Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor
Abstract
1. Introduction
2. Materials and Methods
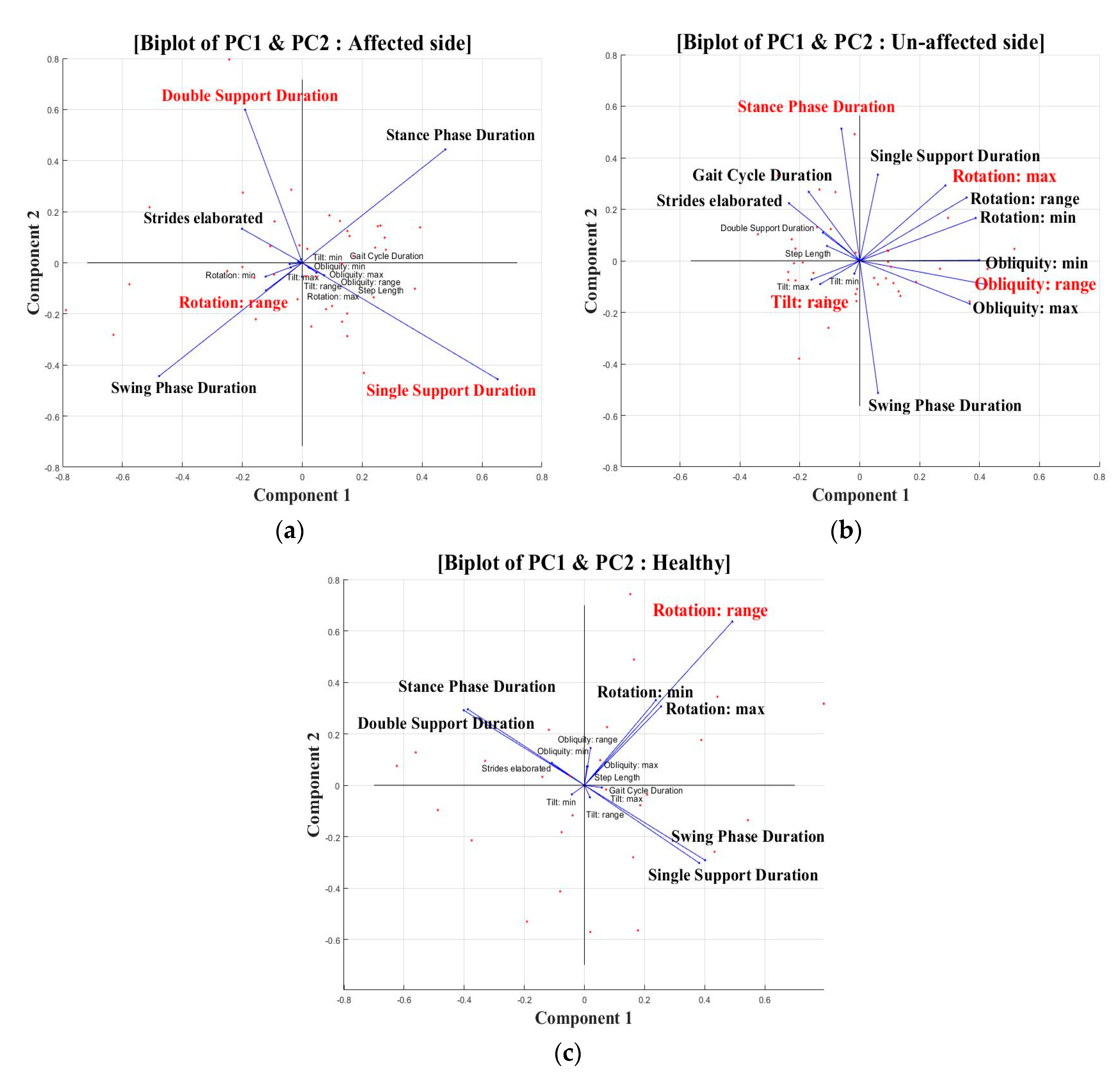
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olney, S.J.; Richards, C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture 1996, 4, 136–148. [Google Scholar] [CrossRef]
- Styliani, F.; Nikolaos, A.; Vassilios, G.; Paraskevi, M.; Nikolaos, P.; Erasmia, G.; Ioannis, I.; Konstantinos, V.; Aikaterini, T.; Haritomeni, P. Reproducibility of gait kinematics and kinetics in chronic stroke patients. NeuroRehabiltation 2018, 42, 53–61. [Google Scholar]
- Wrisley, D.M.; Marchetti, G.F.; Kuharsky, D.K.; Whitney, S.L. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004, 84, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000, 80, 896–903. [Google Scholar] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 15, 2999–3006. [Google Scholar] [CrossRef]
- Glowinski, S.; Łosiński, K.; Kowiański, P.; Waśkow, M.; Bryndal, A.; Grochulska, A. Inertial sensors as a tool for diagnosing discopathy lumbosacral pathologic gait: A preliminary research. Diagnostics 2020, 10, 342. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Saeys, W.; Hallemans, A.; Velghe, S.; Viskens, P.J.; Vereeck, L.; De Hertogh, W.; Truijen, S. Trunk biomechanics during hemiplegic gait after stroke: A systematic review. Gait Posture 2017, 54, 133–143. [Google Scholar] [CrossRef]
- Chau, T. A review of analytical techniques for gait data. Part 1: Fuzzy, statistical and fractal methods. Gait Posture 2001, 13, 49–66. [Google Scholar] [CrossRef]
- Olney, S.J.; Griffin, M.P.; McBride, I.D. Multivariate examination of data from gait analysis of persons with stroke. Phys. Ther. 1998, 78, 814–828. [Google Scholar] [CrossRef]
- Carriero, A.; Zavatsky, A.; Stebbins, J.; Theologis, T.; Shefelbine, S.J. Determination of gait patterns in children with spastic diplegic cerebral palsy using principal components. Gait Posture 2009, 29, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chu, H.; Park, C.; Kang, G.H.; Seo, J.; Sung, K.K.; Lee, S. Comparison of recovery patterns of gait patterns according to the paralyzed side in Korean stroke patients. Medicine 2018, 97, e12095. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Kim, J.K.; Shin, J.Y.; Ku, B.C.; Bae, J.H.; Yeom, S.Y.; Lee, S. Change in radial artery pulse wave in stroke hemiplegic patients. Medicine 2018, 97, e0204. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, E.; Merlo, A.; Fioretti, S.; Zemp, D.; Campanini, I.; Quadri, P. A statistical approach to discriminate between non-faller, rare fallers and frequent fallers in older adults based on posturopgraphic data. Clin Biomech 2016, 32, 8–13. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.T.; Novak, A.C.; Brouwer, B.; Li, Q. Estimation of spatio-temporal parameters for post-stroke hemiparetic gait using inertial sensors. Gait Posture 2013, 37, 354–358. [Google Scholar] [CrossRef]
- Carrie, L.; Steven, A.; Richard, R. Muscle work is increased in pre-swing during hemiparetic walking. Clin Biomech 2011, 26, 859–866. [Google Scholar]
- Patterson, K.; Gage, W.; Brooks, D.; Black, S.; Mcllroy, W. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Clin Biomech 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Dodd, K.; Morris, M. Lateral pelvic displacement during gait: Abnormalities after stroke and changes during the first month of rehabilitation. Arch. Phys. Med. Rehabil. 2003, 84, 1200–1205. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Tang, S.; Wu, C.; Cheng, P.; Hong, W. Gait Performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J. Phys. Med. Rehabil. 2003, 82, 925–935. [Google Scholar] [CrossRef]
- Hacmon, R.; Krasovsky, T.; Lamontagne, A.; Levin, M. Deficits in intersegmental trunk coordination during walking and related to clinical balance and gait function in chronic stroke. J. Neurol. Phys. Ther. 2012, 36, 173–181. [Google Scholar] [CrossRef]
- Iosa, M.; Morone, G.; Fusco, A.; Pratesi, L.; Bragoni, M.; Coiro, P.; Multari, M.; Venturiero, V.; De Angelis, D.; Paolucci, S. Effects of walking endurance reduction on gait stability on patients with stroke. Stroke Res. Treat. 2011, 2012, 810415. [Google Scholar] [CrossRef] [PubMed]
- Mizuike, C.; Ohigi, S.; Morita, S. Analysis of stroke patient walking dynamics using a tri-axial accelerometer. Gait Posture 2009, 30, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.O.; Torricelli, D.; Molina-Rueda, F.; Alguacil-Diego, I.M.; De-la-Cuerda, R.C.; Santos, C.; Moreno, J.C.; Miangolarra-Page, J.C.; Pons, J.L. Combining muscle synergies and biomechanical analysis to assess gait in stroke patients. J. Biomech. 2017, 63, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; Olney, S. Hemiparetic gait following stroke. PartⅡ: Recovery and physical therapy. Gait Posture 1996, 4, 149–162. [Google Scholar] [CrossRef]
| Variable | Patients | Control |
|---|---|---|
| No. | 40 | 28 |
| Age (years) | 65.1 ± 9.4 | 65.9 ± 7.8 |
| Sex (women/men) | 22/18 | 16/12 |
| Hemiparetic side (left/right) | 14/26 | - |
| Time post-stroke (months) | 3.0 ± 1.9 | - |
| K-NIHSS | 0.9 ± 1.5 | - |
| MMT-lower extremity | 38.8 ± 3.8/50 | 50/50 |
| (affected/unaffected) |
| Gait Measure | Affected Side | Unaffected Side | Healthy (L&R Mean) |
|---|---|---|---|
| Gait event | |||
| Gait cycle Duration (sec) | * 1.19 (0.15) | % 1.19 (0.15) | *,% 1.04 (0.08) |
| Step length (%) | 49.99 (2.55) | 49.89 (2.54) | 50.00 (0.00) |
| Stance duration (%) | # 60.19 (3.96) | # 62.85 (5.37) | 60.98 (2.19) |
| Swing duration (%) | # 39.81 (3.98) | # 37.16 (5.37) | 39.02 (2.19) |
| Double support duration (%) | * 12.89 (3.60) | 11.95 (2.51) | * 10.97 (2.14) |
| Single support duration (%) | *,# 35.69 (5.02) | # 38.33 (4.30) | * 39.04 (2.14) |
| Strides elaborated (no.) | * 11.93 (3.27) | % 12.10 (3.07) | *,% 9.45 (1.24) |
| Tilt | |||
| Tilt: minimum (°) | 1.49 (0.64) | 1.49 (0.66) | 1.86 (0.98) |
| Tilt: maximum (°) | 0.72 (0.52) | 0.74 (0.56) | 0.89 (0.58) |
| Tilt: range (°) | * 2.20 (0.73) | % 2.21 (0.79) | *,% 2.74 (0.88) |
| Obliquity | |||
| Obliquity: minimum (°) | 1.02 (0.60) | 1.08 (0.58) | 1.15 (0.52) |
| Obliquity: maximum (°) | 1.08 (0.57) | 1.04 (0.58) | 1.15 (0.51) |
| Obliquity: range (°) | 2.09 (1.05) | 2.12 (1.04) | 2.30 (1.04) |
| Rotation | |||
| Rotation: minimum (°) | 3.17 (1.57) | 3.27 (1.70) | 3.60 (1.73) |
| Rotation: maximum (°) | 3.33 (1.86) | 3.30 (1.84) | 3.55 (1.71) |
| Rotation: range (°) | 6.50 (3.15) | 6.56 (3.31) | (3.42) |
| Groups | PCs | Highest PC Variable | PC Coefficient | PC Scores (%) (Variation Explained) |
|---|---|---|---|---|
| Affected side | PC1 | Single support duration | 0.65 | 41.7 |
| PC2 | Double support duration | 0.59 | 25.8 | |
| PC3 | Rotation: range | 0.75 | 14.4 | |
| Unaffected side | PC1 | Obliquity: range | 0.43 | 28.2 |
| PC2 | Stance/Swing phase duration | 0.51/−0.51 | 18.4 | |
| PC3 | Tilt: range | 0.63 | 13.1 | |
| PC4 | Rotation: maximum | 0.37 | 10.9 | |
| Healthy side | PC1 | Rotation: range | 0.49 | 50.1 |
| PC2 | Rotation: range | 0.64 | 39.5 |
| Gait Measure | Hip | |||||
|---|---|---|---|---|---|---|
| Flexion | Extension | Abduction | Adduction | Internal Rotation | External Rotation | |
| Gait cycle duration | −0.30 | −0.30 | −0.30 | −0.30 | −0.30 | −0.30 |
| Step length | −0.00 | −0.00 | −0.00 | −0.00 | −0.00 | −0.00 |
| Stance phase duration | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| Swing phase | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 |
| Double support duration | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Single support duration | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Stride elaborated | −0.24 | −0.24 | −0.24 | −0.24 | −0.24 | −0.24 |
| Tilt: min | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Tilt: max | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 |
| Tilt: range | −0.11 | −0.11 | −0.11 | −0.11 | −0.11 | −0.11 |
| Obliquity: min | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Obliquity: max | * 0.36 | * 0.36 | * 0.36 | * 0.36 | * 0.36 | * 0.36 |
| Obliquity: range | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Rotation: min | −0.11 | −0.11 | −0.11 | −0.11 | −0.11 | −0.11 |
| Rotation: max | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 | −0.14 |
| Rotation: range | −0.15 | −0.15 | −0.15 | −0.15 | −0.15 | −0.15 |
| Gait Measure | Knee | Ankle | ||
|---|---|---|---|---|
| Flexion | Extension | Dorsi-Flexion | Plantar-Flexion | |
| Gait cycle duration | −0.30 | −0.30 | −0.16 | −0.16 |
| Step length | −0.00 | −0.00 | −0.10 | −0.10 |
| Stance phase duration | 0.14 | 0.14 | 0.27 | 0.27 |
| Swing phase | −0.14 | −0.14 | −0.27 | −0.27 |
| Double support duration | 0.05 | 0.05 | −0.16 | −0.16 |
| Single support duration | 0.03 | 0.03 | 0.34 | 0.34 |
| Stride elaborated | −0.24 | −0.24 | −0.23 | −0.23 |
| Tilt: min | 0.05 | 0.05 | 0.15 | 0.15 |
| Tilt: max | −0.14 | −0.14 | −0.33 | −0.33 |
| Tilt: range | −0.11 | −0.11 | −0.15 | −0.15 |
| Obliquity: min | 0.06 | 0.06 | 0.05 | 0.05 |
| Obliquity: max | * 0.36 | * 0.36 | 0.30 | 0.30 |
| Obliquity: range | 0.23 | 0.23 | 0.19 | 0.19 |
| Rotation: min | −0.11 | −0.11 | −0.18 | −0.18 |
| Rotation: max | −0.14 | −0.14 | −0.10 | −0.10 |
| Rotation: range | −0.15 | −0.15 | −0.15 | −0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.-W.; Kim, S.-G.; Kim, J.I.; Ku, B.; Kim, K.; Lee, S.; Kim, J.U. Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor. Sensors 2020, 20, 7338. https://doi.org/10.3390/s20247338
Seo J-W, Kim S-G, Kim JI, Ku B, Kim K, Lee S, Kim JU. Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor. Sensors. 2020; 20(24):7338. https://doi.org/10.3390/s20247338
Chicago/Turabian StyleSeo, Jeong-Woo, Seul-Gee Kim, Joong Il Kim, Boncho Ku, Kahye Kim, Sangkwan Lee, and Jaeuk U. Kim. 2020. "Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor" Sensors 20, no. 24: 7338. https://doi.org/10.3390/s20247338
APA StyleSeo, J.-W., Kim, S.-G., Kim, J. I., Ku, B., Kim, K., Lee, S., & Kim, J. U. (2020). Principal Characteristics of Affected and Unaffected Side Trunk Movement and Gait Event Parameters during Hemiplegic Stroke Gait with IMU Sensor. Sensors, 20(24), 7338. https://doi.org/10.3390/s20247338








