Assessment of Biomechanical Response to Fatigue through Wearable Sensors in Semi-Professional Football Referees
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedures
- A 5 min warm-up phase of jogging on treadmill at fixed speed (i.e., 10 km/h);
- Pre-fatigue acquisition, in which MIMUs were placed on the participant’s body using elastic bands, and the athlete performed one series of 5 CMJs separated by a 10 s of rest time (at the end of this acquisition sensors were removed to facilitate the following phase);
- Fatigue phase induced in athletes by using the YYIR1 (which consisted of 2 × 20 m shuttle runs at increasing speeds, with a 10 s period of active recovery, allowing us to quantify the individual’s capacity to perform repeated intense exercise and to examine changes in performance [20]);
- Post-fatigue acquisitions in which the same CMJ test conducted in the pre-fatigue acquisition (i.e., 5 CMJs separated by a 10 s of rest time) was repeated after 5 min from the end of fatigue exercises.
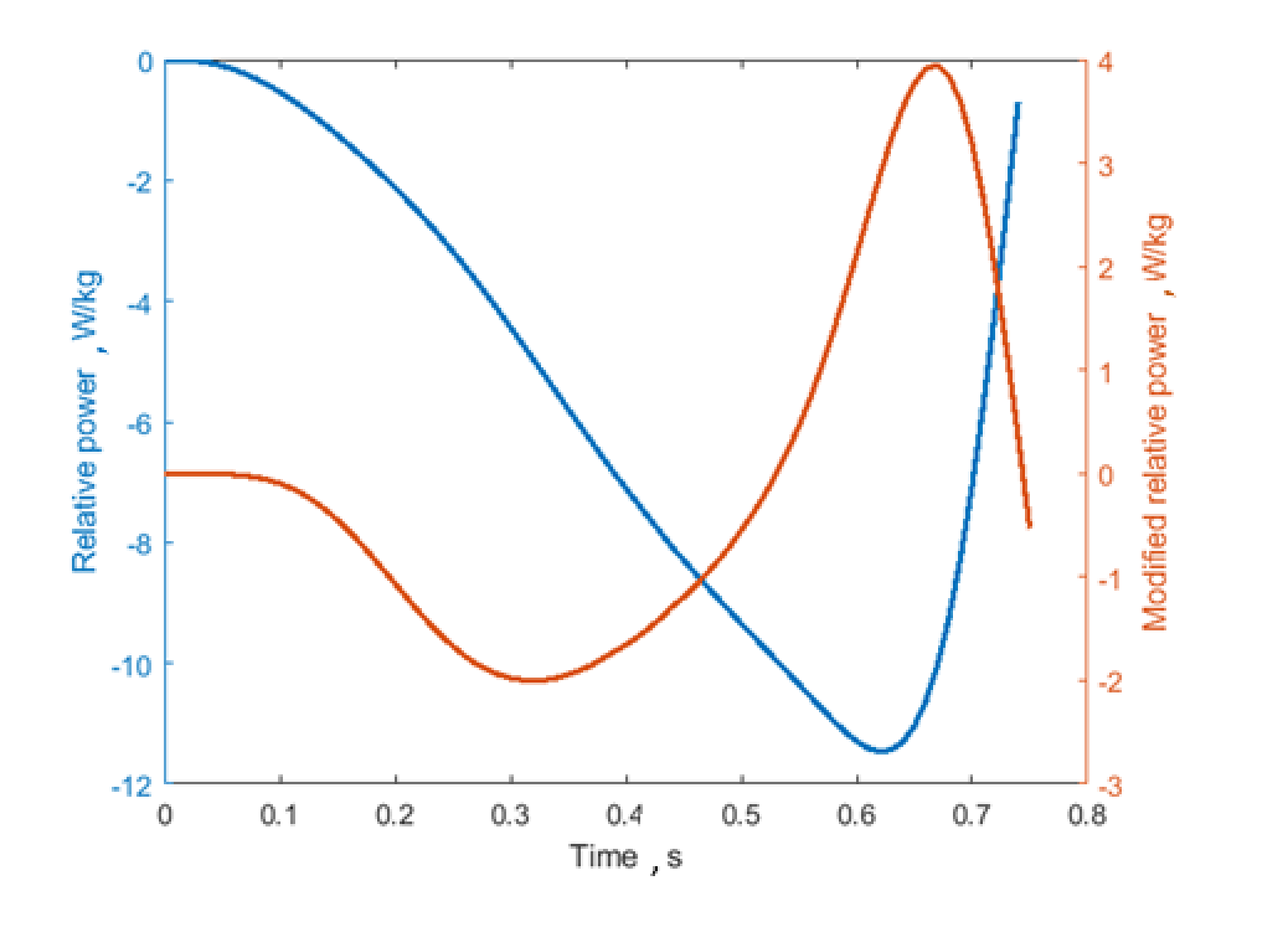
2.3. Segmentation of the Jump
- The start of the eccentric phase was identified by analysing the acceleration curve and automatically finding when it goes below a specific threshold (i.e., mean +3 standard deviations of the first 100 samples);
- The end of the eccentric phase corresponds to the zero crossing of the velocity (second red line in Figure 2);
- The end of the concentric jump coincides with the peak in the acceleration curve of the foot sensor. The peak of the curve was automatically detected by finding the maximum value of the acceleration.
2.4. Measurements
- Displacement parameters: the mean displacement of the centre of mass during the eccentric phase (i.e., jump depth) is analysed in this work. In our opinion, monitoring the mean position of the barycentre could be useful to understand how much the athlete is loading the jump due to fatigue.
- Power and energy parameters: allow us to quantify the lower-limb explosive ability, resulting in extremely important indicators for fatigue detection. An in-depth analysis of the scientific literature allowed us to find the following parameters [5,17,24]: minimum and maximum relative power exerted, mean relative power over time, mean power velocity (MPV), mean and minimum energy.
- Time parameters [17,24]: allow us to pinpoint specific events in time during the execution of the exercise (i.e., when the maximum power is exerted or how long the athlete stays in contact with the ground). Therefore, negative power peak time (NPPT), positive power peak time (PPPT) and the total duration of the entire eccentric phase () were reported in this study.
3. Results
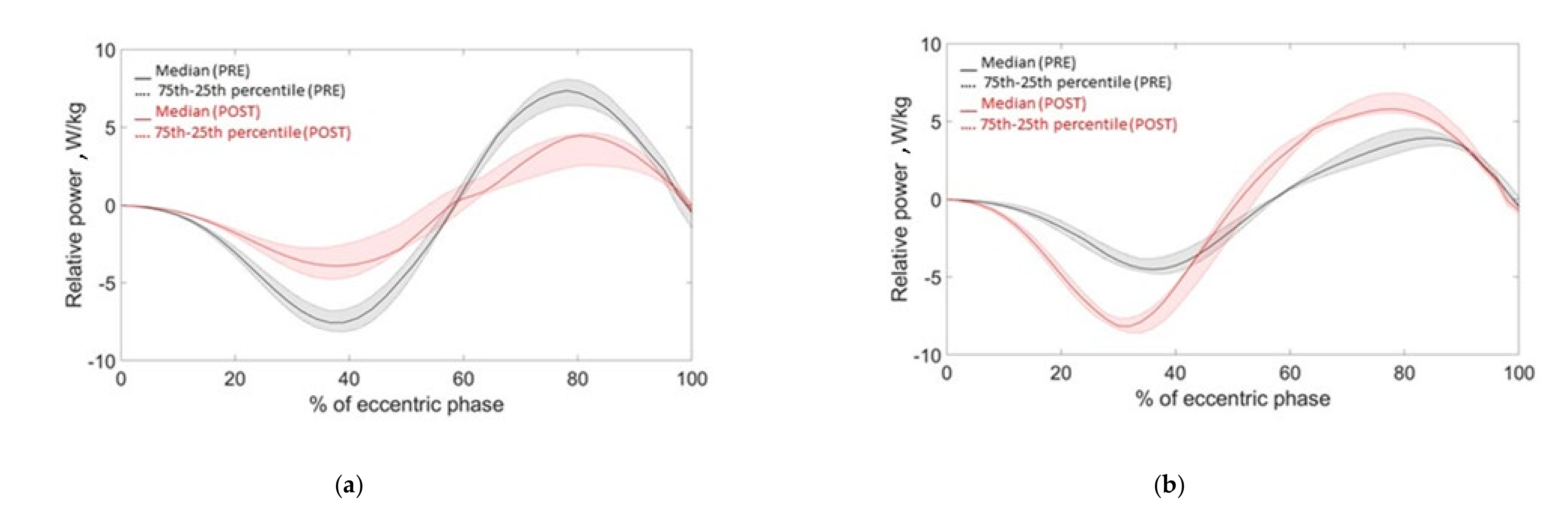
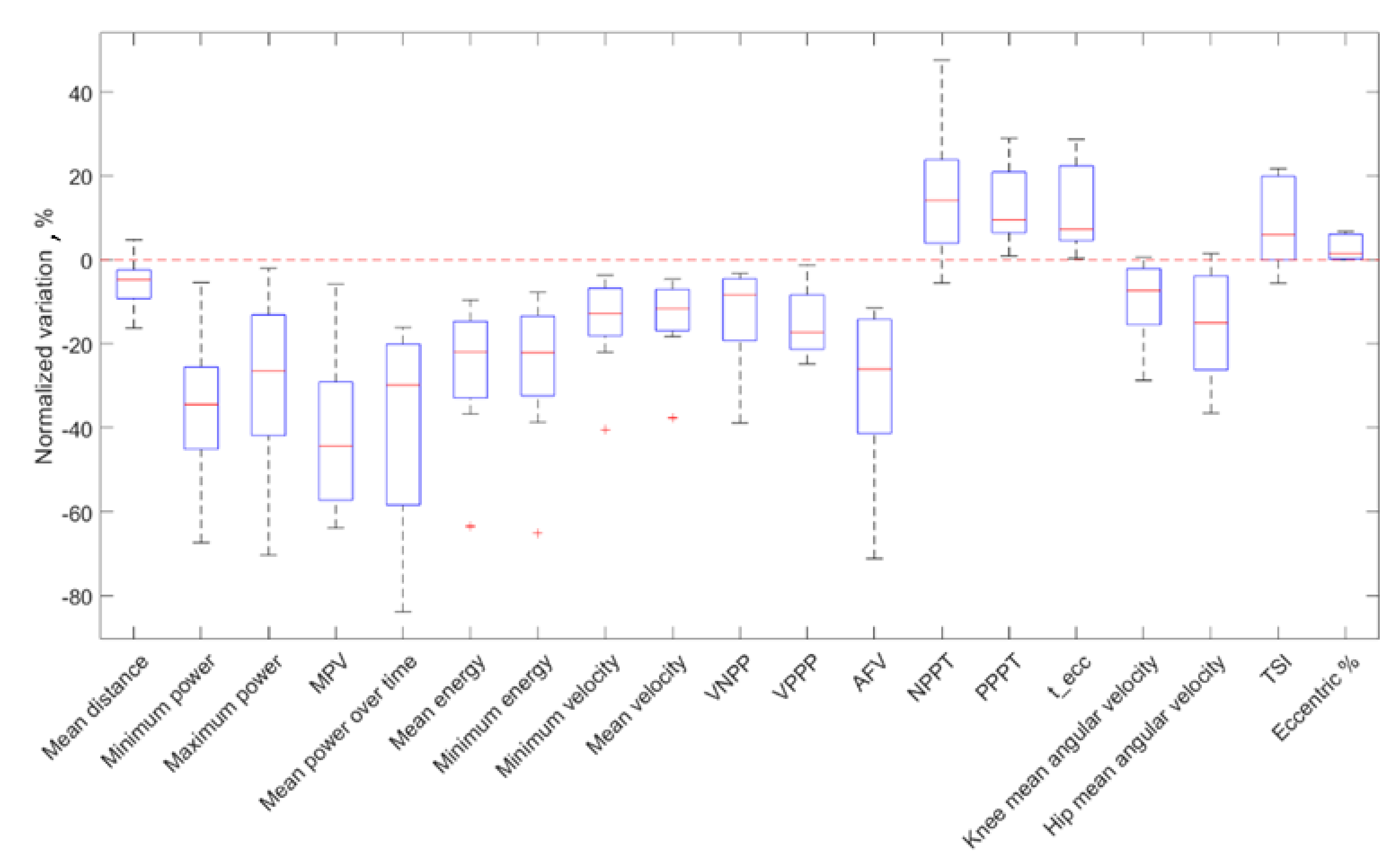
3.1. Fatigue Group
3.2. PAP Group
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flück, M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J. Exp. Biol. 2006, 209, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Kilduff, L.P.; Kingsley, M.I.C.; Owen, N. Postactivation potentiation in professional rugby players: Optimal recovery. J. Strength Cond. Res. 2007, 21, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Duncan, N.; Marin, P.; Brown, L.; Loenneke, J.P.; Wilson, S.M.C.; Jo, E.; Lowery, R.; Ugrinowitsch, C. Meta-analysis of postactivation potentiation and power: Effects of conditioning activity, volume, gender, rest periods, and training status. J. Strength Cond. Res. 2013, 27, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G. Postactivation potentiation: Role in human performance. Exerc. Sport Sci. Rev. 2002, 30, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Claudino, J.G.; Cronin, J.; Mezêncio, B.; McMaster, D.T.; McGuigan, M.; Tricoli, V.; Amadio, A.C.; Serrão, J.C. The countermovement jump to monitor neuromuscular status: A meta-analysis. J. Sci. Med. Sport 2017, 20, 397–402. [Google Scholar] [CrossRef]
- Gibson, H.; Edwards, R.H.T. Muscular Exercise and Fatigue. Sport. Med. 1985, 2, 120–132. [Google Scholar] [CrossRef]
- Jan, E.; Hägglund, M.; Waldén, M. Epidemiology of muscle injuries in professional football (soccer). Am. J. Sports Med. 2011, 39, 1226–1232. [Google Scholar]
- Greig, M.; Walker-Johnson, C. The influence of soccer-specific fatigue on functional stability. Phys. Ther. Sport 2007, 8, 185–190. [Google Scholar] [CrossRef]
- Borresen, J.; Lambert, M.I. The quantification of training load, the training response and the effect on performance. Sports Med. 2009, 39, 779–795. [Google Scholar] [CrossRef]
- Crewe, H.; Tucker, R.; Noakes, T.D. The rate of increase in rating of perceived exertion predicts the duration of exercise to fatigue at a fixed power output in different environmental conditions. Eur. J. Appl. Physiol. 2008, 103, 569. [Google Scholar] [CrossRef]
- Rodacki, A.L.F.; Fowler, N.E.; Bennett, S.J. Vertical jump coordination: Fatigue effects. Med. Sci. Sports Exerc. 2002, 34, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Kumar, D.K.; Polus, B.; Fraser, S. Strategies to identify changes in SEMG due to muscle fatigue during cycling. J. Med. Eng. Technol. 2007, 31, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I. Quantification of training and competition loads in endurance sports: Methods and applications. Int. J. Sports Physiol. Perform. 2017, 12, S29–S217. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Camomilla, V.; Capranica, L. Countermovement jump performance assessment using a wearable 3D inertial measurement unit. J. Sports Sci. 2011, 29, 139–146. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Clifford, T.; Abbott, W.; Kwiecien, S.Y.; Kremenic, I.J.; DeVita, J.J.; Howatson, G. Countermovement jump recovery in professional soccer players using an inertial sensor. Int. J. Sports Physiol. Perform. 2019, 14, 9–15. [Google Scholar] [CrossRef]
- Milosevic, B.; Farella, E. Wearable inertial sensor for jump performance analysis. In Proceedings of the 2015 Workshop on Wearable Systems and Applications, New York, NY, USA, 18 May 2015; pp. 15–20. [Google Scholar]
- Gathercole, R.J.; Stellingwerff, T.; Sporer, B.C. Effect of acute fatigue and training adaptation on countermovement jump performance in elite snowboard cross athletes. J. Strength Cond. Res. 2015, 29, 37–46. [Google Scholar] [CrossRef]
- Bosco, C.; Ito, A.; Komi, P.V.; Luhtanen, P.; Rahkila, P.; Rusko, H.; Viitasalo, J.T. Neuromuscular function and mechanical efficiency of human leg extensor muscles during jumping exercises. Acta Physiol. Scand. 1982, 114, 543–550. [Google Scholar] [CrossRef]
- Truppa, L.; Guaitolini, M.; Castagna, C.; Mannini, A. The Eccentric Phase of Countermovement Jump: Comparing Motion Capture and Inertial Sensor. In Proceedings of the Seventh National Congress of Bioengineering Proceedings, Trieste, Italy, 9–11 June 2021; pp. 10–13. [Google Scholar]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo intermittent recovery test. Sport. Med. 2008, 38, 37–51. [Google Scholar] [CrossRef]
- Ligorio, G.; Bergamini, E.; Truppa, L.; Guaitolini, M.; Raggi, M.; Mannini, A.; Sabatini, A.M.; Vannozzi, G.; Garofalo, P. A wearable magnetometer-free motion capture system: Innovative solutions for real-world applications. IEEE Sens. J. 2020, 20, 8844–8857. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef]
- James, W.P.T.; Waterlow, J.C. Research on Obesity: A Report of the DHSS/MRC Group; HM Stationery Office: London, UK, 1976. [Google Scholar]
- Gathercole, R.; Sporer, B.; Stellingwerff, T.; Sleivert, G. Alternative Countermovement–Jump Analysis to Quantify Acute Neuromuscular Fatigue Pilot study investigating the effects of a short-term low Fodmap diet in healthy runners with persistent exercise-associated GI symptoms View project. Int. J. Sport Physiol Perform. 2015, 10, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Appell, H.-J.; Soares, J.M.C.; Duarte, J.A.R. Exercise, muscle damage and fatigue. Sport. Med. 1992, 13, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.-L. A tutorial on se (3) transformation parameterizations and on-manifold optimization. Univ. Malaga Tech. Rep. 2010, 3, 6. [Google Scholar]
- Sanchez-Medina, L.; González-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.C.H.; Beaudette, C.M.; Smale, K.B.; Beange, K.H.E.; Graham, R.B. A Subject-Specific Approach to Detect Fatigue-Related Changes in Spine Motion Using Wearable Sensors. Sensors 2020, 20, 2646. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, N.; Lees, A.; Reilly, T. Electromyography of selected lower-limb muscles fatigued by exercise at the intensity of soccer match-play. J. Electromyogr. Kinesiol. 2006, 16, 257–263. [Google Scholar] [CrossRef]
- Castellini, C.; Artemiadis, P.; Wininger, M.; Ajoudani, A.; Alimusaj, M.; Bicchi, A.; Caputo, B.; Craelius, W.; Dosen, S.; Englehart, K. Proceedings of the first workshop on peripheral machine interfaces: Going beyond traditional surface electromyography. Front. Neurorobot. 2014, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, J.B.; Aagaard, P.; Madsen, K. Rapid muscle force capacity changes after soccer match play. Int. J. Sports Med. 2009, 30, 273–278. [Google Scholar] [CrossRef]
- Djaoui, L.; Haddad, M.; Chamari, K.; Dellal, A. Physiology & Behavior Monitoring training load and fatigue in soccer players with physiological markers. Physiol. Behav. 2017, 181, 86–94. [Google Scholar]
- Castagna, C.; Impellizzeri, F.M.; Rampinini, E.; D’Ottavio, S.; Manzi, V. The Yo–Yo intermittent recovery test in basketball players. J. Sci. Med. Sport 2008, 11, 202–208. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sport. Med. 2014, 44, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.F.T.; Polito, L.F.T.; Figueira Jr, A.J.; Miranda, M.L.J.; Chtourou, H.; Miranda, J.M.; Brandão, M.R.F. Psychophysiological indicators of fatigue in soccer players: A systematic review. Sci. Sports 2017, 32, 1–13. [Google Scholar] [CrossRef]
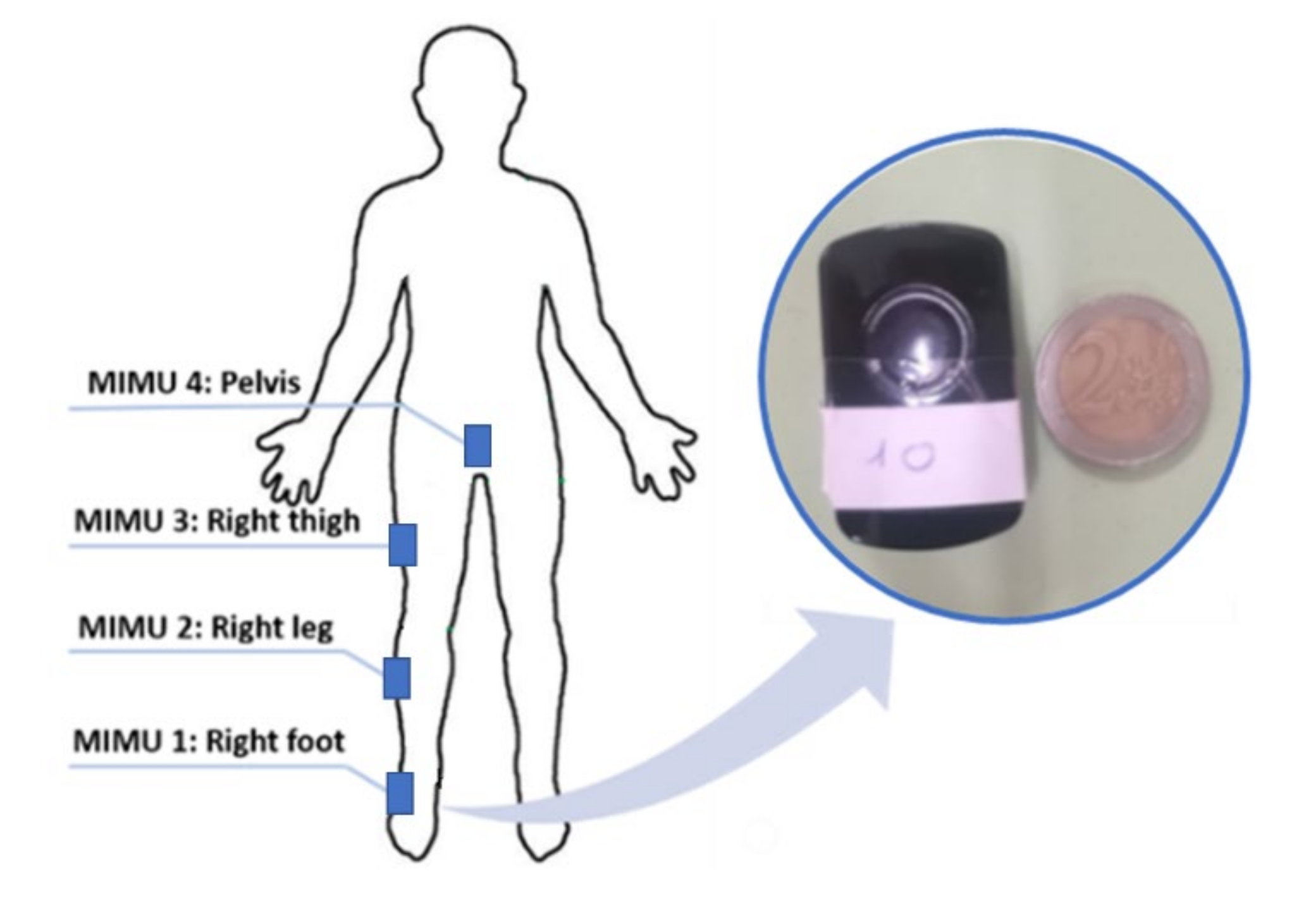

| Physical Quantities | MIMU |
|---|---|
| Measured quantity | |
| TSI | |
| Eccentric % |
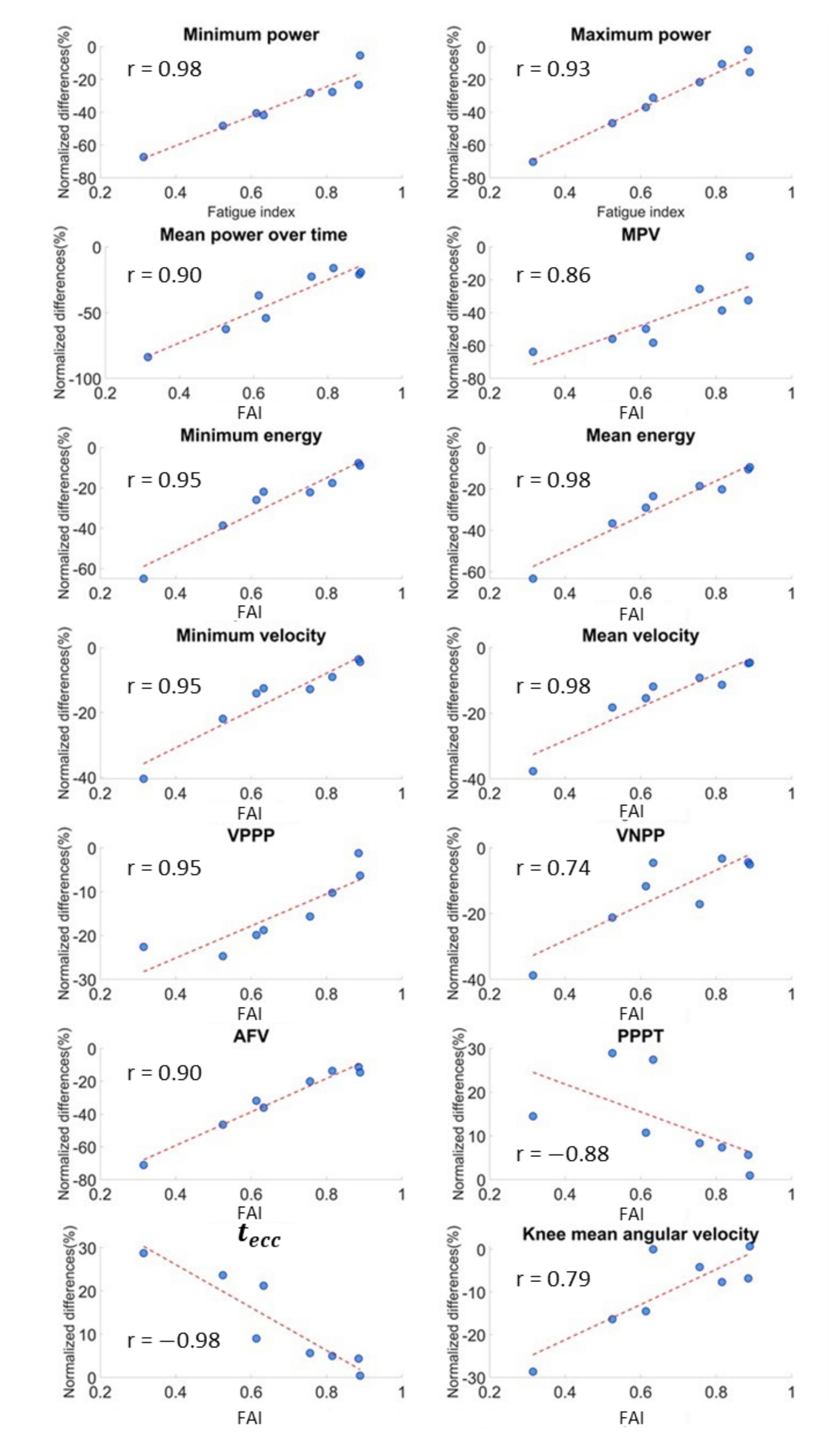
| Parameters | PRE | POST | Wilcoxon Signed Rank Test Significance | Normalized Variations (%) | FAI Spearman Coefficient | Cliff’s Delta ES |
|---|---|---|---|---|---|---|
| Mean distance | −1.85 (0.54) | −1.76 (0.46) | 0.04 * | −4.27 (4.80) | 0.12 | 0.19 (T↓) |
| −4.92 (5.42) | −3.33 (3.30) | <0.01 ** | −34.4 (19.76) | 0.98 ** | 0.38 (M↓) | |
| 5.42 (5.85) | 3.84 (4.56) | <0.01 ** | −26.4 (28.8) | 0.93 ** | −0.34 (M↓) | |
| 0.26 (0.62) | 0.15 (0.34) | <0.01 ** | −29.8 (38.2) | 0.90 ** | −0.38 (M↓) | |
| −28.3 (39.0) | −15.5 (21.4) | <0.01 ** | −44.3 (28.0) | 0.86 ** | 0.47 (M↓) | |
| Mean energy () | −3.57 (0.62) | −2.93 (0.74) | <0.01 ** | −21.9 (18.3) | 0.98 ** | 0.37 (M↓) |
| Minimum energy () | −7.88 (5.12) | −5.97 (5.00) | <0.01 ** | −22.1 (19.1) | 0.95 ** | 0.34 (M↓) |
| −1.24 (0.18) | −1.08 (0.44) | <0.01 ** | −12.7 (11.3) | 0.95 ** | 0.34 (M↓) | |
| −0.70 (0.16) | −0.64 (0.22) | <0.01 ** | −11.1 (9.84) | 0.98 ** | 0.44 (M↓) | |
| −0.91 (0.38) | −0.82 (0.36) | <0.01 ** | −8.37 (14.7) | 0.74 * | 0.28 (S↓) | |
| −0.82 (0.38) | −0.64 (0.34) | <0.01 ** | −17.2 (12.9) | 0.95 ** | 0.31 (M↓) | |
| −15.00 (15.9) | −10.10 (11.4) | <0.01 ** | −26.0 (27.2) | 0.90 ** | 0.34 (M↓) | |
| NPPT () | 20.25 (5.44) | 23.25 (3.38) | 0.04 * | 14.2 (19.98) | −0.19 | 0.5 (L↑) |
| PPPT () | 47.75 (15.8) | 57.75 (15.9) | <0.01 ** | 9.53 (14.4) | −0.88 ** | 0.25 (S↑) |
| () | 55.00 (15.8) | 60.00 (16.1) | <0.01 ** | 7.32 (17.8) | −0.98 ** | 0.28 (S↑) |
| Knee mean angular velocity (deg/s) | 113.9 (31.8) | 107.8 (29.2) | 0.02 * | −7.23 (9.20) | 0.79 * | −0.39 (M↓) |
| Hip mean angular velocity (deg/s) | 114.7 (56.2) | 101.9 (28.2) | 0.02 * | −16.2 (13.2) | 0.21 | −0.55 (L↓) |
| TSI | 0.419 (0.10) | 0.488 (0.18) | 0.02 * | 6.04 (18.5) | −0.57 | 0.43 (M↑) |
| Eccentric % | 0.711 (0.04) | 0.744 (0.08) | 0.02 * | 1.51 (5.04) | −0.43 | 0.43 (M↑) |
| Parameters | Weight | Muscular Mass |
|---|---|---|
| Maximum power | - | 0.83 * |
| Minimum energy | 0.79 * | 0.88 ** |
| Minimum velocity | 0.79 * | 0.88 ** |
| VPPP | - | 0.82 * |
| VNPP | 0.86 * | 0.83 ** |
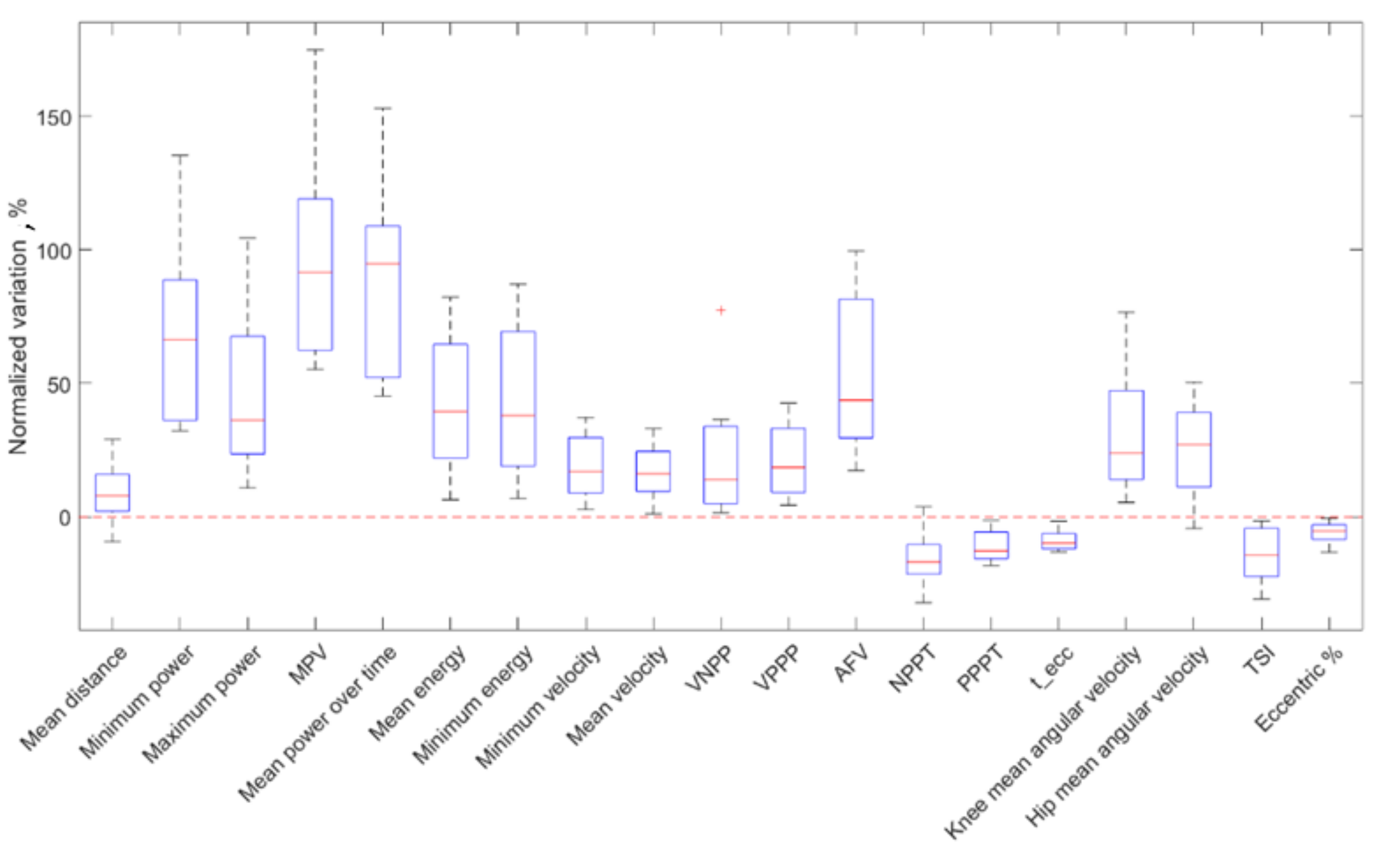
| Parameters | PRE | POST | Wilcoxon Signed Rank Test Significance | Normalized Variations (%) | Cliff’s Delta ES |
|---|---|---|---|---|---|
| −1.27 (0.30) | −1.31 (0.32) | 0.08 | 7.98 (13.8) | −0.19 (T↑) | |
| Minimum power (W) | −2.77 (1.38) | −4.34 (1.96) | <0.01 ** | 66.4 (52.6) | −0.78 (L↑)) |
| Maximum power (W) | 3.47 (1.26) | 4.66 (1.74) | <0.01 ** | 36.3 (44.2) | 0.72 (L↑) |
| 0.16 (0.08) | 0.34 (0.20) | <0.01 ** | 94.8 (57.0) | 0.72 (L↑) | |
| −14.37 (6.80) | −28.83 (14.0) | <0.01 ** | 91.6 (56.6) | −0.81 (L↑) | |
| Mean energy () | −2.02 (0.66) | −2.49 (0.378) | <0.01 ** | 39.4 (42.6) | −0.62 (L↑) |
| Minimum energy () | −4.36 (1.62) | −5.43 (1.76) | <0.01 ** | 37.8 (50.4) | −0.59 (L↑) |
| −0.92 (0.18) | −1.03 (0.16) | <0.01 ** | 16.9 (20.8) | −0.59 (L↑) | |
| −0.54 (0.08) | −0.58 (0.10) | <0.01 ** | 16.2 (14.9) | −0.69 (L↑) | |
| −0.71 (0.16) | −0.78 (0.12) | <0.01 ** | 13.9 (28.8) | −0.58 (L↑) | |
| −0.60 (0.14) | −0.67 (0.16) | <0.01 ** | 18.5 (42.0) | −0.63 (L↑) | |
| −9.55 (3.06) | −13.13 (4.64) | <0.01 ** | 43.6 (52.2) | −0.84 (L↑) | |
| NPPT () | 20.38 (3.76) | 17.38 (4.50) | 0.02 * | −17.0 (11.1) | −0.56 (L↓) |
| PPPT () | 44.50 (6.76) | 38.88 (11.0) | <0.01 ** | −12.9 (9.96) | −0.40 (M↓) |
| () | 50.88 (0.08) | 45.25 (10.0) | <0.01 ** | −9.92 (5.66) | −0.34 (M↓) |
| Knee mean angular velocity (°/sec) | 108.5 (20.5) | 124.6 (29.6) | <0.01 ** | 27.1 (33.4) | 0.81 (L↑) |
| Hip mean angular velocity (°/sec) | 108.7 (22.8) | 131.8 (38.4) | <0.01 ** | 27.0 (27.2) | 0.68 (L↑) |
| TSI | 0.486 (0.08) | 0.407 (0.12) | <0.01 ** | −14.5 (15.6) | −0.50 (L↓) |
| Eccentric % | 0.743 (0.04) | 0.691 (0.80) | <0.01 ** | −5.48 (5.54) | −0.56 (L↑) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truppa, L.; Guaitolini, M.; Garofalo, P.; Castagna, C.; Mannini, A. Assessment of Biomechanical Response to Fatigue through Wearable Sensors in Semi-Professional Football Referees. Sensors 2021, 21, 66. https://doi.org/10.3390/s21010066
Truppa L, Guaitolini M, Garofalo P, Castagna C, Mannini A. Assessment of Biomechanical Response to Fatigue through Wearable Sensors in Semi-Professional Football Referees. Sensors. 2021; 21(1):66. https://doi.org/10.3390/s21010066
Chicago/Turabian StyleTruppa, Luigi, Michelangelo Guaitolini, Pietro Garofalo, Carlo Castagna, and Andrea Mannini. 2021. "Assessment of Biomechanical Response to Fatigue through Wearable Sensors in Semi-Professional Football Referees" Sensors 21, no. 1: 66. https://doi.org/10.3390/s21010066
APA StyleTruppa, L., Guaitolini, M., Garofalo, P., Castagna, C., & Mannini, A. (2021). Assessment of Biomechanical Response to Fatigue through Wearable Sensors in Semi-Professional Football Referees. Sensors, 21(1), 66. https://doi.org/10.3390/s21010066






