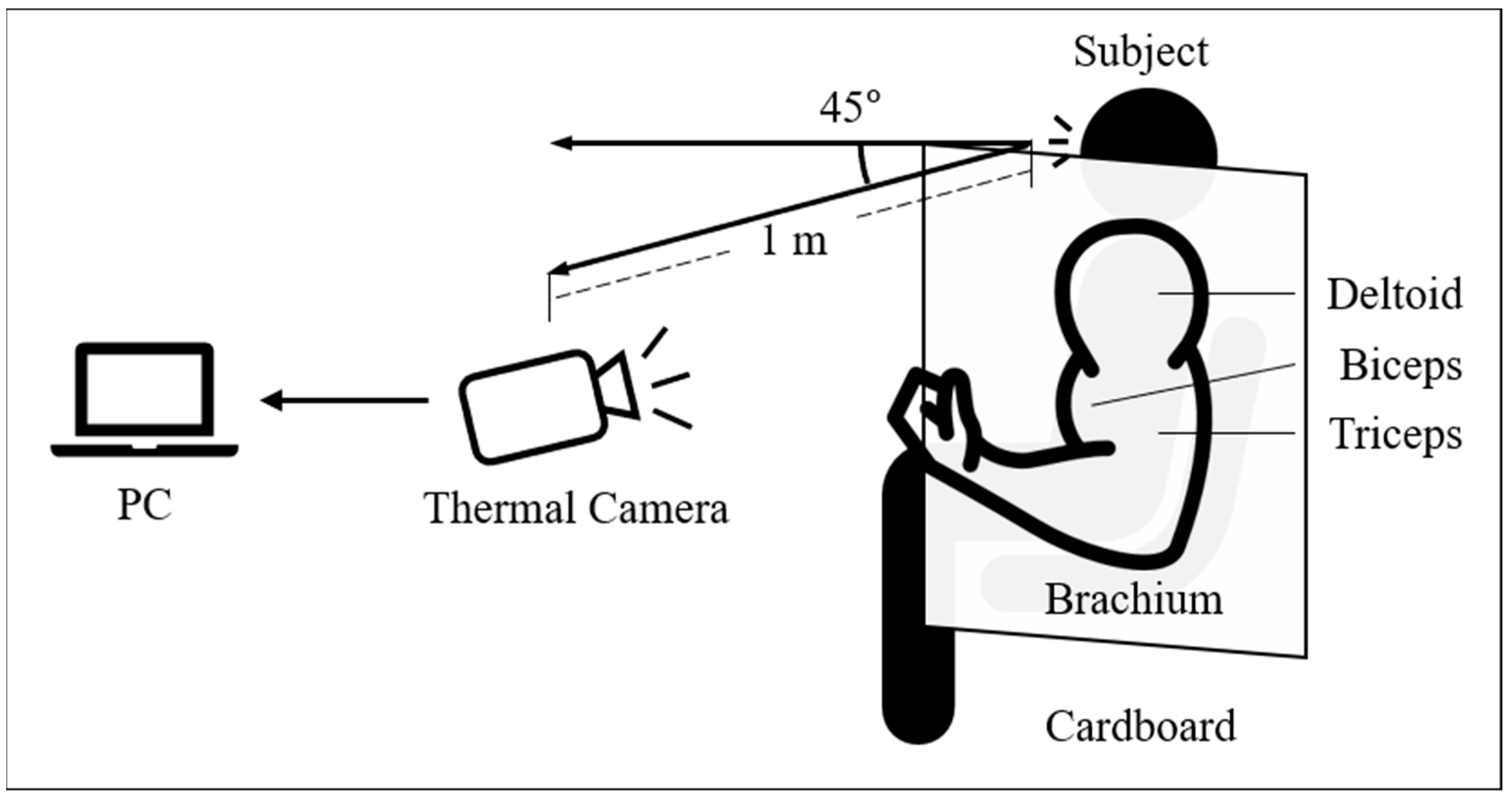
2.1. Experimental Setup
2.2. Experimental Procedures
2.3. Data Processing
2.4. Classification of Target Muscle Activation
3. Results
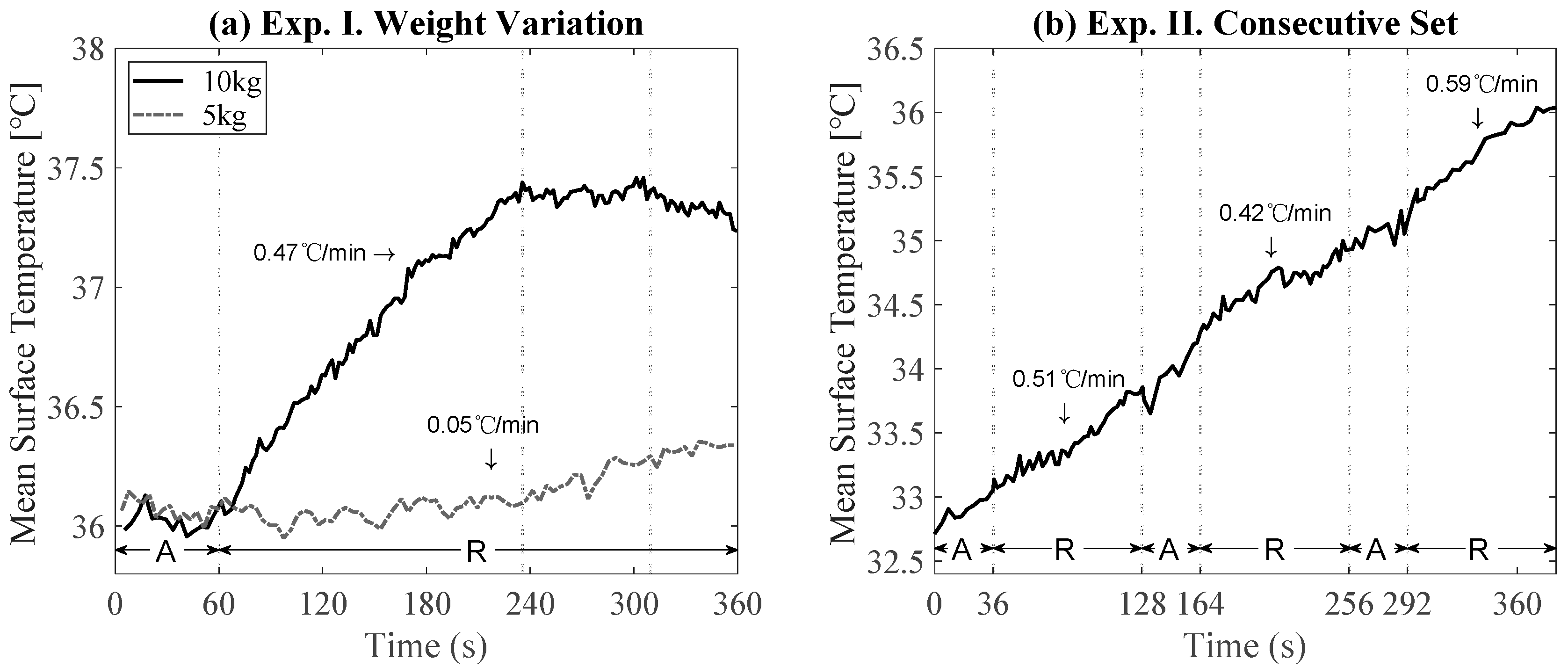
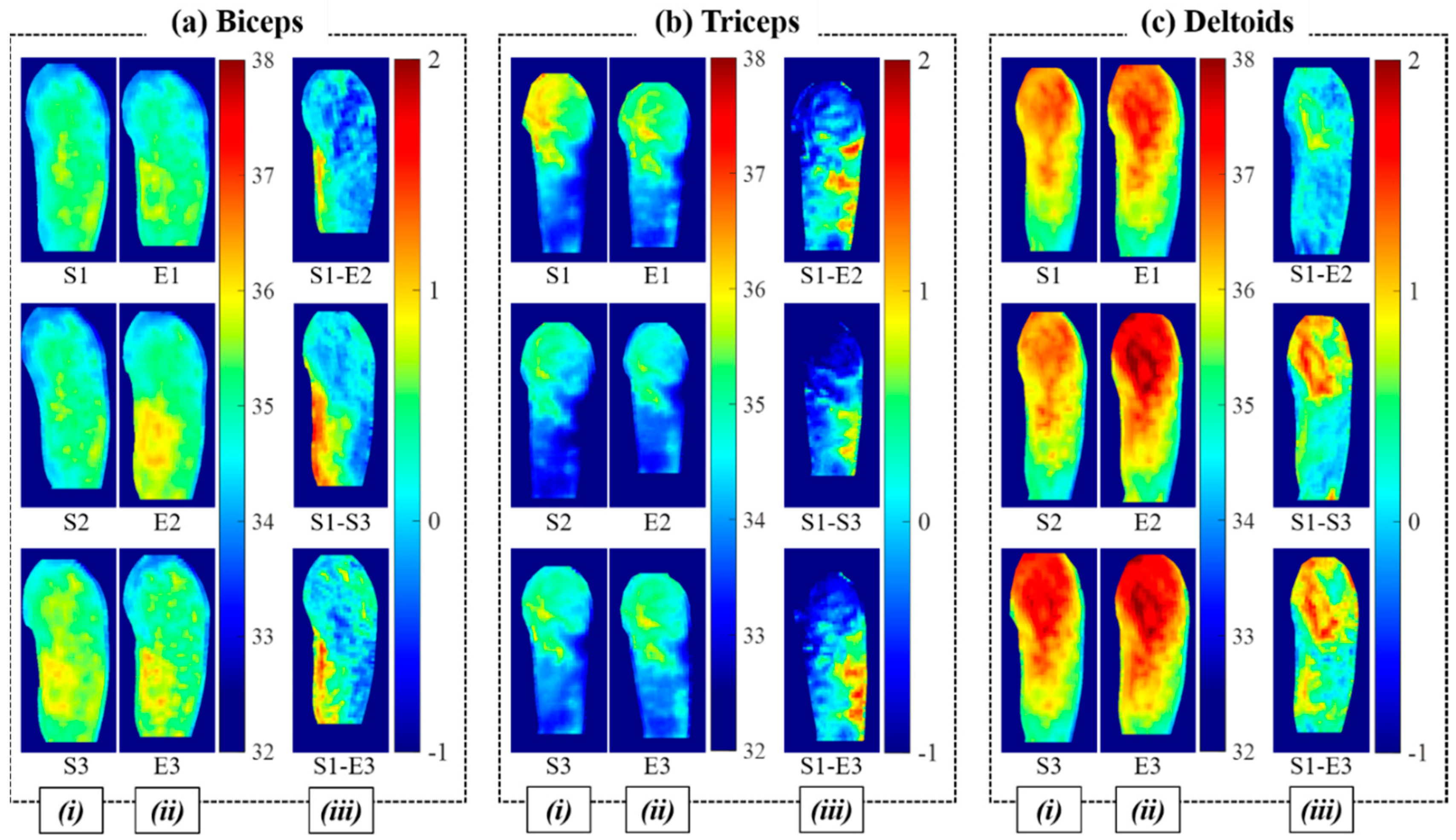
3.1. Effects of Weight, Duration, and Muscle Activation for Training
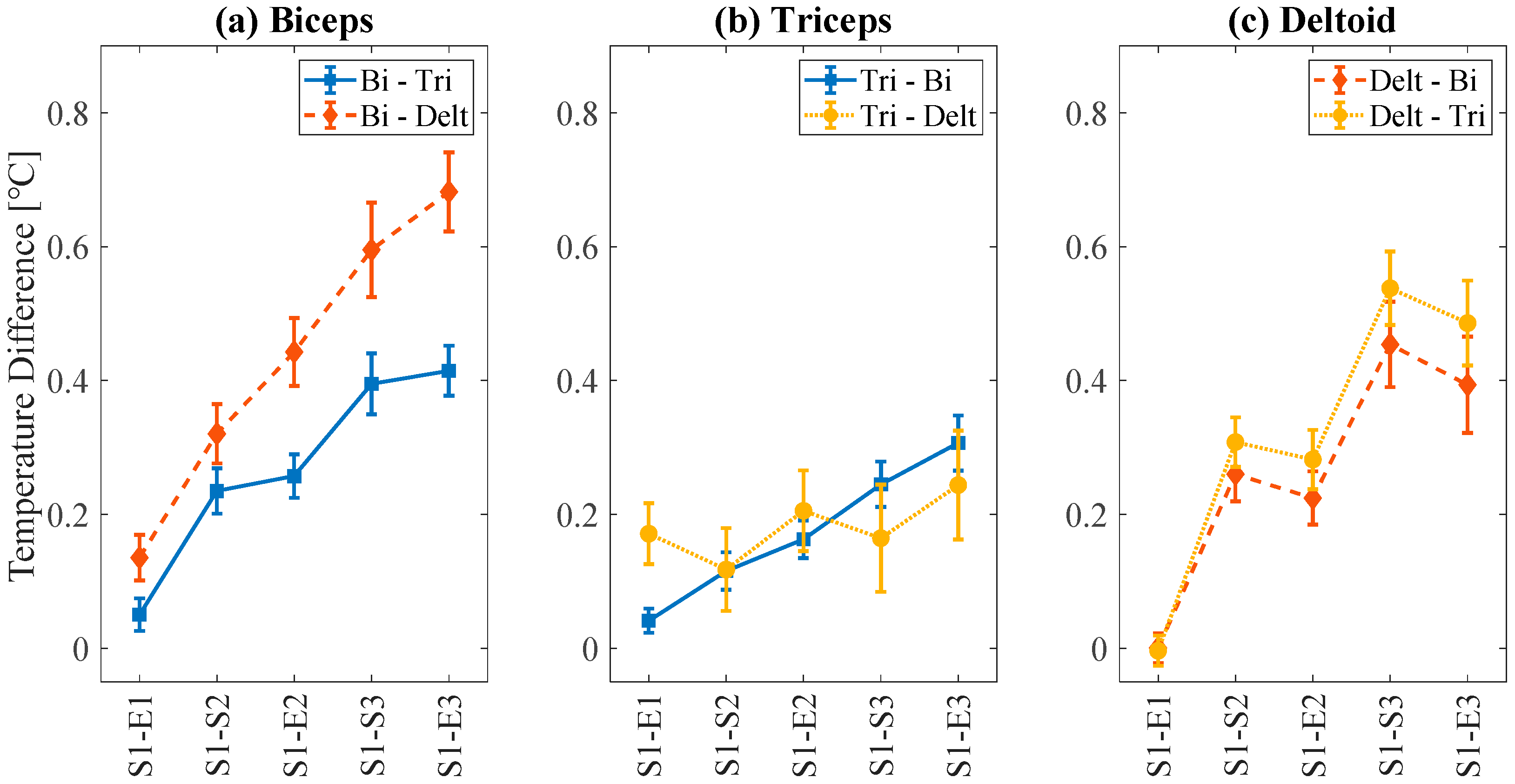
3.2. Statistical Analysis
3.3. Classification of Muscle Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, B.R.; Duthie, G.M.; Thornton, H.R.; Dascombe, B.J. Training Monitoring for Resistance Exercise: Theory and Applications. Sports Med. 2016, 46, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Peiffer, J.J.; Newton, M.J.; Scott, B.R. A comparison of traditional and novel metrics to quantify resistance training. Sci. Rep. 2017, 7, 5606. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; McCaulley, G.O.; Cormie, P.; Nuzzo, J.L.; Cavill, M.J.; Triplett, N.T. Comparison of Methods to Quantify Volume During Resistance Exercise. J. Strength Cond. Res. 2009, 23, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Haff, G.G. Quantifying Workloads in Resistance Training: A Brief Review; UK Strength and Conditioning Association: Lytham, UK, 2010; p. 19. [Google Scholar]
- Chiu, L.Z.F. Biomechanical Methods to Quantify Muscle Effort during Resistance Exercise. J. Strength Cond. Res. 2018, 32, 502–513. [Google Scholar] [CrossRef]
- Taha, Z.; Lee, C.M.; Ahamed, N.U.; Joseph, S.; Omar, S.F.S. Performance Analysis in Strength Training: An Innovative Instrumentation. Procedia Eng. 2016, 147, 455–460. [Google Scholar] [CrossRef][Green Version]
- Gielen, F.L.H.; Wallinga-de Jonge, W.; Boon, K.L. Electrical conductivity of skeletal muscle tissue: Experimental results from different musclesin vivo. Med. Biol. Eng. Comput. 1984, 22, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kamen, G.; Gabriel, D.A. Essentials of Electromyography; Human Kinetics: Leeds, UK, 2010. [Google Scholar]
- Krustrup, P.; Ferguson, R.A.; Kjaer, M.; Bangsbo, J. ATP and heat production in human skeletal muscle during dynamic exercise: Higher efficiency of anaerobic than aerobic ATP resynthesis. J. Physiol. 2003, 549 Pt 1, 255–269. [Google Scholar] [CrossRef]
- González-Alonso, J.; Quistorff, B.; Krustrup, P.; Bangsbo, J.; Saltin, B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J. Physiol. 2000, 524, 603–615. [Google Scholar] [CrossRef]
- Ali, S.S.; Khan, A.Y.; Michael, S.G.; Tankha, P.; Tokuno, H. Use of Digital Infrared Thermal Imaging in the Electromyography Clinic: A Case Series. Cureus 2019. [Google Scholar] [CrossRef] [PubMed]
- Chudecka, M.; Lubkowska, A. Temperature changes of selected body’s surfaces of handball players in the course of training estimated by thermovision, and the study of the impact of physiological and morphological factors on the skin temperature. J. Therm. Biol. 2010, 35, 379–385. [Google Scholar] [CrossRef]
- Priego Quesada, J.I.; Carpes, F.P.; Bini, R.R.; Salvador Palmer, R.; Pérez-Soriano, P.; Cibrián Ortiz de Anda, R.M. Relationship between skin temperature and muscle activation during incremental cycle exercise. J. Therm. Biol. 2015, 48, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Galindo, V.L.; Estal-Martínez, A.; Adamczyk, J.G.; Brito, C.J.; Arnaiz-Lastras, J.; Sillero-Quintana, M. Skin temperature response to unilateral training measured with infrared thermography. J. Exerc. Rehabil. 2017, 13, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Zagrodny, B.; Wojnicz, W.; Ludwicki, M.; Awrejcewicz, J. Could Thermal Imaging Supplement Surface Electromyography Measurements for Skeletal Muscles? IEEE Trans. Instrum. Meas. 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Daud, S.A.; Elamvazuthi, I.; Bt Zulkifli, Z.; Parasuraman, S.; Ahamed Khan, M.K.A. Analysis of thermal imaging in determining muscle contractions of upper extremities. In Proceedings of the 2017 IEEE 3rd International Symposium in Robotics and Manufacturing Automation (ROMA), Kuala Lumpur, Malaysia, 19–21 September 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–5. [Google Scholar]
- Formenti, D.; Ludwig, N.; Rossi, A.; Trecroci, A.; Alberti, G.; Gargano, M.; Merla, A.; Ammer, K.; Caumo, A. Skin temperature evaluation by infrared thermography: Comparison of two image analysis methods during the nonsteady state induced by physical exercise. Infrared Phys. Technol. 2017, 81, 32–40. [Google Scholar] [CrossRef]
- Ferreira, J.J.A.; Mendonça, L.C.S.; Nunes, L.A.O.; Andrade Filho, A.C.C.; Rebelatto, J.R.; Salvini, T.F. Exercise-Associated Thermographic Changes in Young and Elderly Subjects. Ann. Biomed. Eng. 2008, 36, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Kuniszyk-Joóźkowiak, W.; Jaszczuk, J.; Czaplicki, A. Changes in electromyographic signals and skin temperature during standardised effort in volleyball players. Acta Bioeng. Biomech. 2018. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. arXiv 2015, arXiv:1512.03385. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.-J.; Kai, L.; Li, F.-F. ImageNet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops (CVPR Workshops), Miami, FL, USA, 20–25 June 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 248–255. [Google Scholar]
- Hadžić, V.; Širok, B.; Malneršič, A.; Čoh, M. Can infrared thermography be used to monitor fatigue during exercise? A case study. J. Sport Health Sci. 2019, 8, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.d.A.; Amorim, P.R.d.S.; Brito, C.J.; de Moura, A.G.; Moreira, D.G.; Costa, C.M.A.; Sillero-Quintana, M.; Marins, J.C.B. Measuring skin temperature before, during and after exercise: A comparison of thermocouples and infrared thermography. Physiol. Meas. 2014, 35, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A.; Alberti, G. Thermal Imaging of Exercise-Associated Skin Temperature Changes in Trained and Untrained Female Subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Eaton-Evans, J. Nutritional Assessment: Anthropometry. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 227–232. [Google Scholar]
- Costello, J.T.; Culligan, K.; Selfe, J.; Donnelly, A.E. Muscle, Skin and Core Temperature after −110 °C Cold Air and 8 °C Water Treatment. PLoS ONE 2012, 7, e48190. [Google Scholar] [CrossRef] [PubMed]





| Experiment | Training Weight [kg] | Recovery Time [s] | # of Sets per Session | # of Sessions | # of Repetitions per Set | # of Subjects | Target Muscle |
|---|---|---|---|---|---|---|---|
| I. Weight | 5 and 10 | 300 | 1 set | 1 | 15 | 1 | Biceps |
| II. Duration | 10 | 92 | 3 sets | 1 | 8 | 1 | Biceps |
| III. Muscle | 5 or 10 | 92 | 3 sets | 3 | 12 | 5 | All Three |
| Dataset | Biceps | Triceps | Deltoid | Vague | Total |
|---|---|---|---|---|---|
| Train | 33 | 19 | 31 | 38 | 121 |
| Test | 4 | 3 | 2 | 4 | 13 |
| Heat Map Pairs | Count | Percentage |
|---|---|---|
| (S1-E3) | 36 | 51% |
| (S1-S3) | 24 | 34% |
| (S1-E2) | 4 | 5% |
| (S1-S2) | 2 | 3% |
| (S1-E1) | 4 | 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Seo, J.; Seo, K.; Kim, D.; Park, S. Detection of Muscle Activation during Resistance Training Using Infrared Thermal Imaging. Sensors 2021, 21, 4505. https://doi.org/10.3390/s21134505
Jung H, Seo J, Seo K, Kim D, Park S. Detection of Muscle Activation during Resistance Training Using Infrared Thermal Imaging. Sensors. 2021; 21(13):4505. https://doi.org/10.3390/s21134505
Chicago/Turabian StyleJung, Haemin, Jeongwung Seo, Kangwon Seo, Dohwi Kim, and Suhyun Park. 2021. "Detection of Muscle Activation during Resistance Training Using Infrared Thermal Imaging" Sensors 21, no. 13: 4505. https://doi.org/10.3390/s21134505
APA StyleJung, H., Seo, J., Seo, K., Kim, D., & Park, S. (2021). Detection of Muscle Activation during Resistance Training Using Infrared Thermal Imaging. Sensors, 21(13), 4505. https://doi.org/10.3390/s21134505







