Quantitative 2D Magnetorelaxometry Imaging of Magnetic Nanoparticles Using Optically Pumped Magnetometers
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Experimental OPM Setups for MRX Quantification and 2D Imaging
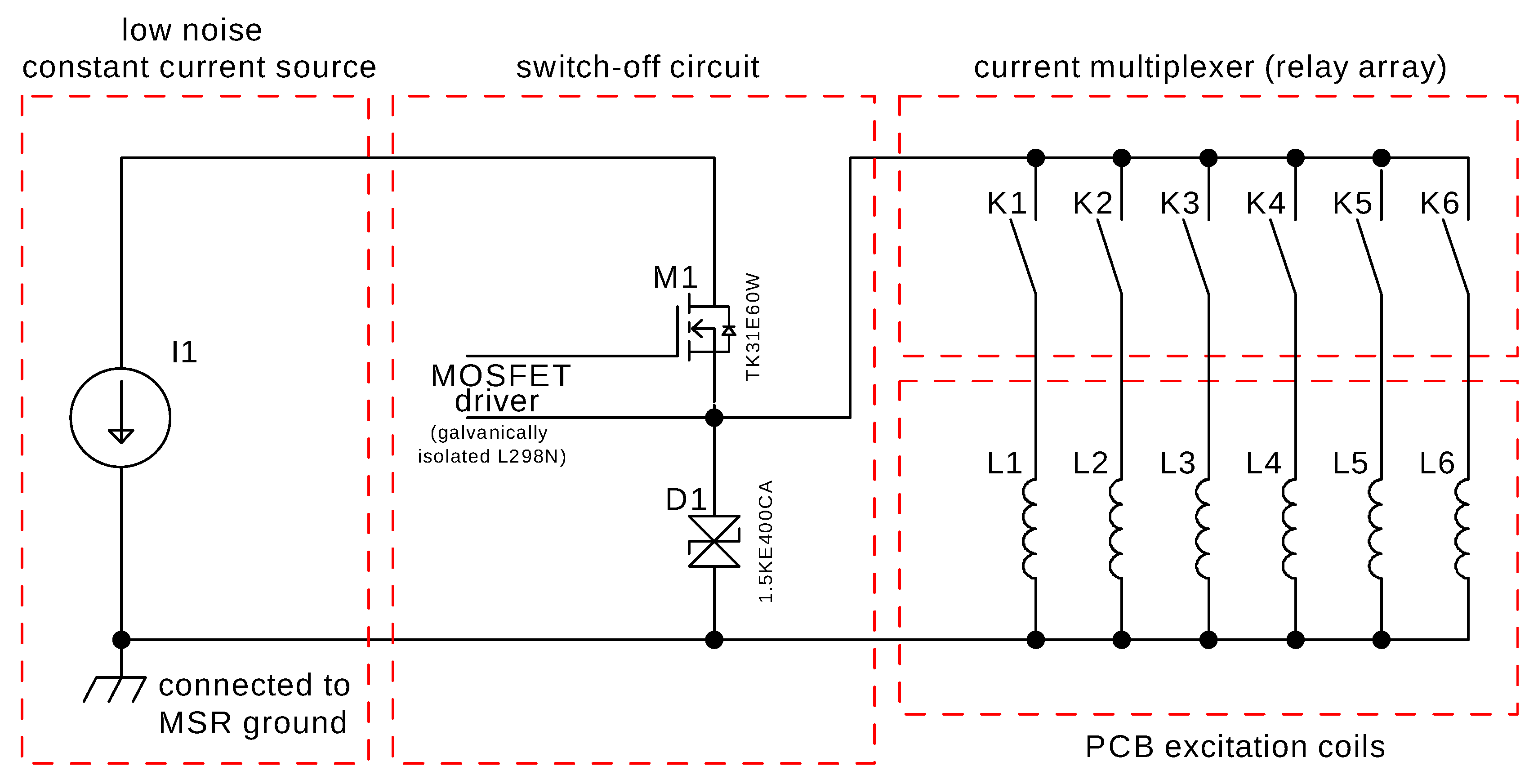
2.2. MRX Excitation Coil Circuit
2.3. OPM
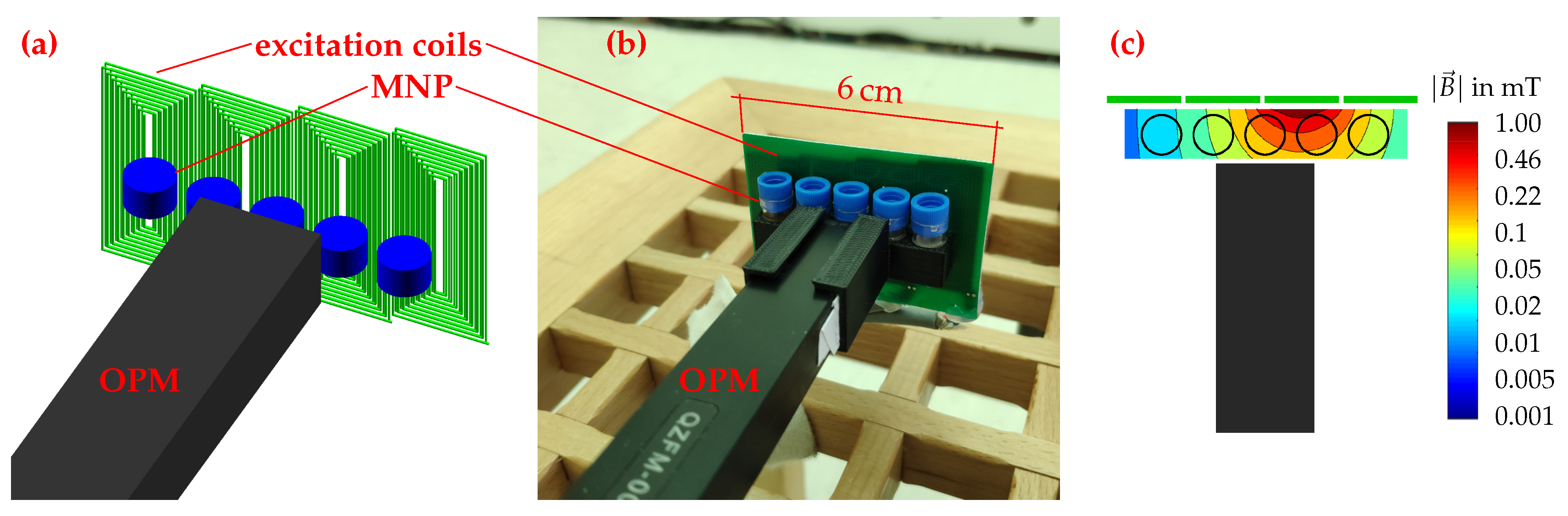
2.4. Setup and Procedure for MNP Quantification and 1D Reconstruction
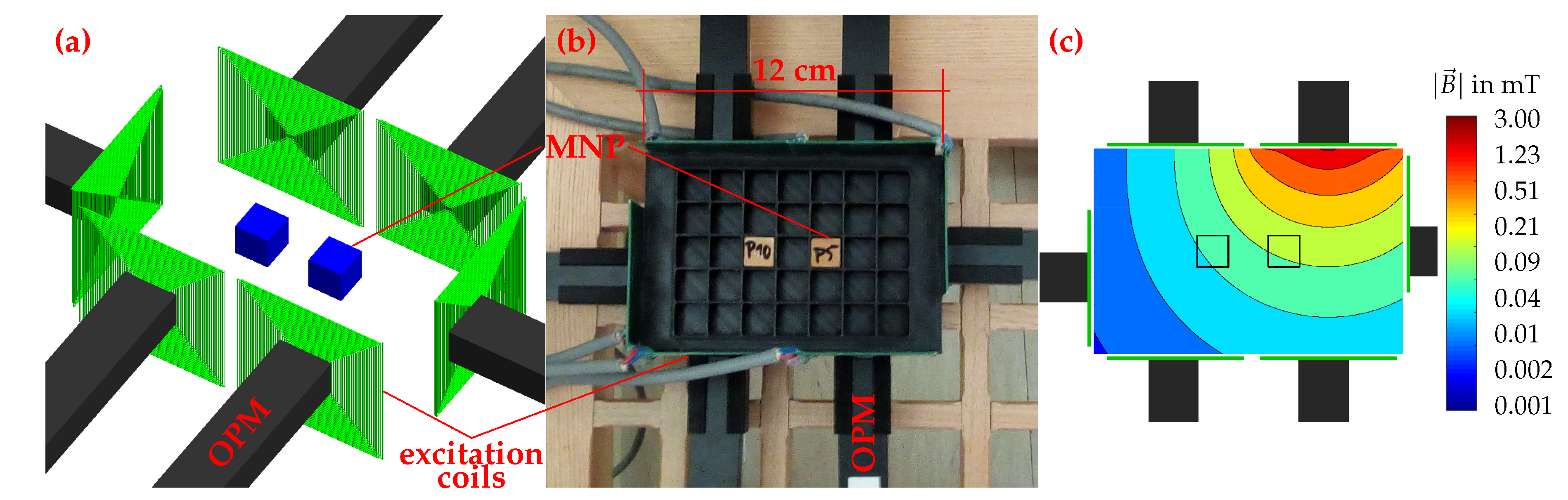
2.5. Setup and Procedure for 2D Imaging
2.6. MRX Model
2.7. Data Acquisition and Preprocessing
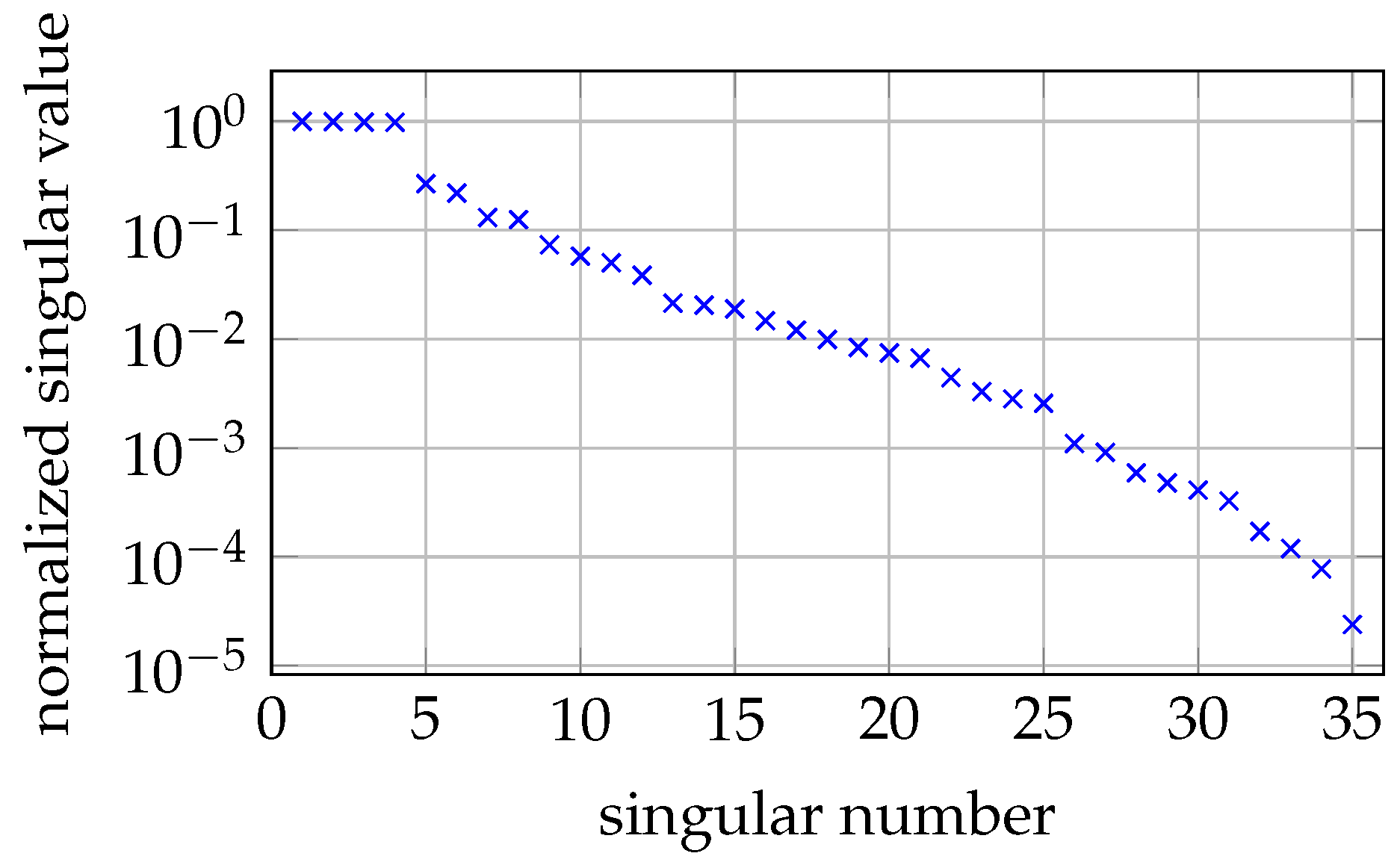
2.8. System Model and Reconstruction
3. Results
3.1. OPM
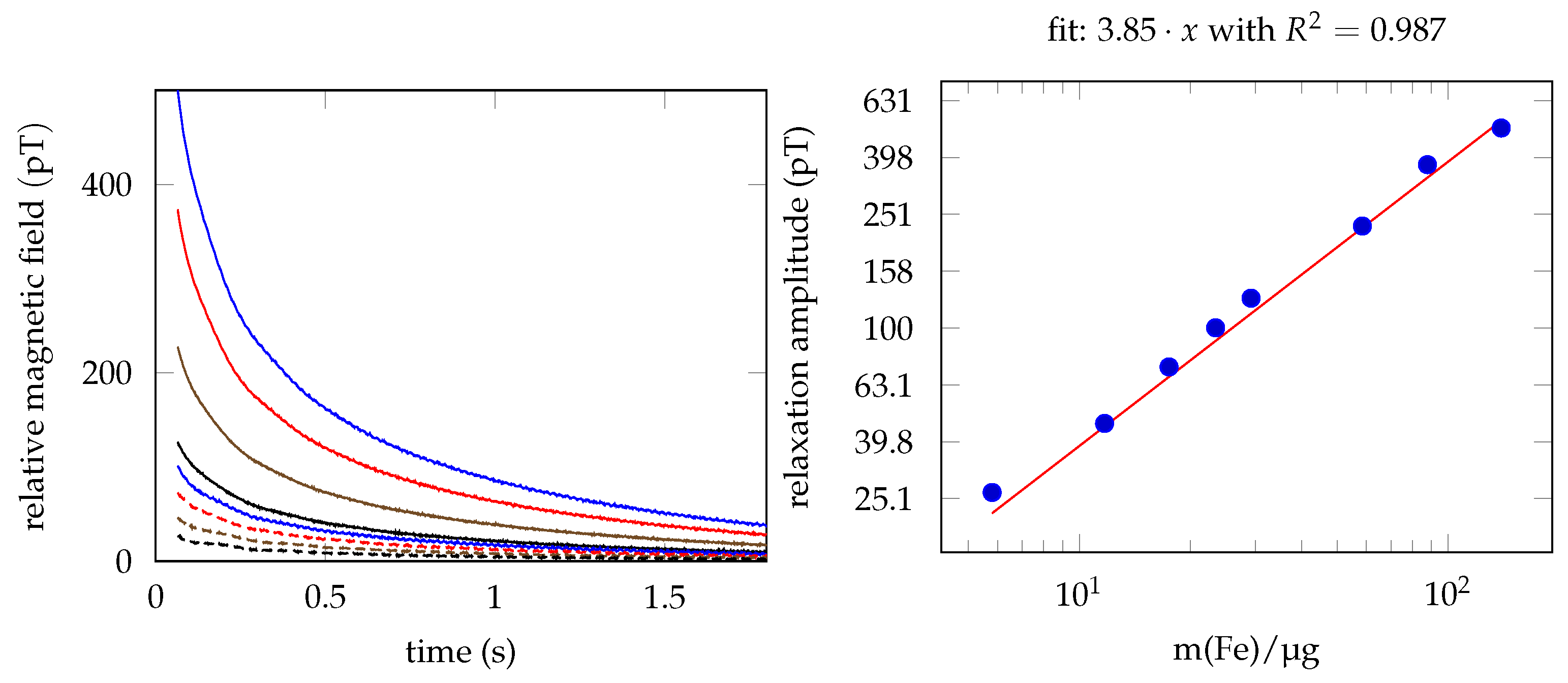
3.2. MNP Quantification
3.3. 1D Reconstruction
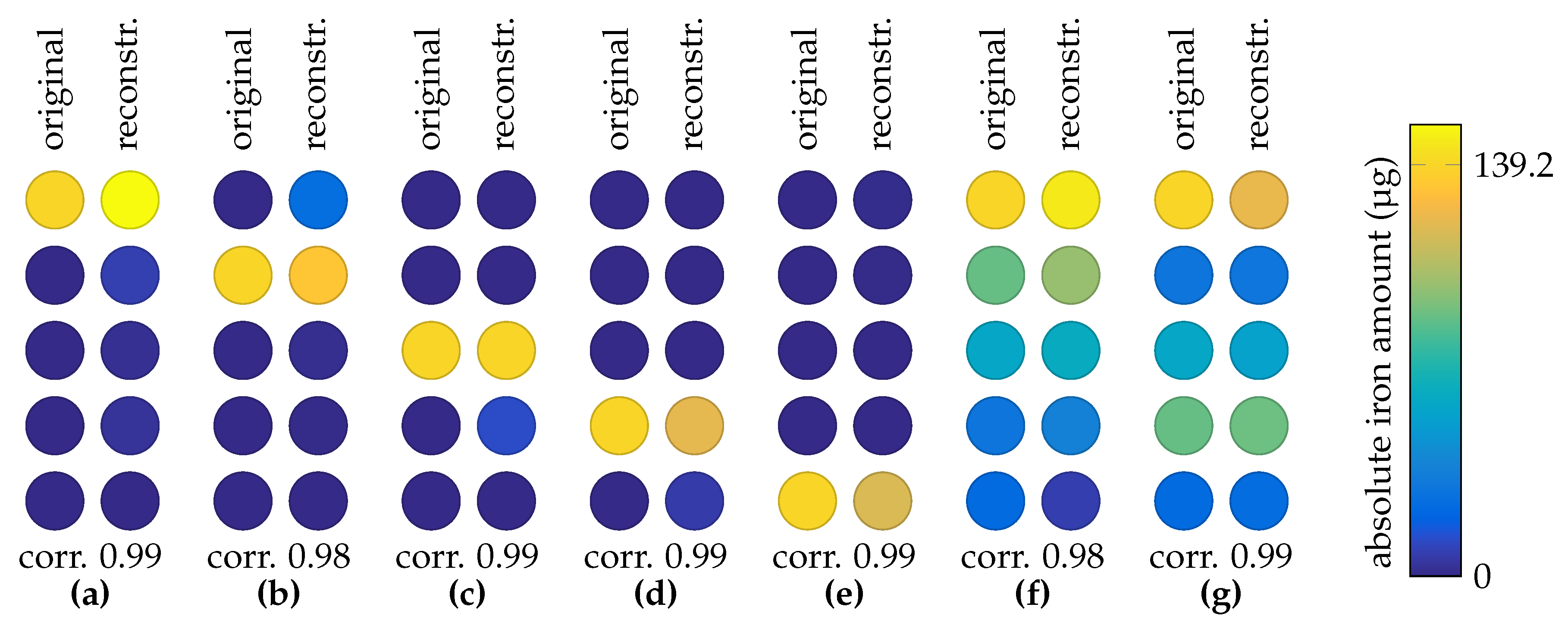
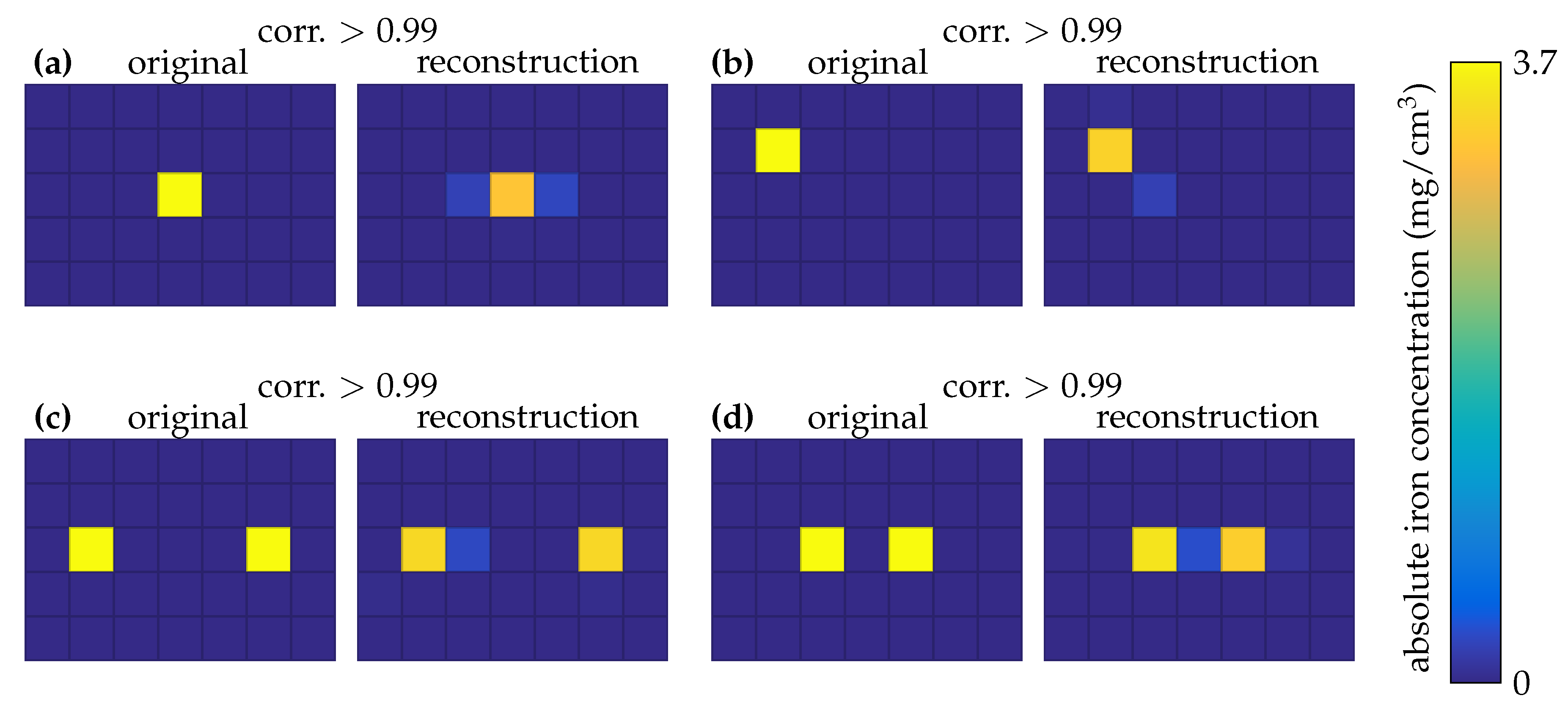
3.4. 2D MRX Imaging
4. Discussion
5. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MOSFET | Metal Oxide Semiconductor Field-Effect Transistor |
| MNP | Magnetic nanoparticle |
| MRX | Magnetorelaxometry |
| MRXI | Magnetorelaxometry imaging |
| OPM | Optically pumped magnetometer |
| PCB | Printed circuit board |
| SERF | Spin-exchange-relaxation-free |
| SQUID | Superconducting quantum interference device |
References
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Chantrell, R.; Hoon, S.; Tanner, B. Time-dependent magnetization in fine-particle ferromagnetic systems. J. Magn. Magn. Mater. 1983, 38, 133–141. [Google Scholar] [CrossRef]
- Ludwig, F.; Heim, E.; Mäuselein, S.; Eberbeck, D.; Schilling, M. Magnetorelaxometry of magnetic nanoparticles with fluxgate magnetometers for the analysis of biological targets. J. Magn. Magn. Mater. 2005, 293, 690–695. [Google Scholar] [CrossRef]
- Knappe, S.; Sander, T.H.; Kosch, O.; Wiekhorst, F.; Kitching, J.; Trahms, L. Cross-validation of microfabricated atomic magnetometers with superconducting quantum interference devices for biomagnetic applications. Appl. Phys. Lett. 2010, 97, 133703. [Google Scholar] [CrossRef]
- Dolgovskiy, V.; Fescenko, I.; Sekiguchi, N.; Colombo, S.; Lebedev, V.; Zhang, J.; Weis, A. A magnetic source imaging camera. Appl. Phys. Lett. 2016, 109, 023505. [Google Scholar] [CrossRef]
- Wiekhorst, F.; Seliger, C.; Jurgons, R.; Steinhoff, U.; Eberbeck, D.; Trahms, L.; Alexiou, C. Quantification of magnetic nanoparticles by magnetorelaxometry and comparison to histology after magnetic drug targeting. J. Nanosci. Nanotechnol. 2006, 6, 3222–3225. [Google Scholar] [CrossRef]
- Heim, E.; Gerloff, M.; Ludwig, F.; Schilling, M. Quantitative and qualitative characterization of magnetic nanoparticles by magnetorelaxometry using a laboratory MRX analyzer. In World Congress on Medical Physics and Biomedical Engineering; Springer: Munich, Germany, 2009; pp. 650–652. [Google Scholar]
- Liebl, M.; Wiekhorst, F.; Eberbeck, D.; Radon, P.; Gutkelch, D.; Baumgarten, D.; Steinhoff, U.; Trahms, L. Magnetorelaxometry procedures for quantitative imaging and characterization of magnetic nanoparticles in biomedical applications. Biomed. Tech. 2015, 60, 427–443. [Google Scholar] [CrossRef]
- Coene, A.; Leliaert, J.; Liebl, M.; Loewa, N.; Steinhoff, U.; Crevecoeur, G.; Dupré, L.; Wiekhorst, F. Multi-color magnetic nanoparticle imaging using magnetorelaxometry. Phys. Med. Biol. 2017, 62, 3139. [Google Scholar] [CrossRef]
- Leliaert, J.; Schmidt, D.; Posth, O.; Liebl, M.; Eberbeck, D.; Coene, A.; Steinhoff, U.; Wiekhorst, F.; Van Waeyenberge, B.; Dupré, L. Interpreting the magnetorelaxometry signal of suspended magnetic nanoparticles with Kaczmarz’algorithm. J. Phys. D Appl. Phys. 2017, 50, 195002. [Google Scholar] [CrossRef]
- Liebl, M.; Steinhoff, U.; Wiekhorst, F.; Haueisen, J.; Trahms, L. Quantitative imaging of magnetic nanoparticles by magnetorelaxometry with multiple excitation coils. Phys. Med. Biol. 2014, 59, 6607. [Google Scholar] [CrossRef]
- Baumgarten, D.; Braune, F.; Supriyanto, E.; Haueisen, J. Plane-wise sensitivity based inhomogeneous excitation fields for magnetorelaxometry imaging of magnetic nanoparticles. J. Magn. Magn. Mater. 2015, 380, 255–260. [Google Scholar] [CrossRef]
- Kominis, I.; Kornack, T.; Allred, J.; Romalis, M. A subfemtotesla multichannel atomic magnetometer. Nature 2003, 422, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Wiekhorst, F.; Steinhoff, U.; Eberbeck, D.; Trahms, L. Magnetorelaxometry assisting biomedical applications of magnetic nanoparticles. Pharm. Res. 2012, 29, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Dolgovskiy, V.; Lebedev, V.; Colombo, S.; Weis, A.; Michen, B.; Ackermann-Hirschi, L.; Petri-Fink, A. A quantitative study of particle size effects in the magnetorelaxometry of magnetic nanoparticles using atomic magnetometry. J. Magn. Magn. Mater. 2015, 379, 137–150. [Google Scholar] [CrossRef]
- Baffa, O.; Matsuda, R.; Arsalani, S.; Prospero, A.; Miranda, J.; Wakai, R. Development of an Optical Pumped Gradiometric System to Detect Magnetic Relaxation of Magnetic Nanoparticles. J. Magn. Magn. Mater. 2018, 475, 533–538. [Google Scholar] [CrossRef]
- Crevecoeur, G.; Baumgarten, D.; Steinhoff, U.; Haueisen, J.; Trahms, L.; Dupré, L. Advancements in magnetic nanoparticle reconstruction using sequential activation of excitation coil arrays using magnetorelaxometry. IEEE Trans. Magn. 2012, 48, 1313–1316. [Google Scholar] [CrossRef]
- Thiel, F.; Schnabel, A.; Knappe-Grüneberg, S.; Stollfuß, D.; Burghoff, M. Demagnetization of magnetically shielded rooms. Rev. Sci. Instrum. 2007, 78, 035106. [Google Scholar] [CrossRef]
- Liebl, M. Quantitative Bildgebung Magnetischer Nanopartikel Mittels Magnetrelaxometrischer Tomographie für Biomedizinische Anwendungen. Ph.D. Thesis, Technische Universität Ilmenau, Ilmenau, Germany, 2016. Available online: https://nbn-resolving.org/urn:nbn:de:gbv:ilm1-2016000744 (accessed on 9 December 2019).
- Dupont-Roc, J.; Haroche, S.; Cohen-Tannoudji, C. Detection of very weak magnetic fields (10−9gauss) by 87Rb zero-field level crossing resonances. Phys. Lett. A 1969, 28, 638–639. [Google Scholar] [CrossRef]
- Osborne, J.; Orton, J.; Alem, O.; Shah, V. Fully integrated standalone zero field optically pumped magnetometer for biomagnetism. Steep Dispersion Engineering and Opto-Atomic Precision Metrology XI. In Proceedings of the International Society for Optics and Photonics, San Francisco, CA, USA, 29 January–1 February 2018; p. 105481G. [Google Scholar]
- Eberbeck, D.; Wiekhorst, F.; Wagner, S.; Trahms, L. How the size distribution of magnetic nanoparticles determines their magnetic particle imaging performance. Appl. Phys. Lett. 2011, 98, 182502. [Google Scholar] [CrossRef]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677. [Google Scholar] [CrossRef]
- Wiekhorst, F.; Liebl, M.; Steinhoff, U.; Trahms, L.; Lyer, S.; Dürr, S.; Alexiou, C. Magnetorelaxometry for in-vivo quantification of magnetic nanoparticle distributions after magnetic drug targeting in a rabbit carcinoma model. In Magnetic Particle Imaging; Springer: Berlin, Germany, 2012; pp. 301–305. [Google Scholar]
- Néel, L. Théorie du traînage magnétique des ferromagnétiques en grains fins avec applications aux terres cuites. Ann. Géophys. 1949, 5, 99–136. [Google Scholar]
- Eberbeck, D.; Wiekhorst, F.; Steinhoff, U.; Trahms, L. Aggregation behaviour of magnetic nanoparticle suspensions investigated by magnetorelaxometry. J. Phys. Condens. Matter 2006, 18, S2829. [Google Scholar] [CrossRef]
- Shliomis, M. Effective viscosity of magnetic suspensions. Zh. Eksp. Teor. Fiz. 1971, 61, 2411–2418. [Google Scholar]
- Byrd, R.H.; Schnabel, R.B.; Shultz, G.A. Approximate solution of the trust region problem by minimization over two-dimensional subspaces. Math. Program. 1988, 40, 247–263. [Google Scholar] [CrossRef]
- Golub, G.; Van Loan, C. Matrix Computations; The Johns Hopkins University Press: Baltimore, MD, USA, 1996; ISBN 978-0801854149. [Google Scholar]
- Engl, H.W.; Hanke, M.; Neubauer, A. Regularization of Inverse Problems; Springer Science & Business Media: Berlin, Germany, 1996; p. 117. [Google Scholar]
- Leliaert, J.; Coene, A.; Crevecoeur, G.; Vansteenkiste, A.; Eberbeck, D.; Wiekhorst, F.; Van Waeyenberge, B.; Dupré, L. Regarding the Néel relaxation time constant in magnetorelaxometry. J. Appl. Phys. 2014, 116, 163914. [Google Scholar] [CrossRef]
- Van Durme, R.; Coene, A.; Crevecoeur, G.; Dupré, L. Model-based optimal design of a magnetic nanoparticle tomographic imaging setup. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 369–372. [Google Scholar]
- Schier, P.; Liebl, M.; Steinhoff, U.; Handler, M.; Wiekhorst, F.; Baumgarten, D. Optimizing Excitation Coil Currents for Advanced Magnetorelaxometry Imaging. J. Math. Imaging Vision 2020, 62, 238–252. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaufenthaler, A.; Schier, P.; Middelmann, T.; Liebl, M.; Wiekhorst, F.; Baumgarten, D. Quantitative 2D Magnetorelaxometry Imaging of Magnetic Nanoparticles Using Optically Pumped Magnetometers. Sensors 2020, 20, 753. https://doi.org/10.3390/s20030753
Jaufenthaler A, Schier P, Middelmann T, Liebl M, Wiekhorst F, Baumgarten D. Quantitative 2D Magnetorelaxometry Imaging of Magnetic Nanoparticles Using Optically Pumped Magnetometers. Sensors. 2020; 20(3):753. https://doi.org/10.3390/s20030753
Chicago/Turabian StyleJaufenthaler, Aaron, Peter Schier, Thomas Middelmann, Maik Liebl, Frank Wiekhorst, and Daniel Baumgarten. 2020. "Quantitative 2D Magnetorelaxometry Imaging of Magnetic Nanoparticles Using Optically Pumped Magnetometers" Sensors 20, no. 3: 753. https://doi.org/10.3390/s20030753
APA StyleJaufenthaler, A., Schier, P., Middelmann, T., Liebl, M., Wiekhorst, F., & Baumgarten, D. (2020). Quantitative 2D Magnetorelaxometry Imaging of Magnetic Nanoparticles Using Optically Pumped Magnetometers. Sensors, 20(3), 753. https://doi.org/10.3390/s20030753




