Upconversion Spectral Rulers for Transcutaneous Displacement Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sensor Fabrication
2.3. Luminescent Measurements
3. Results and Discussion
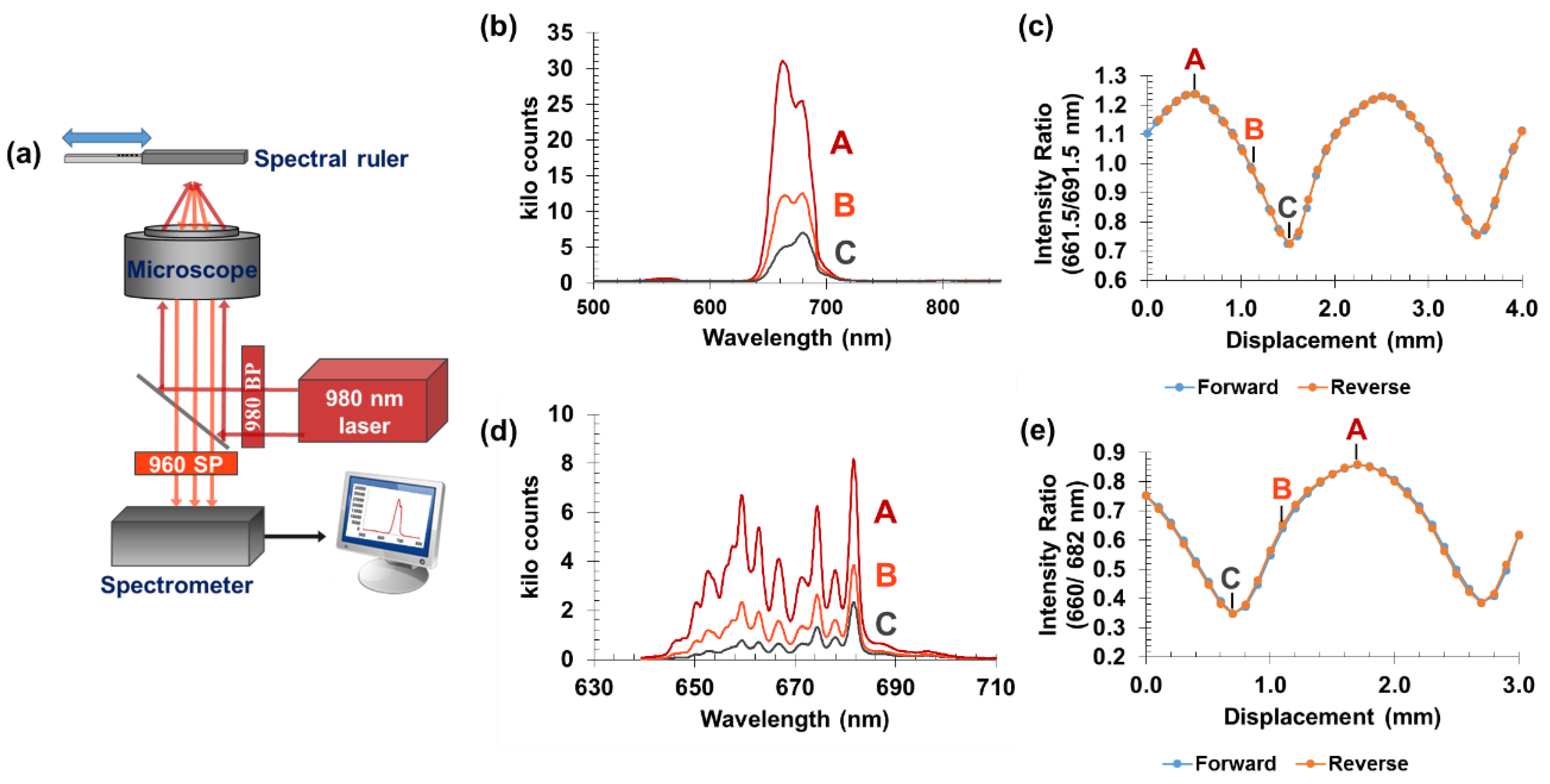
3.1. Sensor Operation
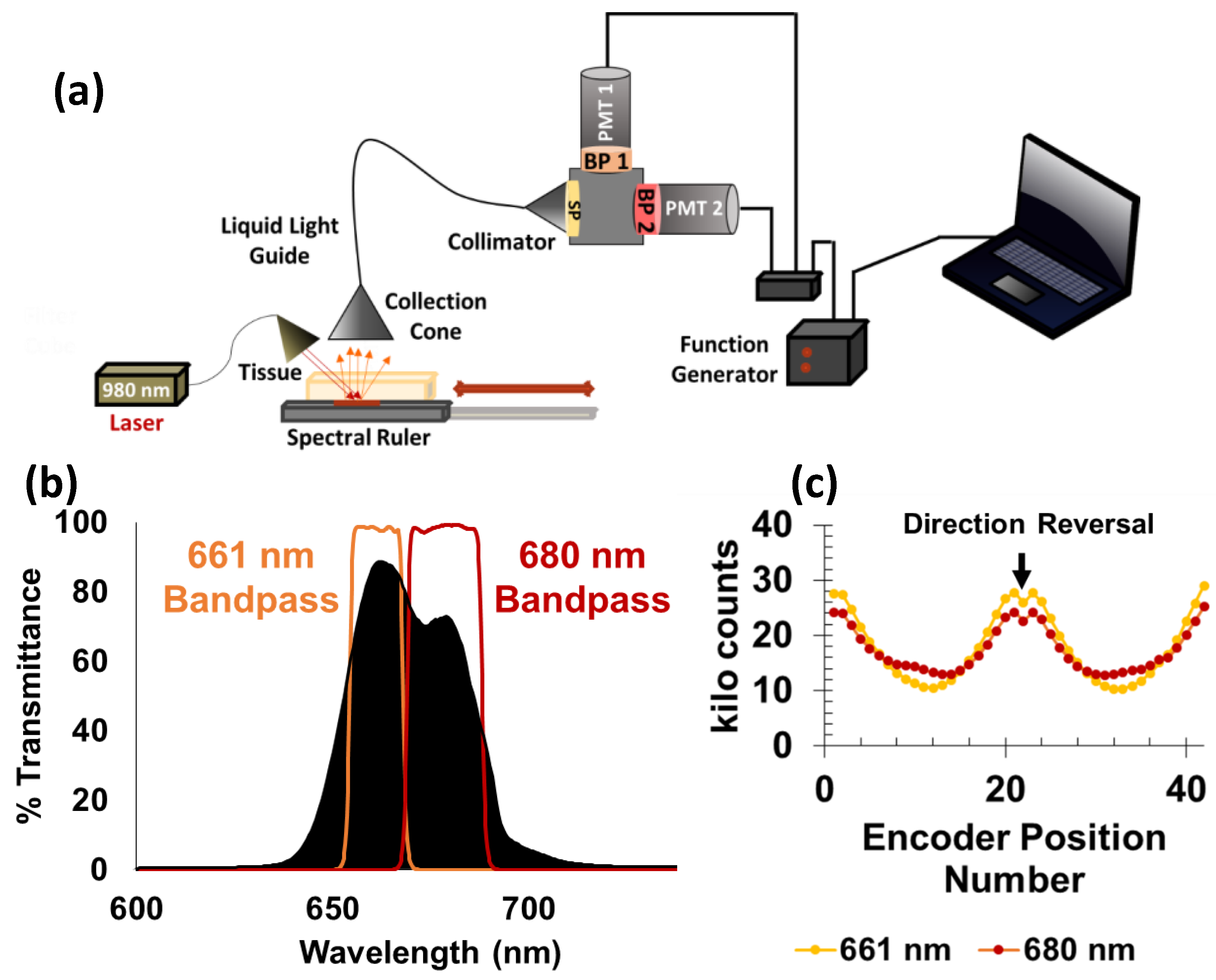
3.2. Fiber-Coupled PMT-Based System
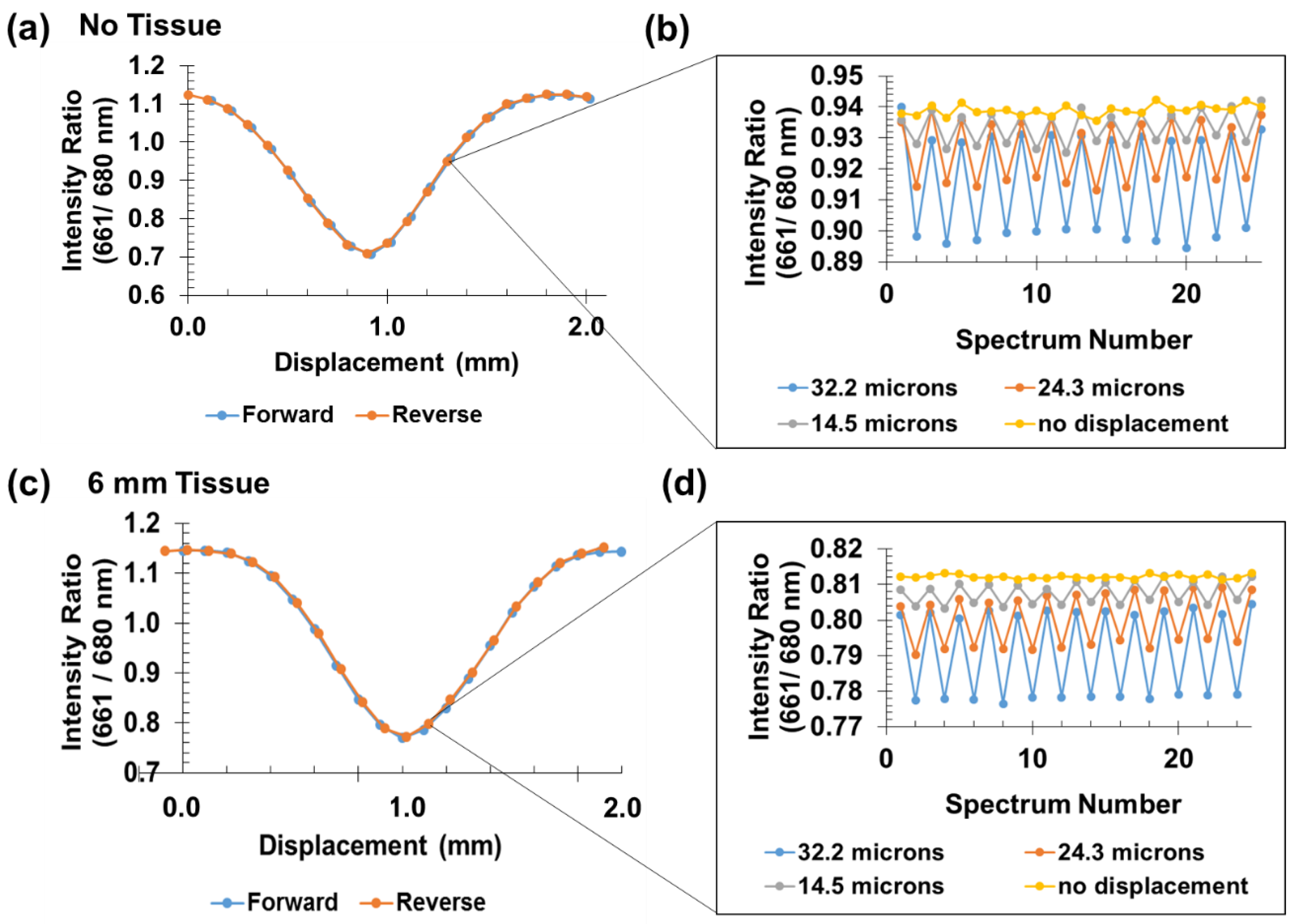
3.3. Small Signal Analysis
3.4. Tendon Measurements
4. Conclusions and Future Work
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bey, M.J.; Derwin, K.A. Measurement of in vivo tendon function. J. Shoulder Elb. Surg. 2012, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; de Sa, D.; Karlsson, J.; Musahl, V.; Ayeni, O.R. Anterior Cruciate Ligament Rupture: A Family Affair. Orthop. J. Sports Med. 2015, 3, 2325967115616783. [Google Scholar] [CrossRef]
- Wojtys, E.M. Anterior Cruciate Ligament Injury. Sports Health 2015, 7, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Fleming, B.C.; Beynnon, B.D. In vivo measurement of ligament/tendon strains and forces: A review. Ann. Biomed. Eng. 2004, 32, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Jacob, H.; Nyffeler, R.; Gerber, C. In vivo tendon force measurement of 2-week duration in sheep. J. Biomech. 2004, 37, 135–140. [Google Scholar] [CrossRef]
- Komi, P.V. Relevance of in vivo force measurements to human biomechanics. J. Biomech. 1990, 23, 23–34. [Google Scholar] [CrossRef]
- Komi, P.; Salonen, M.; Järvinen, M.; Kokko, O. In vivo registration of Achilles tendon forces in man. I. Methodological development. Int. J. Sports Med. 1987, 8, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Sigal, L.; Njus, G.O.; Njus, N.M.; Singerman, R.J.; Brand, R.A. Dynamic performance characteristics of the liquid metal strain gage. J. Biomech. 1986, 19, 165–173. [Google Scholar] [CrossRef]
- Holden, J.P.; Grood, E.S.; Korvick, D.L.; Cummings, J.F.; Butler, D.L.; Bylski-Austrow, D.I. In vivo forces in the anterior cruciate ligament: Direct measurements during walking and trotting in a quadruped. J. Biomech. 1994, 27, 517–526. [Google Scholar] [CrossRef]
- West, J.R.; Juncosa, N.; Galloway, M.T.; Boivin, G.P.; Butler, D.L. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J. Biomech. 2004, 37, 1647–1653. [Google Scholar] [CrossRef]
- Gregor, R.; Roy, R.; Whiting, W.; Lovely, R.; Hodgson, J.; Edgerton, V. Mechanical output of the cat soleus during treadmill locomotion: In vivo vs in situ characteristics. J. Biomech. 1988, 21, 721–732. [Google Scholar] [CrossRef]
- Herzog, W.; Archambault, J.; Leonard, T.; Nguyen, H. Evaluation of the implantable force transducer for chronic tendon-force recordings. J. Biomech. 1996, 29, 103–109. [Google Scholar] [CrossRef]
- Finni, T.; Komi, P.; Lukkariniemi, J. Achilles tendon loading during walking: Application of a novel optic fiber technique. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, C.R.; Malchau, H.; Yuan, X.; Perinchief, R.; Kärrholm, J.; Börlin, N.; Estok, D.M.; Harris, W.H. Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model. J. Orthop. Res. 2002, 20, 688–695. [Google Scholar] [CrossRef]
- Selvik, G. Roentgen stereophotogrammetric analysis. Acta Radiol. 1990, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ryd, L.; Albrektsson, B.; Carlsson, L.; Dansgard, F.; Herberts, P.; Lindstrand, A.; Regner, L.; Toksvig-Larsen, S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J. Bone Jt. Surg. Br. Vol. 1995, 77, 377–383. [Google Scholar] [CrossRef]
- Kärrholm, J.; Selvik, G.; Elmqvist, L.-G.; Hansson, L.I. Active knee motion after cruciate ligament rupture: Stereoradiography. Acta Orthop. Scand. 1988, 59, 158–164. [Google Scholar] [CrossRef]
- Muramatsu, T.; Muraoka, T.; Takeshita, D.; Kawakami, Y.; Hirano, Y.; Fukunaga, T. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J. Appl. Physiol. 2001, 90, 1671–1678. [Google Scholar] [CrossRef]
- Oliveira, L.; Peixinho, C.; Silva, G.; Menegaldo, L. In vivo passive mechanical properties estimation of Achilles tendon using ultrasound. J. Biomech. 2015. [Google Scholar] [CrossRef]
- Seynnes, O.R.; Bojsen-Møller, J.; Albracht, K.; Arndt, A.; Cronin, N.J.; Finni, T.; Magnusson, S.P. Ultrasound-based testing of tendon mechanical properties: A critical evaluation. J. Appl. Physiol. 2015, 118, 133–141. [Google Scholar] [CrossRef]
- Chen, H.; Rogalski, M.M.; Anker, J.N. Advances in functional X-ray imaging techniques and contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 13469–13486. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cong, W.; Durairaj, K.; Qian, X.; Shen, H.; Sinn, P.; Hoffman, E.; McLennan, G.; Henry, M. In vivo mouse studies with bioluminescence tomography. Opt. Express 2006, 14, 7801–7809. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Suckey, M.M.; Benza, D.; Arifuzzaman, M.; Millhouse, P.W.; Anderson, D.; Heath, J.; Des Jardins, J.D.; Anker, J.N. Luminescent Spectral Rulers for Noninvasive Displacement Measurement through Tissue. ACS Sens. 2020, 5, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, N.; Rogalski, M.M.; Benza, D.; Lake, J.; Urban, M.; Pelham, H.; Anker, J.N.; DesJardins, J.D. Development of an Optically-Based Tension-Indicating Implanted Orthopedic Screw with a Luminescent Spectral Ruler; SPIE BiOS, 2017; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; p. 100810N. [Google Scholar]
- Moore, T.L.; Wang, F.; Chen, H.; Grimes, S.W.; Anker, J.N.; Alexis, F. Polymer-Coated Radioluminescent Nanoparticles for Quantitative Imaging of Drug Delivery. Adv. Funct. Mater. 2014, 24, 5815–5823. [Google Scholar] [CrossRef]
- Santelli, J.; Lechevallier, S.; Calise, D.; Marsal, D.; Siegfried, A.; Vincent, M.; Martinez, C.; Cussac, D.; Mauricot, R.; Verelst, M. Multimodal gadolinium oxysulfide nanoparticles for bioimaging: A comprehensive biodistribution, elimination and toxicological study. Acta Biomater. 2020, 108, 172–261. [Google Scholar] [CrossRef] [PubMed]
- Arion, H. Carboxymethylcellulose hydrogel-filled breast implants. Our experience in 15 years. Ann. Chir. Plast. Esthet. 2001, 46, 55–59. [Google Scholar] [CrossRef]
- Bifari, E.N.; Bahadar Khan, S.; Alamry, K.A.; Asiri, A.M.; Akhtar, K. Cellulose acetate based nanocomposites for biomedical applications: A review. Curr. Pharm. Des. 2016, 22, 3007–3019. [Google Scholar] [CrossRef]
- Wu, X.; Suvarnapathaki, S.; Walsh, K.; Camci-Unal, G. Paper as a scaffold for cell cultures: Teaching an old material new tricks. MRS Commun. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Wu, X.; Walsh, K.; Suvarnapathaki, S.; Lantigua, D.; McCarthy, C.; Camci-Unal, G. Mineralized paper scaffolds for bone tissue engineering. Biotechnol. Bioeng. 2020, 118, 1411–1418. [Google Scholar] [CrossRef]
- Hassani, F.A.; Lee, C. A triboelectric energy harvester using low-cost, flexible, and biocompatible ethylene vinyl acetate (EVA). J. Microelectromechanical Syst. 2015, 24, 1338–1345. [Google Scholar] [CrossRef]
- Seitz, H.; Marlovits, S.; Schwendenwein, I.; Vecsei, V.; Losert, U. Biocompatibility of polyethylene terephthalate—PET—(Trevira strong)—an in vivo study of the sheep knee. Biomed. Technik. Biomed. Eng. 1996, 41, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, Y.; Ai, C.; Jiang, J.; Cai, J.; Sheng, D.; Wan, F.; Liu, X.; Hao, Y.; Chen, J. Enhance the biocompatibility and osseointegration of polyethylene terephthalate ligament by plasma spraying with hydroxyapatite in vitro and in vivo. Int. J. Nanomed. 2018, 13, 3609. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Raval, Y.; Chen, H.; Tzeng, T.R.J.; DesJardins, J.D.; Anker, J.N. Development of luminescent pH sensor films for monitoring bacterial growth through tissue. Adv. Healthc. Mater. 2014, 3, 197–204. [Google Scholar] [CrossRef]
- Wang, F.; Raval, Y.; Tzeng, T.R.J.; Anker, J.N. X-ray Excited Luminescence Chemical Imaging of Bacterial Growth on Surfaces Implanted in Tissue. Adv. Healthc. Mater. 2015, 4, 903–910. [Google Scholar] [CrossRef]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Xu, C.T.; Svenmarker, P.; Liu, H.; Wu, X.; Messing, M.E.; Wallenberg, L.R.; Andersson-Engels, S. High-resolution fluorescence diffuse optical tomography developed with nonlinear upconverting nanoparticles. ACS Nano 2012, 6, 4788–4795. [Google Scholar] [CrossRef]
- Uzair, U.; Johnson, C.; Beladi-Behbahani, S.; Rajamanthrilage, A.C.; Raval, Y.S.; Benza, D.; Ranasinghe, M.; Schober, G.; Tzeng, T.-R.J.; Anker, J.N. Conformal Coating of Orthopedic Plates with X-ray Scintillators and pH Indicators for X-ray Excited Luminescence Chemical Imaging through Tissue. ACS Appl. Mater. Interfaces 2020, 12, 52343–52353. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Moore, T.L.; Qi, B.; Sulejmanovic, D.; Hwu, S.J.; Mefford, O.T.; Alexis, F.; Anker, J.N. Bright X-ray and up-conversion nanophosphors annealed using encapsulated sintering agents for bioimaging applications. J. Mater. Chem. B 2017, 5, 5412–5424. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suckey, M.M.; Benza, D.W.; DesJardins, J.D.; Anker, J.N. Upconversion Spectral Rulers for Transcutaneous Displacement Measurements. Sensors 2021, 21, 3554. https://doi.org/10.3390/s21103554
Suckey MM, Benza DW, DesJardins JD, Anker JN. Upconversion Spectral Rulers for Transcutaneous Displacement Measurements. Sensors. 2021; 21(10):3554. https://doi.org/10.3390/s21103554
Chicago/Turabian StyleSuckey, Melissa M., Donald W. Benza, John D. DesJardins, and Jeffrey N. Anker. 2021. "Upconversion Spectral Rulers for Transcutaneous Displacement Measurements" Sensors 21, no. 10: 3554. https://doi.org/10.3390/s21103554
APA StyleSuckey, M. M., Benza, D. W., DesJardins, J. D., & Anker, J. N. (2021). Upconversion Spectral Rulers for Transcutaneous Displacement Measurements. Sensors, 21(10), 3554. https://doi.org/10.3390/s21103554





