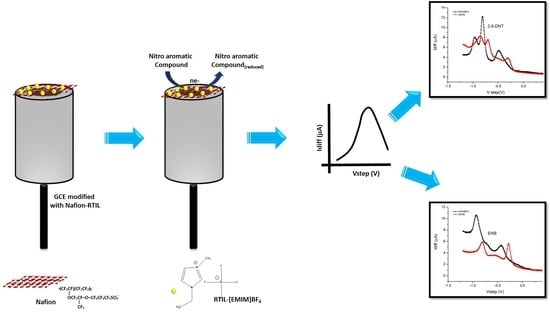
Characterization of Room-Temperature Ionic Liquids to Study the Electrochemical Activity of Nitro Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Methods
2.2. Electrode Preparation
3. Results and Discussion
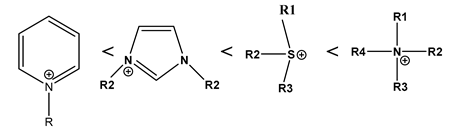
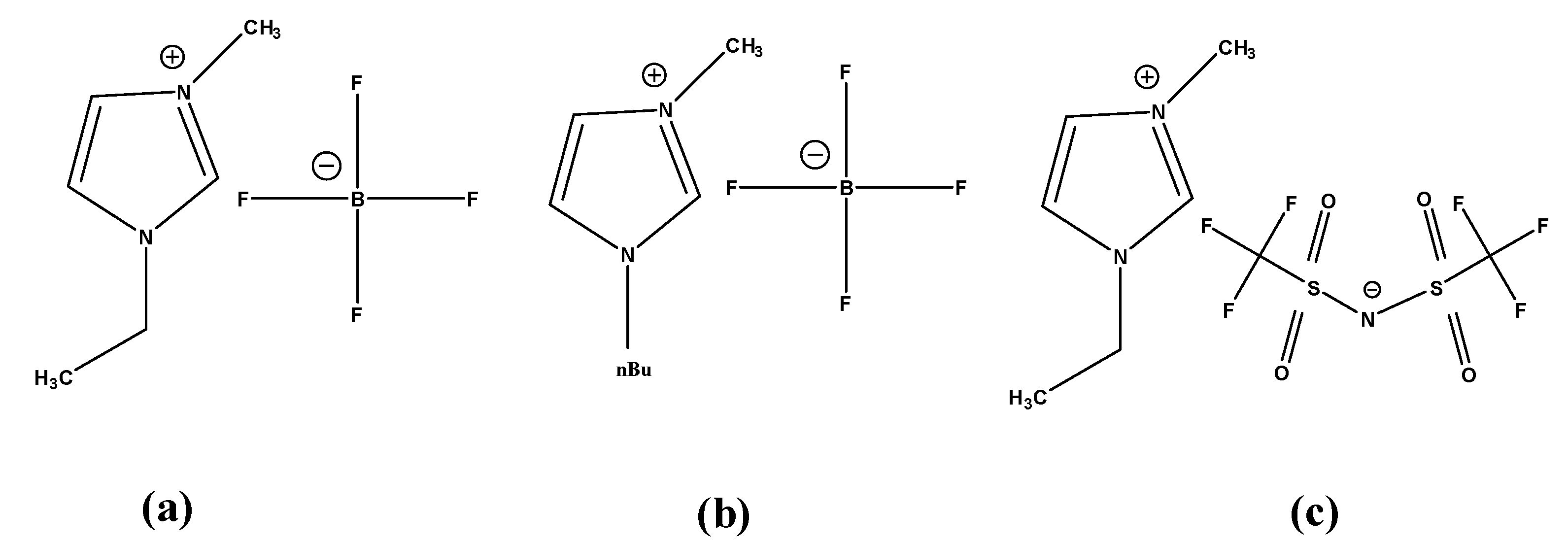
3.1. Selection of Room-Temperature Ionic Liquid (RTIL)
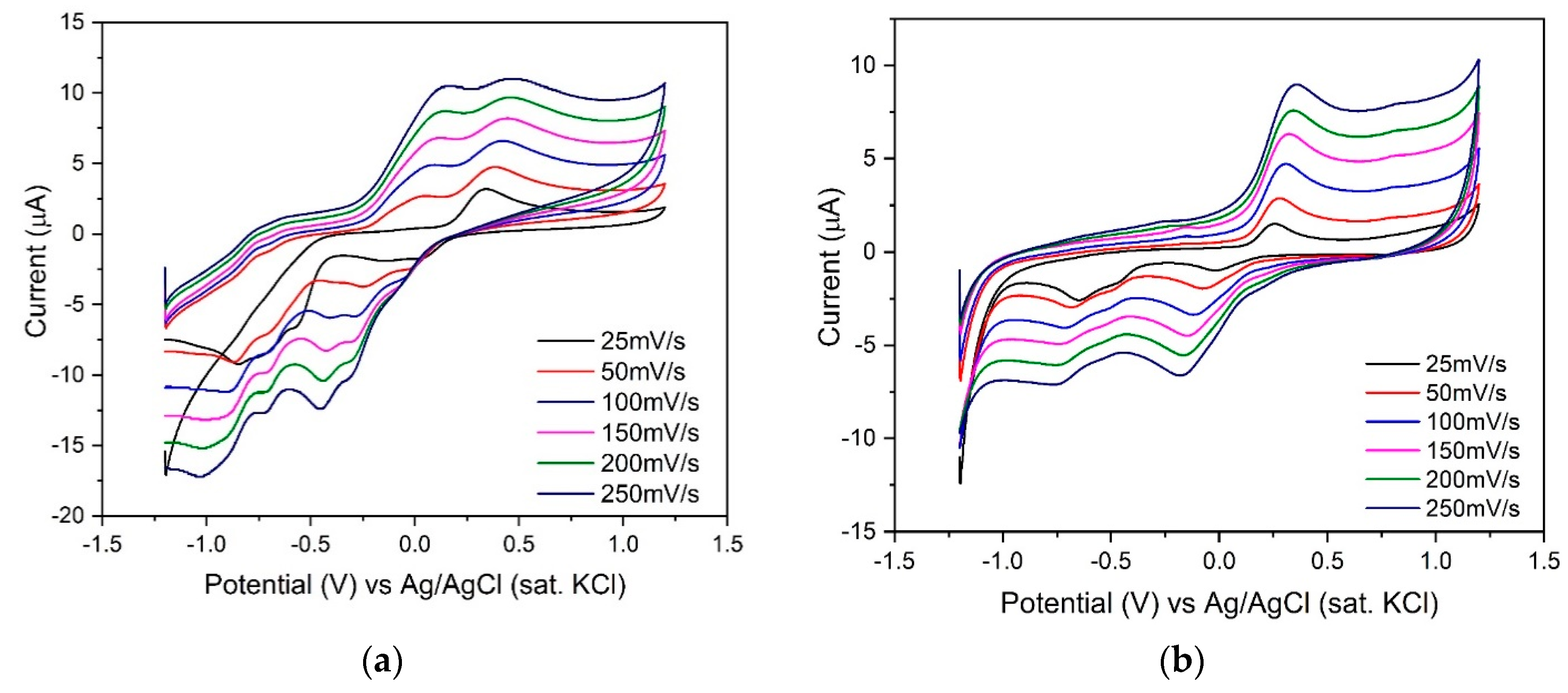
3.2. Cyclic Voltammetry (CV) Characterization of Different RTILs
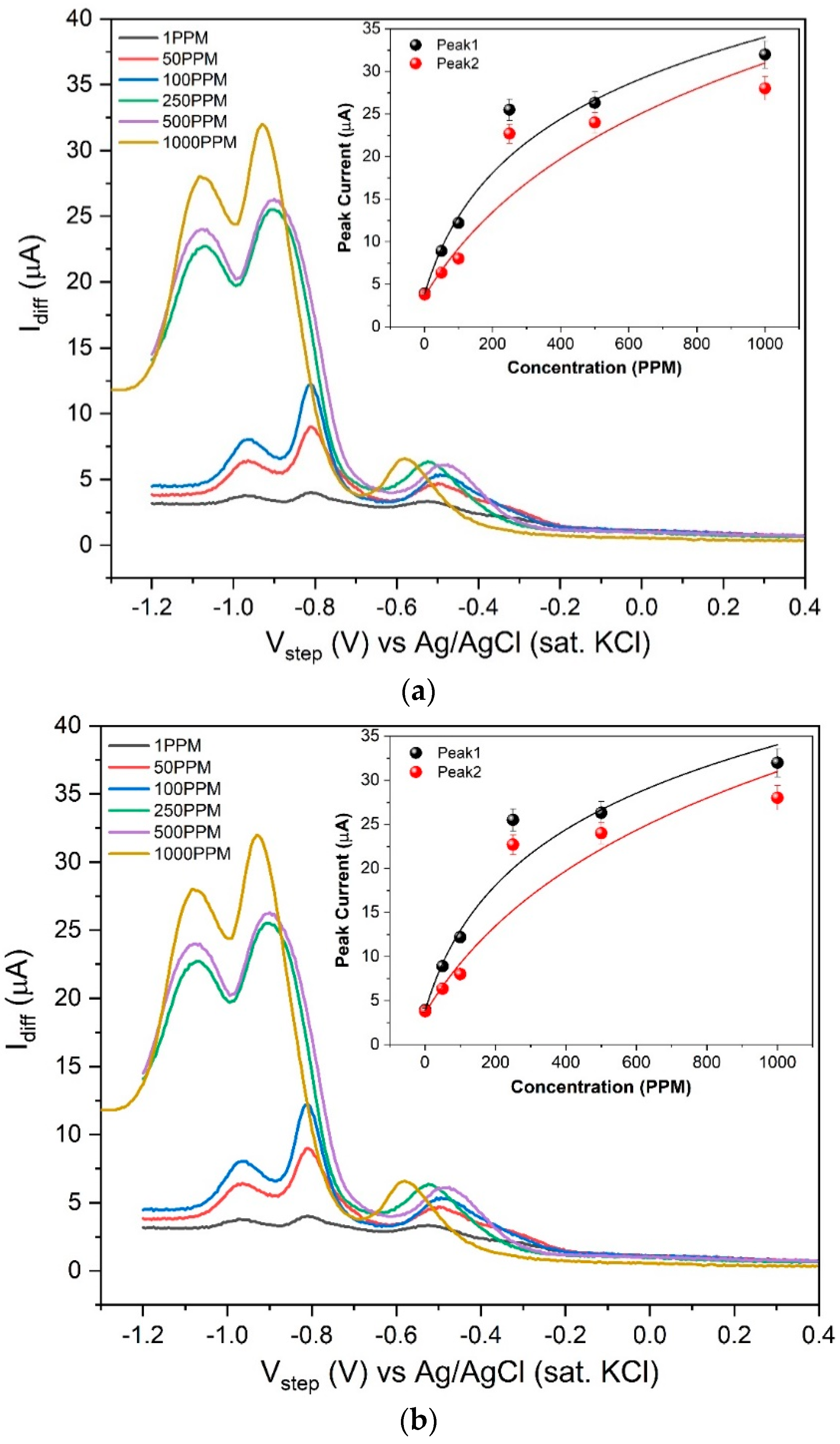
3.3. Square Wave Voltammetry (SQWV) Comparison and Calibration
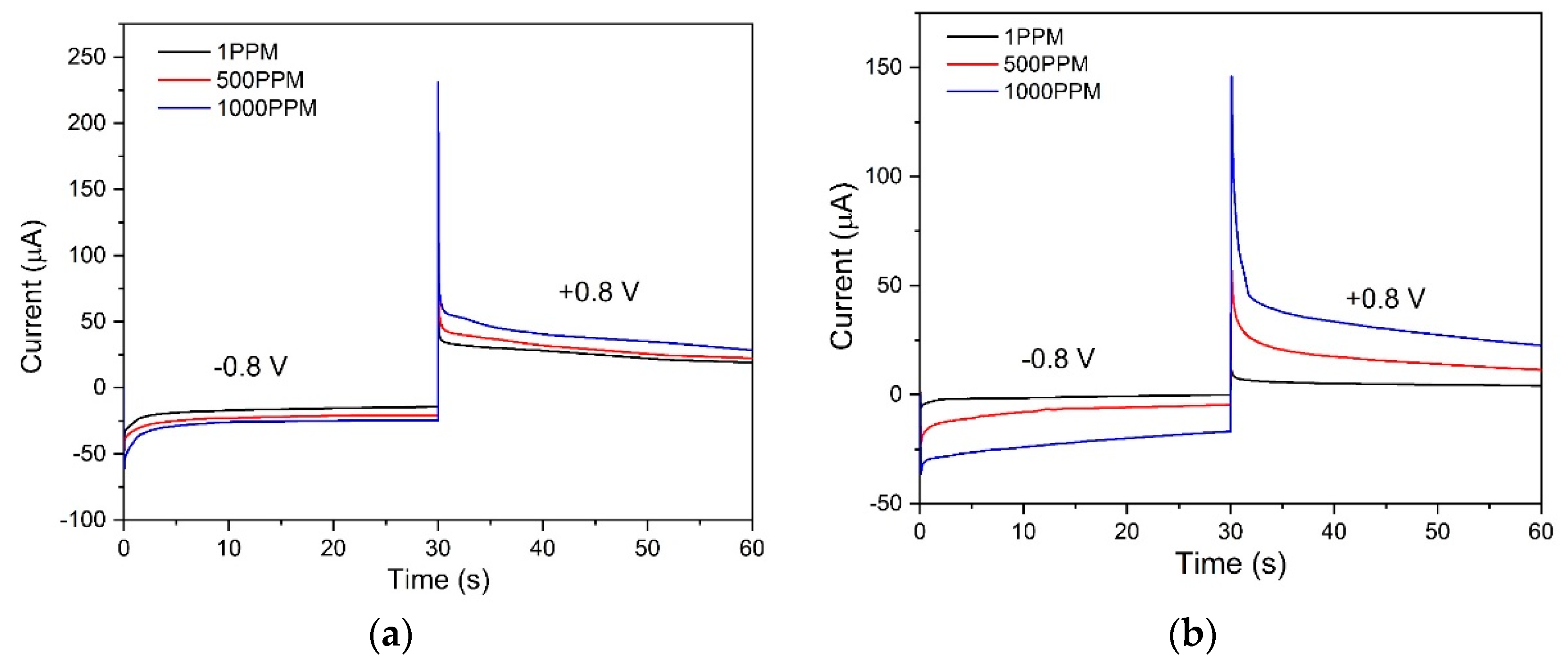
3.4. Open Circuit Potential
3.5. Portable Amperometric Interdigitated Electrode (IDE) Sensor for Detection of Nitroaromatic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Marsh, K.; Boxall, J.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Marsh, K.N.; Deev, A.; Wu, A.C.T.; Tran, E.; Klamt, A. Room temperature ionic liquids as replacements for conventional solvents—A review. Korean J. Chem. Eng. 2002, 19, 357–362. [Google Scholar] [CrossRef]
- Paul, A.; Muthukumar, S.; Prasad, S. Review—Room-Temperature Ionic Liquids for Electrochemical Application with Special Focus on Gas Sensors. J. Electrochem. Soc. 2020, 167, 037511. [Google Scholar] [CrossRef]
- Silvester, D.S.; Compton, R.G. Electrochemistry in Room Temperature Ionic Liquids: A Review and Some Possible Applications. Z. Phys. Chem. 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Eskandani, M.; Guardia, M. de la Room-temperature ionic liquid-based electrochemical nanobiosensors. TrAC Trends Anal. Chem. 2012, 41, 58–74. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Timur, S. Ionic Liquids from Biocompatibility and Electrochemical Aspects toward Applying in Biosensing Devices. Anal. Chem. 2018, 90, 640–648. [Google Scholar] [CrossRef]
- Araque, J.C.; Hettige, J.J.; Margulis, C.J. Modern Room Temperature Ionic Liquids, a Simple Guide to Understanding Their Structure and How It May Relate to Dynamics. J. Phys. Chem. B 2015, 119, 12727–12740. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Liang, C.; Yuan, C.-Y.; Warmack, R.J.; Barnes, C.E.; Dai, S. Ionic Liquids: A New Class of Sensing Materials for Detection of Organic Vapors Based on the Use of a Quartz Crystal Microbalance. Anal. Chem. 2002, 74, 2172–2176. [Google Scholar] [CrossRef]
- Schreiner, C.; Zugmann, S.; Hartl, R.; Gores, H.J. Fractional Walden Rule for Ionic Liquids: Examples from Recent Measurements and a Critique of the So-Called Ideal KCl Line for the Walden Plot. J. Chem. Eng. Data 2010, 55, 1784–1788. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, S. Electrogenerated Chemiluminescence from Ru(Bpy) 3 2+ Ion-Exchanged in Carbon Nanotube/Perfluorosulfonated Ionomer Composite Films. Anal. Chem. 2004, 76, 2683–2688. [Google Scholar] [CrossRef]
- Fan, Z.; Harrison, D.J. Permeability of Glucose and Other Neutral Species through Recast Perfluorosulfonated Ionomer Films. Anal. Chem. 1992, 64, 1304–1311. [Google Scholar] [CrossRef]
- Singh, V.; Nigam, A.; Batra, A.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Application of ionic liquid in electrochemical sensors and biosensors. Int. J. Electrochem. 2012, 2012, 165683. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Liu, Y.; Wang, Y.; Qi, L.; Dong, S. Direct electrochemistry and electrocatalysis of horseradish peroxidase immobilized in Nafion-RTIL composite film. Electrochem. Commun. 2007, 9, 469–472. [Google Scholar] [CrossRef]
- Mondal, A.; Paul, A.; Srivastava, D.N.; Panda, A.B. Defect- and Phase-Induced Acceleration of Electrocatalytic Hydrogen Production by Ultrathin and Small MoS 2-Decorated rGO Sheets. ACS Appl. Nano Mater. 2018, 1, 4622–4632. [Google Scholar] [CrossRef]
- Saha, A.; Paul, A.; Srivastava, D.N.; Panda, A.B. Porous carbon incorporated Β-Mo 2 C hollow sphere: An efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 21655–21664. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Zou, Y.; Liu, X. MoS2 nanosheets direct supported on reduced graphene oxide: An advanced electrocatalyst for hydrogen evolution reaction. PLoS ONE 2017, 12, e0177258. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Tian, Y.; You, J.; Yang, C.; Su, J.; Li, Y.; Gao, Y.; Gu, H. In situ synthesis of MoS2/graphene nanosheets as free-standing and flexible electrode paper for high-efficiency hydrogen evolution reaction. RSC Adv. 2018, 8, 10698–10705. [Google Scholar] [CrossRef]
- Solomon, G.; Mazzaro, R.; You, S.; Natile, M.M.; Morandi, V.; Concina, I.; Vomiero, A. Ag2S/MoS2 Nanocomposites Anchored on Reduced Graphene Oxide: Fast Interfacial Charge Transfer for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 22380–22389. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Liu, X. A Clean and Facile Synthesis Strategy of MoS 2 Nanosheets Grown on Multi-Wall CNTs for Enhanced Hydrogen Evolution Reaction Performance. Sci. Rep. 2017, 7, 8825. [Google Scholar] [CrossRef]
- Lee, J.E.; Jung, J.; Ko, T.Y.; Kim, S.; Kim, S., II; Nah, J.; Ryu, S.; Nam, K.T.; Lee, M.H. Catalytic synergy effect of MoS2/reduced graphene oxide hybrids for a highly efficient hydrogen evolution reaction. RSC Adv. 2017, 7, 5480–5487. [Google Scholar] [CrossRef]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef]
- Topçu, E.; Kıranşan, K.D. Flexible and Free-standing PtNLs-MoS2/Reduced Graphene Oxide Composite Paper: A High-Performance Rolled Paper Catalyst for Hydrogen Evolution Reaction. Chem. Select 2018, 3, 5941–5949. [Google Scholar] [CrossRef]
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef]
- Bhalla, V.; Arora, H.; Singh, H.; Kumar, M. Triphenylene derivatives: Chemosensors for sensitive detection of nitroaromatic explosives. J. Chem. Soc. Dalt. Trans. 2013, 42, 969–974. [Google Scholar] [CrossRef]
- Mohan, M.; Chand, D.K. Visual colorimetric detection of TNT and 2,4-DNT using as-prepared hexaazamacrocycle-capped gold nanoparticles. Anal. Methods 2014, 6, 276–281. [Google Scholar] [CrossRef]
- Jenkins, T.F.; Walsh, M.E. Development of field screening methods for TNT, 2,4-DNT and RDX in soil. Talanta 1992, 39, 419–428. [Google Scholar] [CrossRef]
- Mirasoli, M.; Buragina, A.; Dolci, L.S.; Guardigli, M.; Simoni, P.; Montoya, A.; Maiolini, E.; Girotti, S.; Roda, A. Development of a chemiluminescence-based quantitative lateral flow immunoassay for on-field detection of 2,4,6-trinitrotoluene. Anal. Chim. Acta 2012, 721, 167–172. [Google Scholar] [CrossRef]
- Chen, J.C.; Shih, J.L.; Liu, C.H.; Kuo, M.Y.; Zen, J.M. Disposable electrochemical sensor for determination of nitroaromatic compounds by a single-run approach. Anal. Chem. 2006, 78, 3752–3757. [Google Scholar] [CrossRef]
- Zhang, H.X.; Cao, A.M.; Hu, J.S.; Wan, L.J.; Lee, S.T. Electrochemical sensor for detecting ultratrace nitroaromatic compounds using mesoporous SiO2-modified electrode. Anal. Chem. 2006, 78, 1967–1971. [Google Scholar] [CrossRef]
- McGill, R.A.; Mlsna, T.E.; Chung, R.; Nguyen, V.K.; Stepnowski, J. Design of functionalized silicone polymers for chemical sensor detection of nitroaromatic compounds. Sens. Actuators B Chem. 2000, 65, 5–9. [Google Scholar] [CrossRef]
- Germain, M.E.; Knapp, M.J. Optical explosives detection: From color changes to fluorescence turn-on. Chem. Soc. Rev. 2009, 38, 2543–2555. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, C.; Qin, D.; Xue, Z.; Wang, Y.; Du, J.; Ma, L.; Ma, L.; Lu, X. Pd Nanoparticles on Functionalized Graphene for Excellent Detection of Nitro aromatic Compounds. Electrochim. Acta 2014, 119, 243–250. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Rapid nitroaromatic compounds sensing based on oligopyrene. Sens. Actuators B Chem. 2008, 130, 777–782. [Google Scholar] [CrossRef]
- Shi, G.H.; Shang, Z.B.; Wang, Y.; Jin, W.J.; Zhang, T.C. Fluorescence quenching of CdSe quantum dots by nitroaromatic explosives and their relative compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 247–252. [Google Scholar] [CrossRef]
- Wu, Z.F.; Tan, B.; Feng, M.L.; Lan, A.J.; Huang, X.Y. A magnesium MOF as a sensitive fluorescence sensor for CS2 and nitroaromatic compounds. J. Mater. Chem. A 2014, 2, 6426–6431. [Google Scholar] [CrossRef]
- Berliner, A.; Lee, M.G.; Zhang, Y.; Park, S.H.; Martino, R.; Rhodes, P.A.; Yi, G.R.; Lim, S.H. A patterned colorimetric sensor array for rapid detection of TNT at ppt level. RSC Adv. 2014, 4, 10672–10675. [Google Scholar] [CrossRef]
- Algarra, M.; Campos, B.B.; Miranda, M.S.; Da Silva, J.C.G.E. CdSe quantum dots capped PAMAM dendrimer nanocomposites for sensing nitroaromatic compounds. Talanta 2011, 83, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Nosova, E.V.; Rusinov, V.L.; Chupakhin, O.N. Chemosensors for detection of nitroaromatic compounds (explosives). Russ. Chem. Rev. 2014, 83, 783–819. [Google Scholar] [CrossRef]
- Yinun, J. Detection of explosive by electronic noses. Anal. Chem. 2003, 75, 99–105. [Google Scholar]
- Hanson, R.L.; Henderson, T.R.; Hobbs, C.H.; Clark, C.R.; Carpenter, R.L.; Dutcher, J.S. Detection of n1troaromatic compounds on coal combustion particles. J. Toxicol. Environ. Health 1983, 11, 971–980. [Google Scholar] [CrossRef]
- Kruusma, J.; Toniso, A.; Parna, R.; Nommiste, E.; Vahtruss, M.; Siinor, L.; Tallo, I.; Romann, T.; Lust, E. Influence of Iodide Ions Concentration on the Stability of 1-Ethyl-3-methylimidazolium Tetrafluoroborate | MolybdenumCarbide Derived Carbon Electrode Interface. J. Electrochem. Soc. 2017, 164, A1110–A1119. [Google Scholar] [CrossRef]
- Liu, C.; Bo, X.; Guo, L. A novel electrochemical sensing platform of JUC-62 metal-organic framework/platelet ordered mesoporous carbon for high selective detection of nitro-aromatic compounds. Sens. Actuators B Chem. 2019, 297, 126741–126753. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, C.; Zheng, F.; Li, X.; Sun, C.L.; Chen, W. Nitrogen and sulfur co-doped graphene nanoribbons: A novel metal-free catalyst for high performance electrochemical reduction of 2,4,6-trinitrotoluene (TNT). Carbon 2018, 126, 328–337. [Google Scholar] [CrossRef]
- Upasham, S.; Tanak, A.; Jagannath, B.; Prasad, S. Development of ultra-low volume, multi-bio fluid, cortisol sensing platform. Sci. Rep. 2018, 8, 16745. [Google Scholar] [CrossRef]
- Graef, E.W.; Munje, R.D.; Prasad, S. A Robust Electrochemical CO2 Sensor Utilizing Room Temperature Ionic Liquids. IEEE Trans. Nanotechnol. 2017, 16, 826–831. [Google Scholar] [CrossRef]

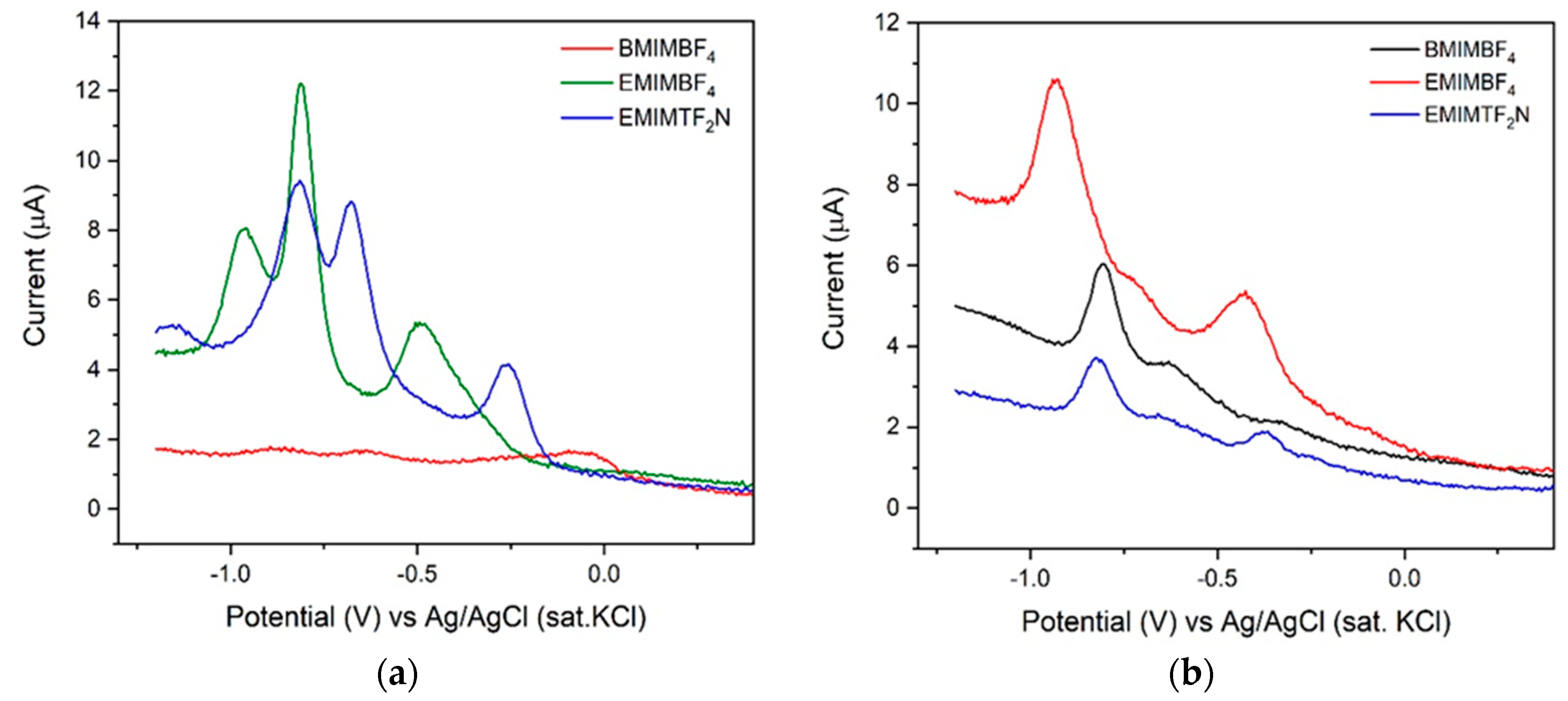
| RTIL | Conductivity | Electrochemical Stability |
|---|---|---|
| [EMIM][BF4] | 12 mS/cm | 4.3 V |
| [BMIM][BF4] | 10 mS/cm | 4.0 V |
| [EMIM][TF2N] | 11 mS/cm | 3.8 V |
| Compound | (V) | (V) | (µA) | (µA) |
|---|---|---|---|---|
| [BMIM][BF4] | −0.036 | −0.504, −0.816, −0.995 | 3.39 | −5.95, −16.39, −18.41 |
| [EMIM][BF4] | −0.137 | −0.221, −0.697, −0.837 | 5.14 | −2.91, −14.28, −19.04 |
| [EMIM][TF2N] | −0.205 | −0.291, −0.717, −0.870 | 4.56 | −1.61, −12.82, −17.60 |
| Compound | (V) | (V) | (µA) | (µA) |
|---|---|---|---|---|
| [BMIM][BF4] | −0.120 | –0.634, −0.804 | 2.26 | −6.41, −7.63 |
| [EMIM][BF4] | −0.234 | –0.482, −0.929 | 2.26 | −7.65, −14.06 |
| [EMIM][TF2N] | −0.164 | –0.362, −0.819 | 1.48 | –1.97, −3.716 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banga, I.; Paul, A.; Muthukumar, S.; Prasad, S. Characterization of Room-Temperature Ionic Liquids to Study the Electrochemical Activity of Nitro Compounds. Sensors 2020, 20, 1124. https://doi.org/10.3390/s20041124
Banga I, Paul A, Muthukumar S, Prasad S. Characterization of Room-Temperature Ionic Liquids to Study the Electrochemical Activity of Nitro Compounds. Sensors. 2020; 20(4):1124. https://doi.org/10.3390/s20041124
Chicago/Turabian StyleBanga, Ivneet, Anirban Paul, Sriram Muthukumar, and Shalini Prasad. 2020. "Characterization of Room-Temperature Ionic Liquids to Study the Electrochemical Activity of Nitro Compounds" Sensors 20, no. 4: 1124. https://doi.org/10.3390/s20041124
APA StyleBanga, I., Paul, A., Muthukumar, S., & Prasad, S. (2020). Characterization of Room-Temperature Ionic Liquids to Study the Electrochemical Activity of Nitro Compounds. Sensors, 20(4), 1124. https://doi.org/10.3390/s20041124