Comments on: “Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients”
Funding
Conflicts of Interest
References
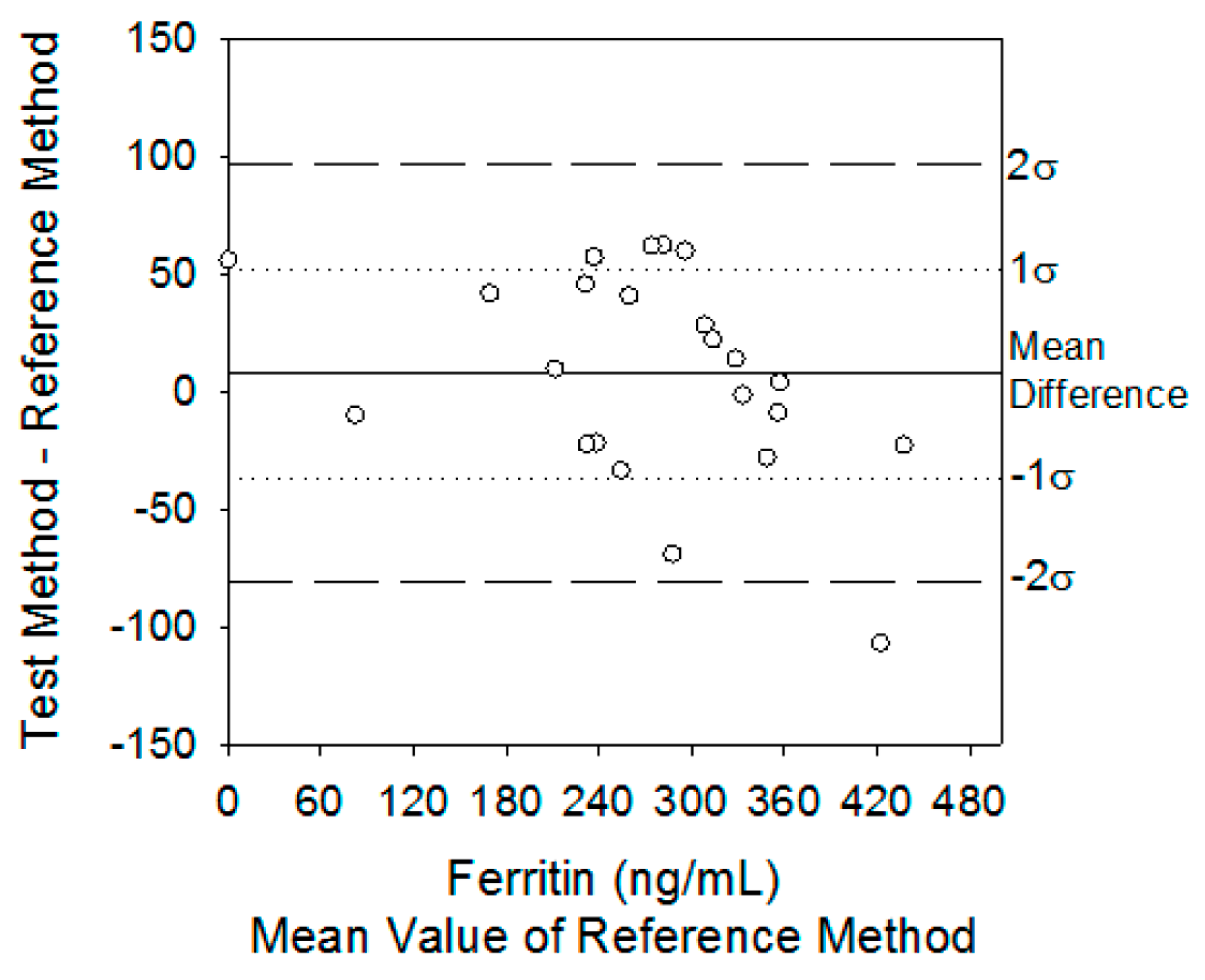
- Peterson, R.; Wilund, K.; Cunningham, B.; Andrade, J. Comparison of methods study between a photonic crystal biosensor and certified ELISA to measure biomarkers of iron deficiency in chronic kidney disease patients. Sensors 2017, 17, 2203. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.H.; Stöckl, D.; Blaabjerg, O.; Pedersen, B.; Birkemose, E.; Thienpont, L.; Lassen, J.F.; Kjeldsen, J. Graphical interpretation of analytical data from comparison of a field method with a reference method by use of difference plots. Clin. Chem. 1997, 43, 2039–2046. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, N.; Naqvi, R.A. Comments on: “Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients”. Sensors 2020, 20, 1126. https://doi.org/10.3390/s20041126
Abbas N, Naqvi RA. Comments on: “Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients”. Sensors. 2020; 20(4):1126. https://doi.org/10.3390/s20041126
Chicago/Turabian StyleAbbas, Naseem, and Rizwan Ali Naqvi. 2020. "Comments on: “Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients”" Sensors 20, no. 4: 1126. https://doi.org/10.3390/s20041126
APA StyleAbbas, N., & Naqvi, R. A. (2020). Comments on: “Comparison of Methods Study between a Photonic Crystal Biosensor and Certified ELISA to Measure Biomarkers of Iron Deficiency in Chronic Kidney Disease Patients”. Sensors, 20(4), 1126. https://doi.org/10.3390/s20041126





