Micro-Fabricated RTD Based Sensor for Breathing Analysis and Monitoring
Abstract
1. Introduction
2. Breathing Analysis Methods Overview
2.1. Flow Measurement Techniques
2.2. Temperature Measurement Techniques
2.3. Humidity Measurement Techniques
2.4. Measurement Techniques
2.5. Strain Measurement Techniques
3. Proposed Sensor Design Optimization & Simulation
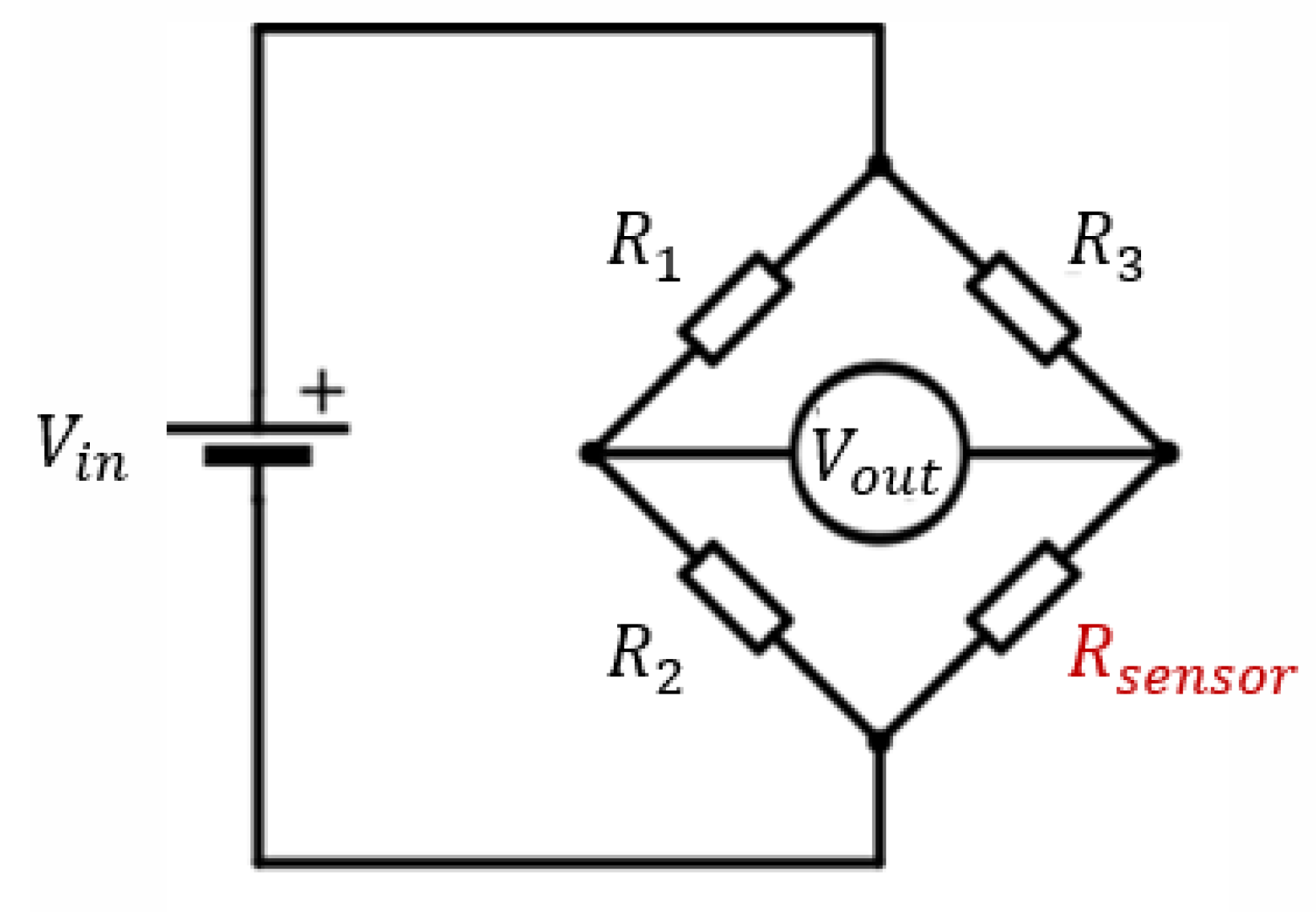
3.1. Sensor Design Optimization
3.2. Final Proposed Design
4. Sensor Physical Design & Optimization
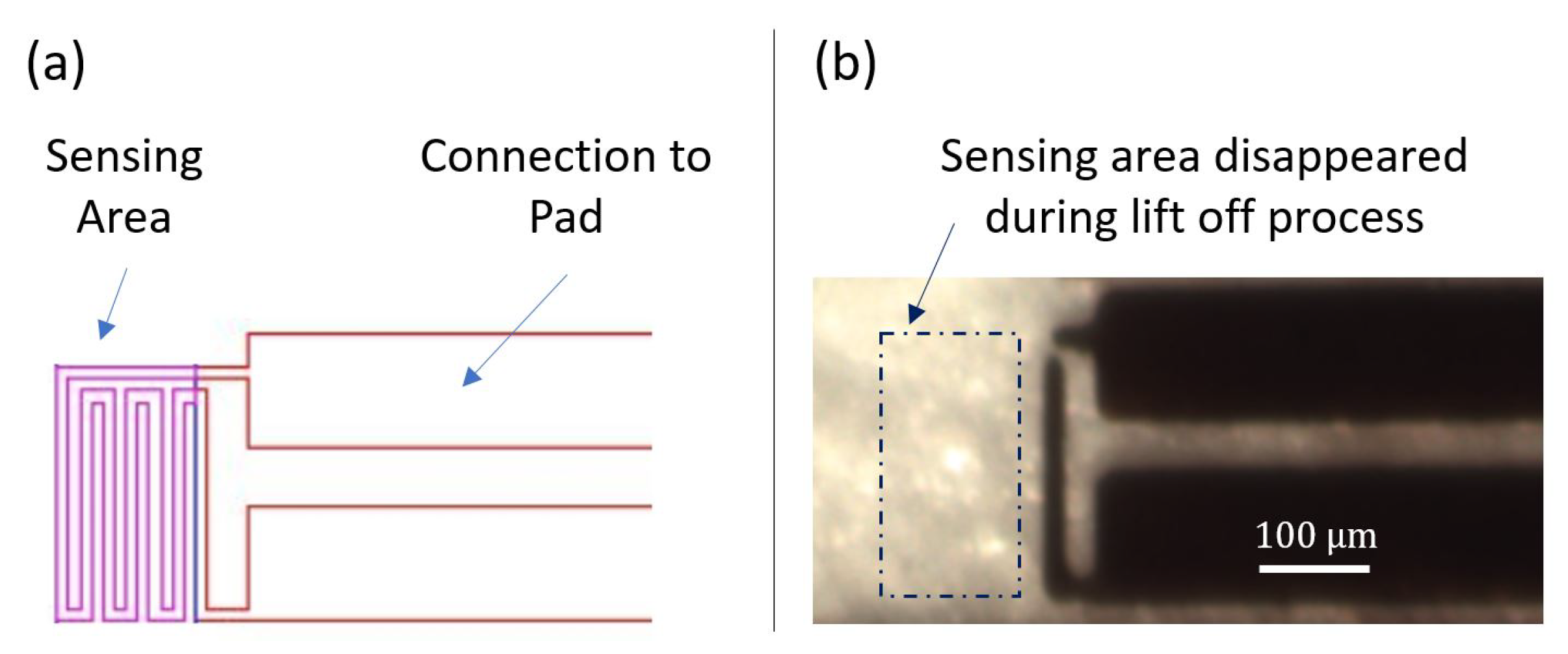
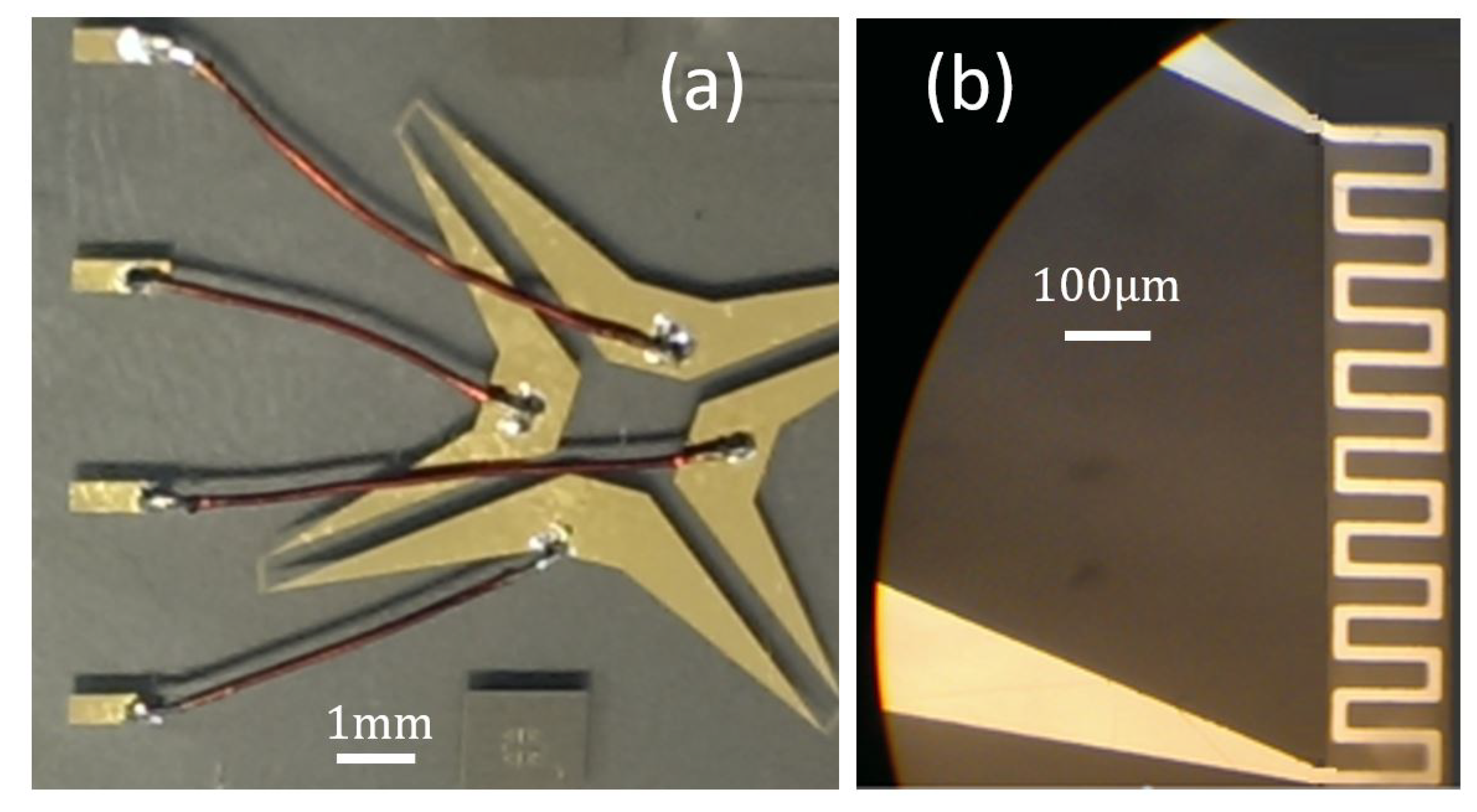
4.1. Fabrication Process
4.2. Inhaling Sensor Proposal
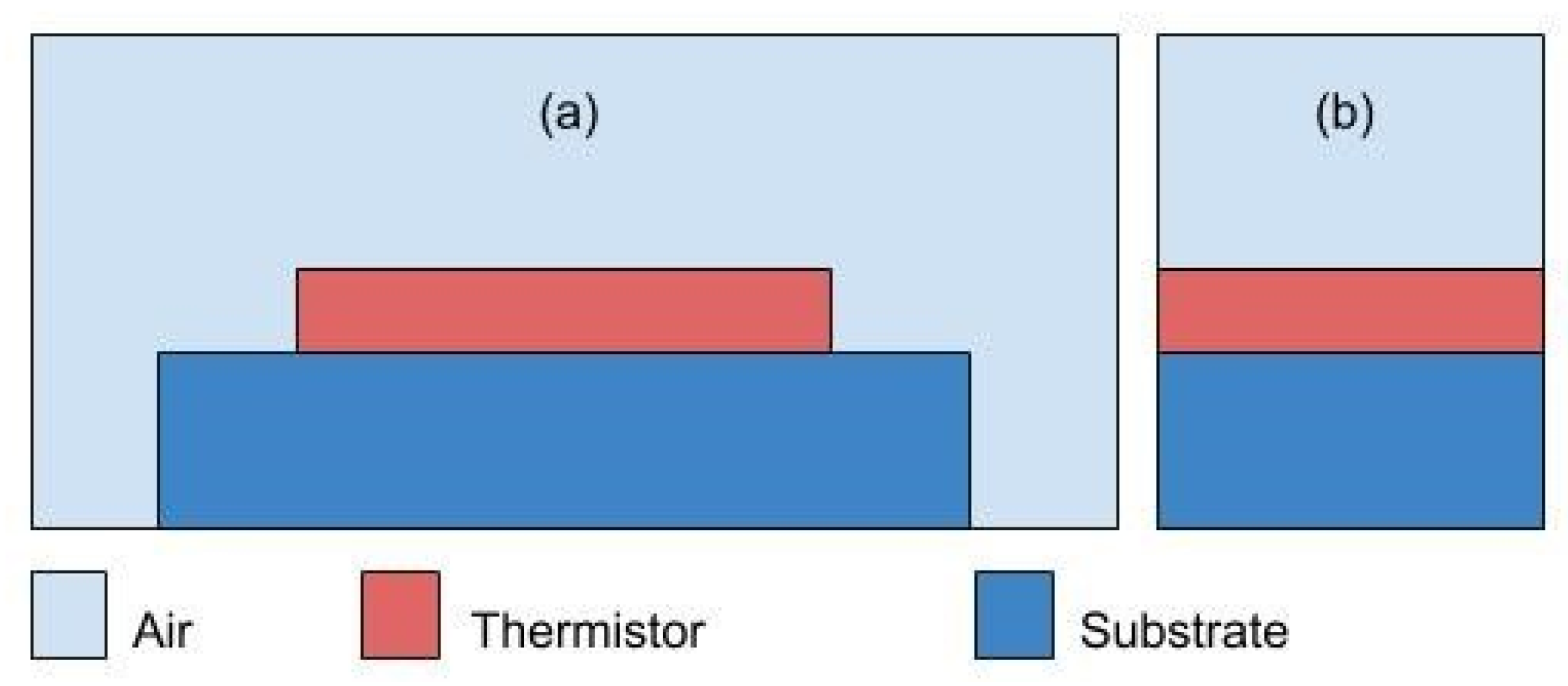
4.2.1. Design Revision 0.1
4.2.2. Design Revision 0.2
4.2.3. Design Revision 0.3
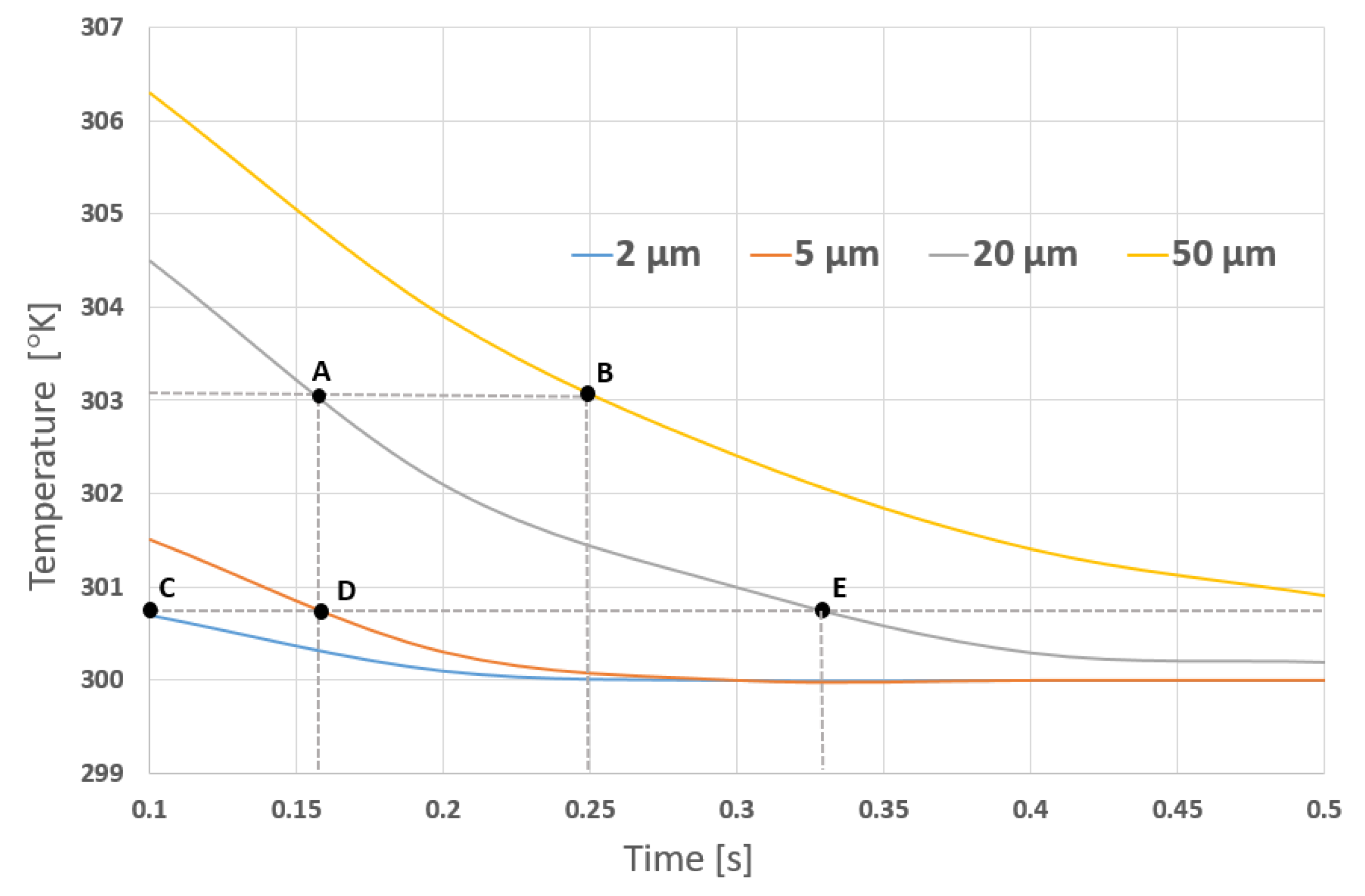
4.2.4. Simulation Results
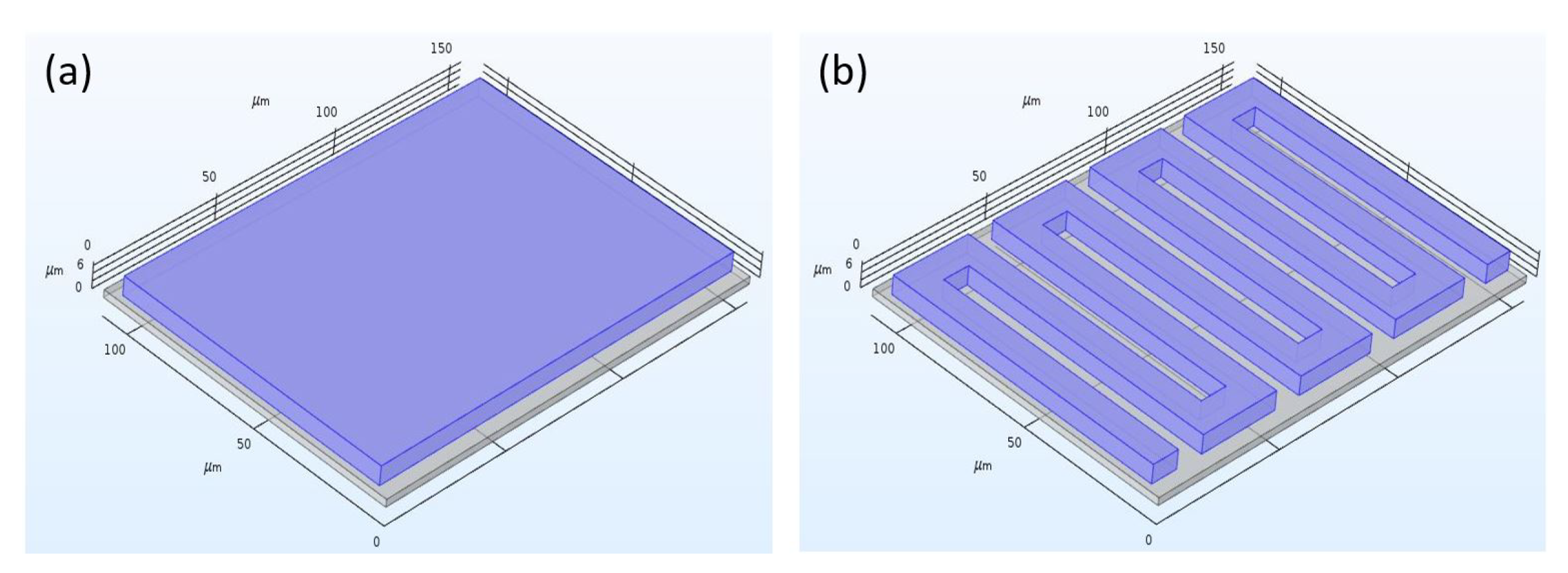
4.3. Enhanced Sensor Proposal
4.3.1. Sensor Design Revision 1.0
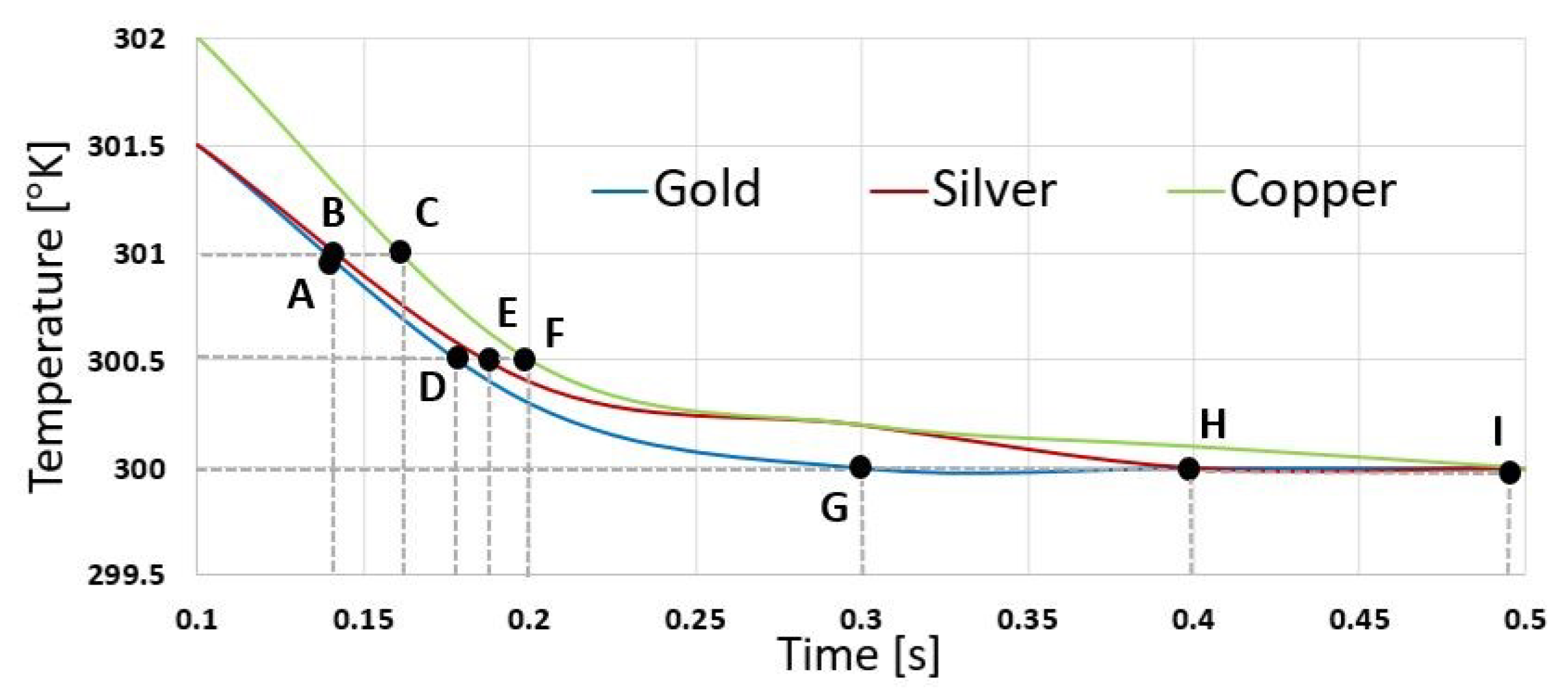
4.3.2. Simulation and Results
5. System Implementation & Results
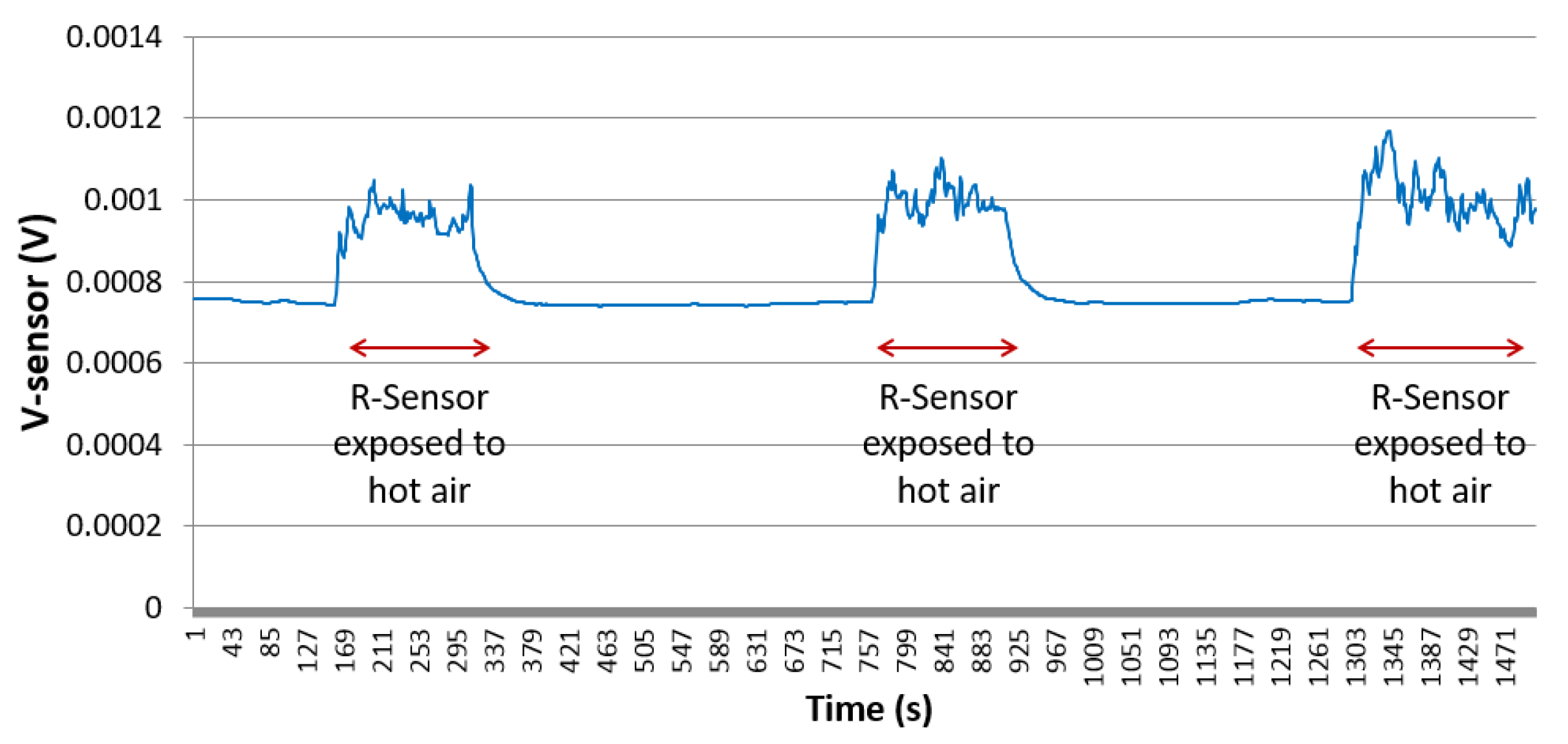
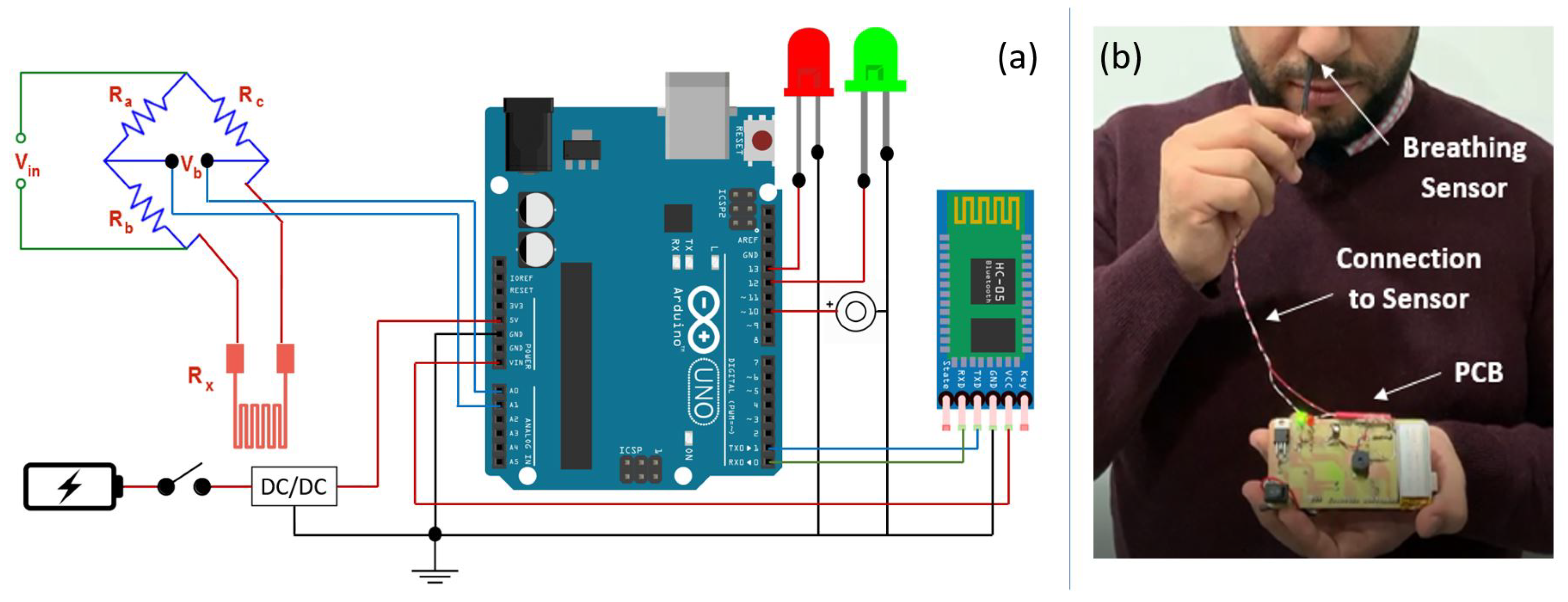
5.1. Experiment Setup
5.2. Experiment Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klum, M.; Urban, M.; Tigges, T.; Pielmus, A.-G.; Feldheiser, A.; Schmitt, T.; Orglmeister, R. Wearable Cardiorespiratory monitoring employing a multimodal digital patch stethoscope:Estimation of ECG, PEP, LVET and respiration using a 55 mm single-lead ECG and phonocardiogram. Sensors 2020, 20, 2033. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings od covid-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Sohrabi, C.; Alsafi, Z.; Neill, N.O.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel Coronavirus(Covid-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Chow, E.; Schwartz, N.; Tobolowsky, F.; Zacks, R.; Huntington-Frazier, M.; Reddy, S.; Rao, A. Symptom Screening at Illness Onset of Health Care Personnel with SARS-CoV-2 Infection in King County, Washington. JAMA 2020, 323, 2087–2089. [Google Scholar] [CrossRef]
- Yilmaz, G.; Rapin, M.; Pessoa, D.; Rocha, B.; de Sousa, A.M.; Rusconi, R.; Carvalho, P.; Wacker, J.; Paiva, R.P.; Chetelat, O. A wearable stethoscope for long term ambulatory respiratory health monitoring. Sensors 2020, 20, 5124. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoring and Personal Healthcare. Adv. Mater. 2016, 28, 22. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2017, 7, 1. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, A.; Cheng, C. Investigation of the impact of infrared sensors on core body temperature monitoring by comparing measurement sites. Sensors 2020, 20, 2885. [Google Scholar] [CrossRef]
- Bian, S.; Zhou, B.; Lukowicz, P. Social distance monitor with a wearable magnetic field proximity sensor. Sensors 2020, 20, 5101. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein Antigen. Sensors 2020, 20, 3121. [Google Scholar] [CrossRef]
- Khan, F.; Ghaffar, A.; Khan, N.; Cho, S.H. An overview of signal processing techniques for remote health monitoring using impulse radio UWB transceiver. Sensors 2020, 20, 2479. [Google Scholar] [CrossRef]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 11, 657–659. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolo, A.; Schena, E.; Sacchetti, M. Remote Respiratory Monitoring in the Time of COVID-19. Front. Physiol. 2020, 11, 635. [Google Scholar] [CrossRef]
- Várady, P.; Micsik, T.; Benedek, S.; Benyó, Z. A novel method for the detection of apnea and hypopnea events in respiration signals. IEEE Trans. Biomed. Eng. 2002, 9, 936–942. [Google Scholar] [CrossRef]
- Li, W.; Xiong, S.; Zhou, X. Lead-Wire-Resistance compensation technique using a single zener diode for Two-Wire resistance temperature detectors (RTDs). Sensors 2020, 20, 2742. [Google Scholar] [CrossRef]
- Lugoda, P.; Costa, J.; Oliveira, C.; Garcia-Garcia, L.; Wickramasinghe, S.; Pouryazdan, A.; Roggen, D.; Dias, T.; Münzenrieder, N. Flexible Temperature sensors integration into e-textiles using different industrial yarn fabrication processes. Sensors 2020, 20, 73. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolo, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensos 2019, 19, 908. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolo, A.; Sacchetti, M.; Schena, E. Contactless Methods For Measuring Respiratory Rate: A Review. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Tardi, G.; Massaroni, C.; Saccomandi, P.; Schena, E. Experimental assessment of a variable orifice flowmeter for respiratory monitoring. J. Sens. 2015, 752540. [Google Scholar] [CrossRef]
- Bruun, H.H. Hot-Wire Anemometry: Principles and Signal Analysis; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- King, L.V., XII. On the convection of heat from small cylinders in a stream of fluid: Determination of the convection constants of small platinum wires with applications to hot-wire anemometry. Philos. Trans. R. Soc. Lond. 1914, 214, 373–432. [Google Scholar]
- Yoshiya, I.; Nakajima, T.; Nagai, I.; Jitsukawa, S. A bidirectional respiratory flowmeter using the hot-wire principle. J. Appl. Physiol. 1975, 38, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Herwaarden, A.W.V.; Sarro, P.M. Thermal sensors based on the seebeck effect. Sens. Actuators 1986, 10, 321–346. [Google Scholar] [CrossRef]
- Neji, B.; Xu, J.; Titus, A.H.; Meltzer, J. Micro-fabricated DC comparison calorimeter for RF power measurement. Sensors 2014, 14, 20245–20261. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.W.; Scroger, M.G. The Calibration of Thermocouples and Thermocouple Materials. Natl. Inst. Stand. Technol. 1989, 250, 35. [Google Scholar]
- Thomson, W., II. On the thermoelastic, thermomagnetic, and pyroelectric properties of matter. Philos. Mag. J. Sci. 1989, 5, 4–27. [Google Scholar] [CrossRef]
- Massaroni, C.; Zaltieri, M.; Presti, D.L.; Nicolo, A.; Tosi, D.; Schena, E. Fiber Bragg Grating Sensors for Cardiorespiratory Monitoring: A Review. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Branson, R.D.; Gentile, M.A. Is humidification always necessary during noninvasive ventilation in the hospital? Respir. Care 2010, 55, 209–216. [Google Scholar]
- Rittersma, Z. Recent achievements in miniaturised humidity sensors-A review of transduction techniques. Sens. Actuators A Phys. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Nurulain, S.; Radin, M.; Suzalina, K.; Manap, H. Spectra comparison for an optical breathing gas sensor development. In Proceedings of the International Conference on Advanced Material Engineering and Technology, Kaohsiung City, Taiwan, 8–9 December 2016; Volume 1835. [Google Scholar]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Fiorillo, A.S.; Critello, C.D.; Pullano, S.A. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-Knitted Strain Sensor for Monitoring Respiratory Rate and Its Electro-Mechanical Modeling. IEEE Sens. J. 2015, 15, 110–122. [Google Scholar] [CrossRef]
- Pecht, M. Handbook of Electronics Package Design; CRC Press: Boca Raton, FL, USA, 2018; Chapter 2; p. 44. [Google Scholar]
- Neji, B. Microfabricated thermal flow rate sensor. In Proceedings of the 5th International Conference on Sensors and Electronic Instrumentation Advances, Tenerife, Spain, 25–27 September 2019; pp. 3–68. [Google Scholar]
- Rahim, R.A.; Nordin, A.N.; Malik, N.A.; Bais, B.; Majlis, B.Y. Fabrication of monolithic Wheatstone bridge circuit for piezoresistive microcantilever sensor. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Montpellier, France, 27–30 April 2015; pp. 1–5. [Google Scholar]
- Neji, B. Micro-calorimetric flow rate measurement device for microfluidic applications. Sens. Transducers J. 2019, 237, 95–102. [Google Scholar]
- Nicolo, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Excercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef]










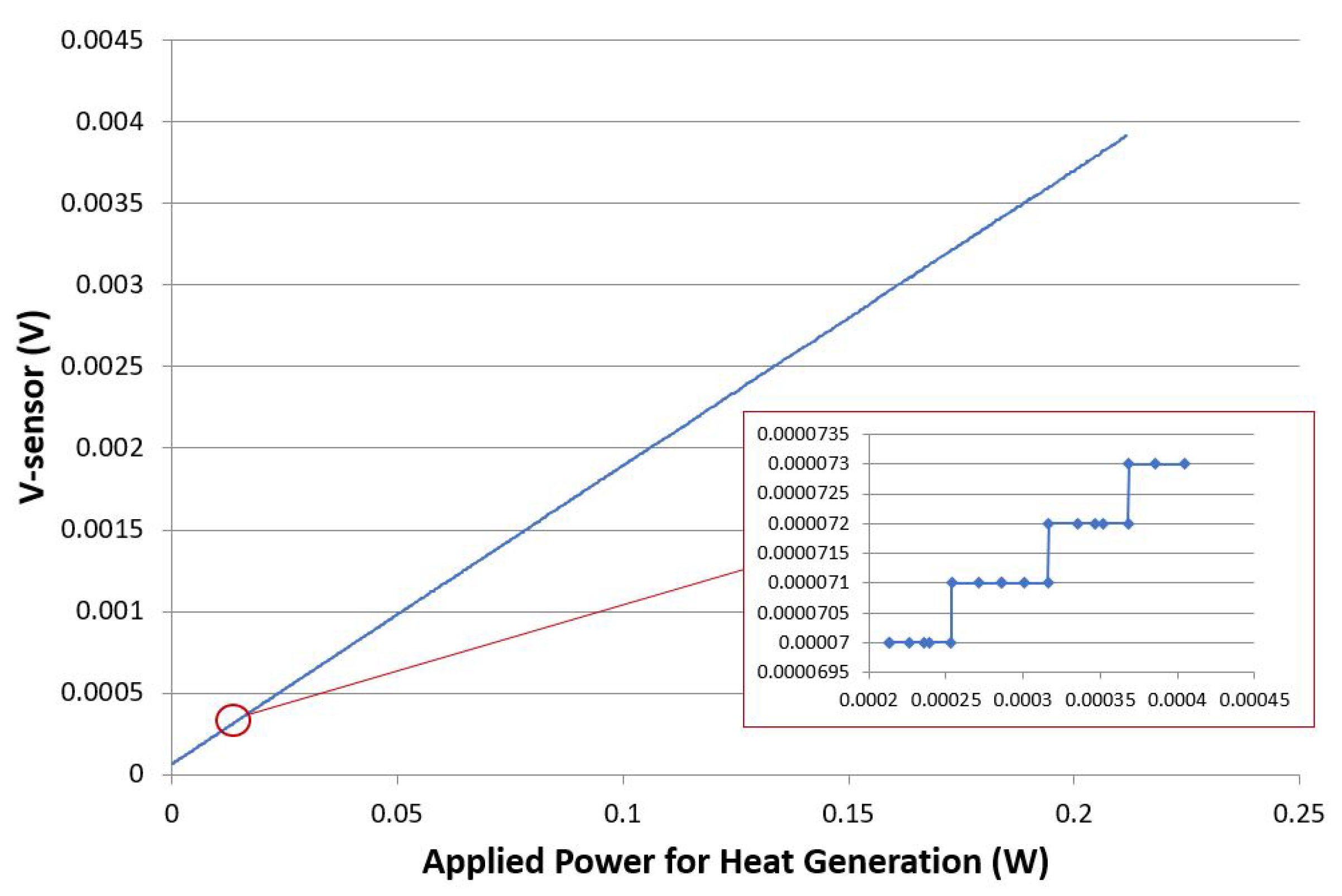

| Average Breathing Frequency (Hz) | Standard Deviation (Hz) | |
|---|---|---|
| Scenario 1: Healthy subject, lying down | ||
| flat on a bed, with no movements | 0.28437 | 0.008615 |
| Scenario 2: Healthy subject, | ||
| walking normally in straight direction | 0.478028 | 0.020604 |
| Scenario 3: Healthy subject, | ||
| walking fast in a straight direction | 0.562761 | 0.026756 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neji, B.; Ferko, N.; Ghandour, R.; Karar, A.S.; Arbess, H. Micro-Fabricated RTD Based Sensor for Breathing Analysis and Monitoring. Sensors 2021, 21, 318. https://doi.org/10.3390/s21010318
Neji B, Ferko N, Ghandour R, Karar AS, Arbess H. Micro-Fabricated RTD Based Sensor for Breathing Analysis and Monitoring. Sensors. 2021; 21(1):318. https://doi.org/10.3390/s21010318
Chicago/Turabian StyleNeji, Bilel, Ndricim Ferko, Raymond Ghandour, Abdullah S. Karar, and Houssam Arbess. 2021. "Micro-Fabricated RTD Based Sensor for Breathing Analysis and Monitoring" Sensors 21, no. 1: 318. https://doi.org/10.3390/s21010318
APA StyleNeji, B., Ferko, N., Ghandour, R., Karar, A. S., & Arbess, H. (2021). Micro-Fabricated RTD Based Sensor for Breathing Analysis and Monitoring. Sensors, 21(1), 318. https://doi.org/10.3390/s21010318









