Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Assessments
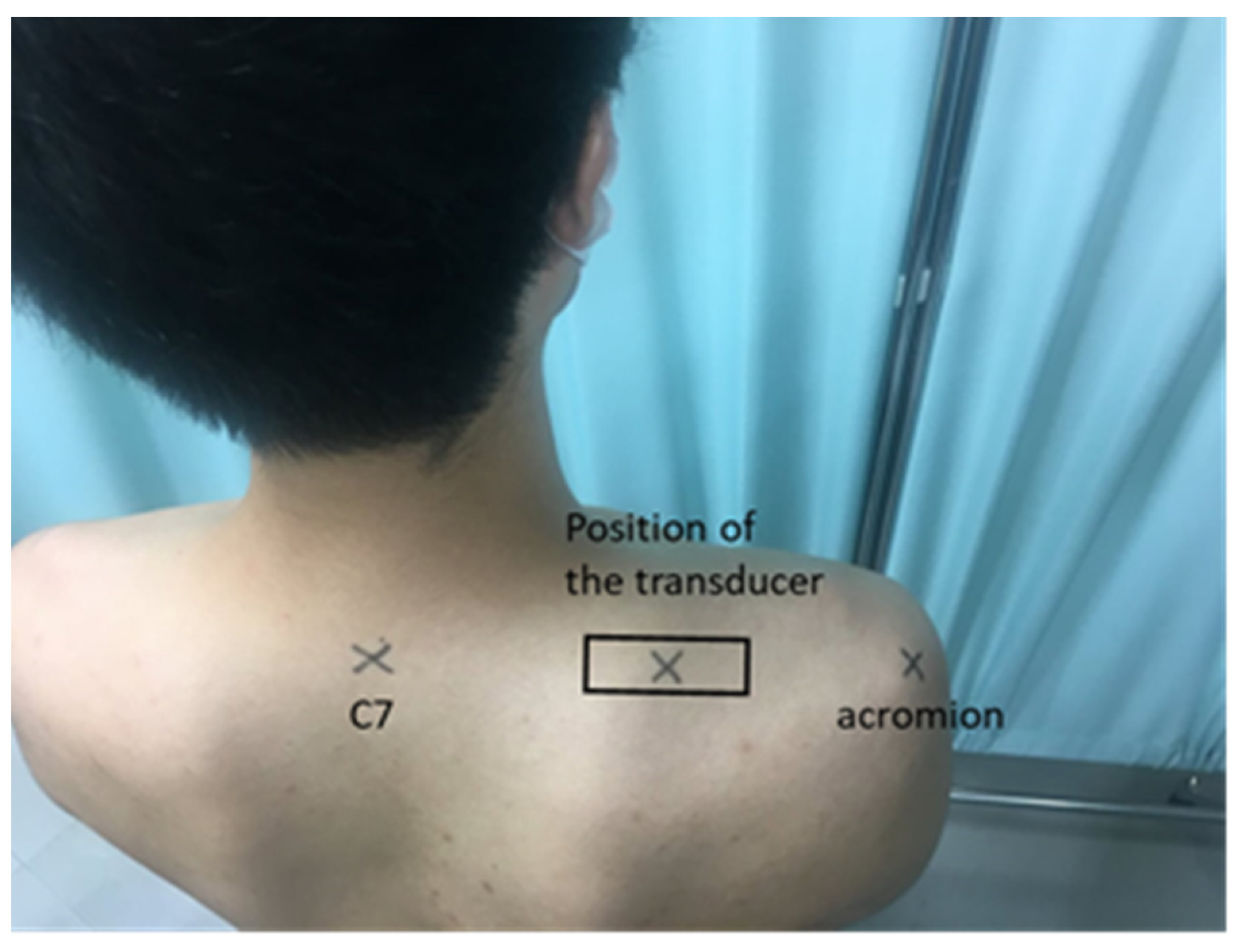
2.3.1. Muscle Hardness Meter
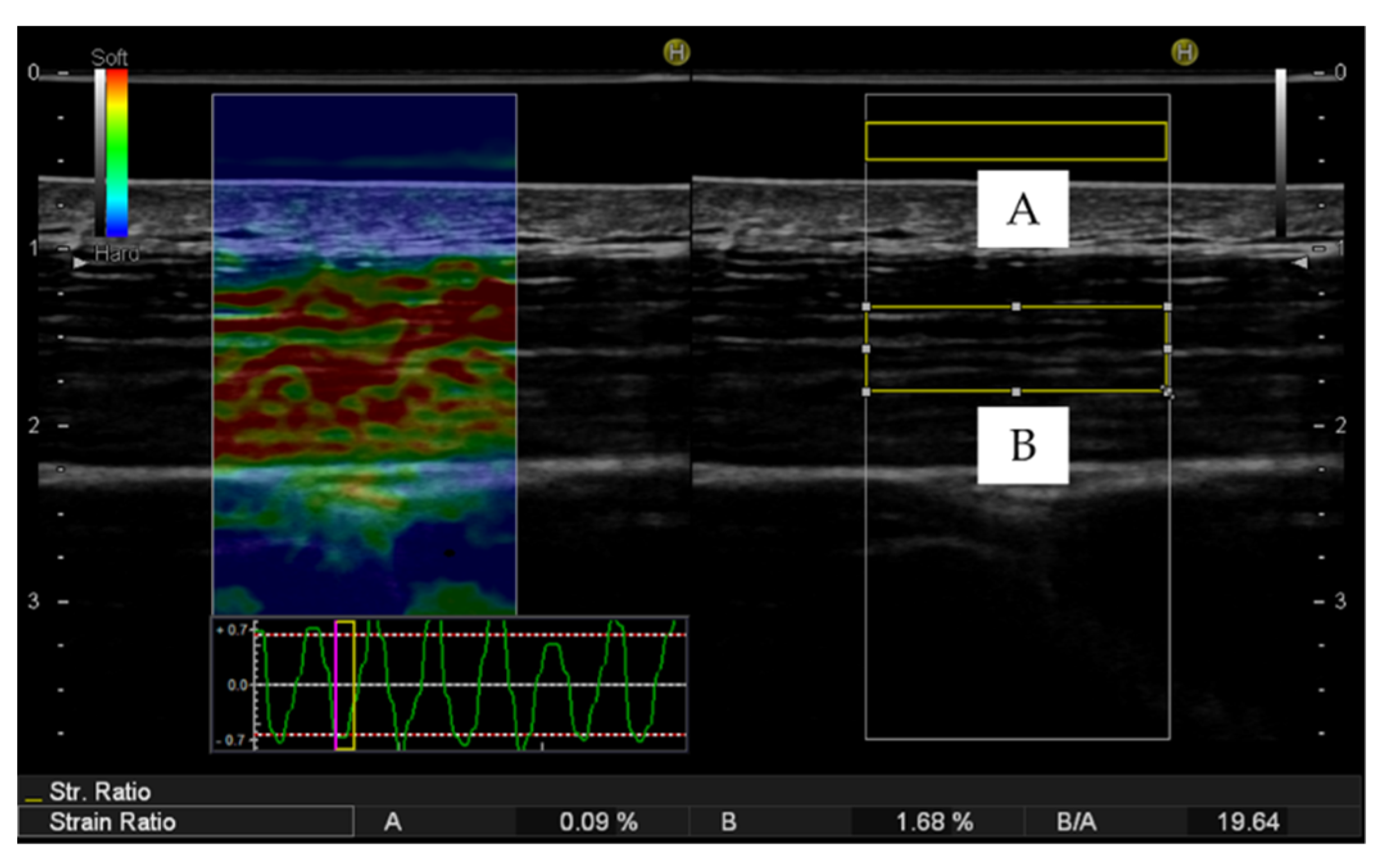
2.3.2. Ultrasound Strain Elastography
2.4. Statistical Analysis
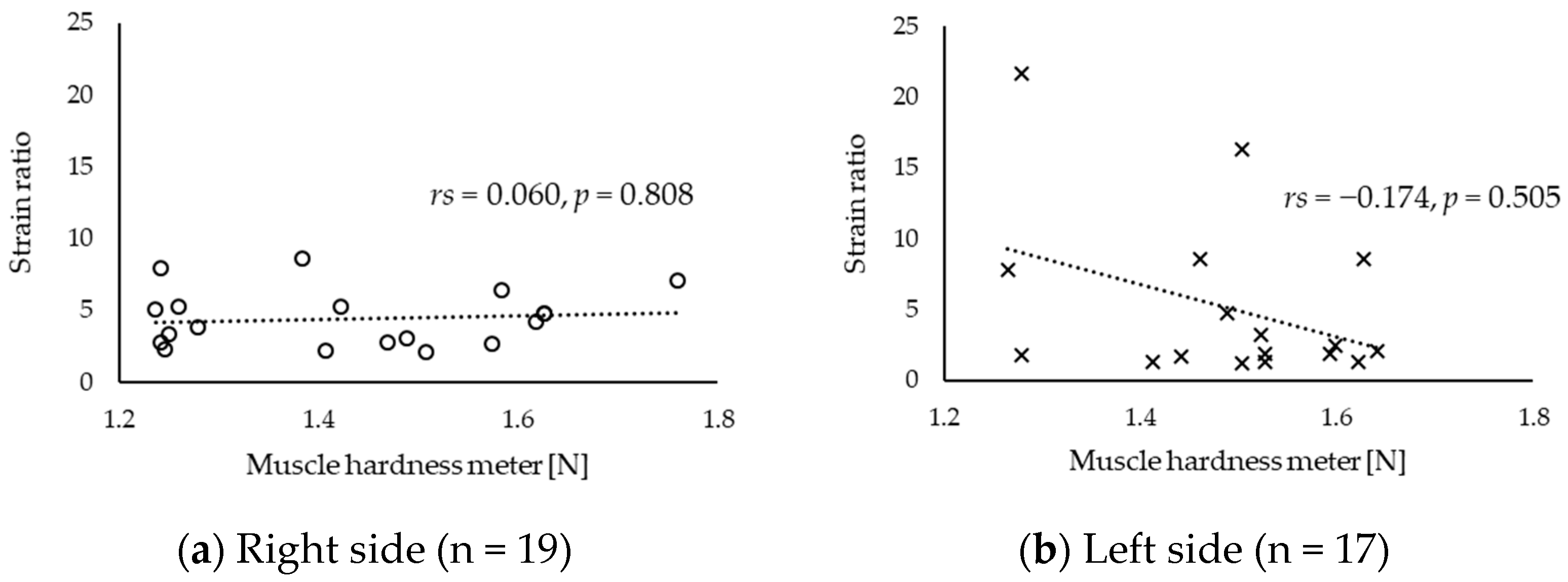
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ming, Z.; Närhi, M.; Siivola, J. Neck and shoulder pain related to computer use. Pathophysiology 2004, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Juul-Kristensen, B.; Kadefors, R.; Hansen, K.; Byström, P.; Sandsjö, L.; Sjøgaard, G. Clinical signs and physical function in neck and upper extremities among elderly female computer users: The NEW study. Eur. J. Appl. Physiol. 2006, 96, 136–145. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Comprehensive Survey of Living Conditions 2019: III Health Status of Household Members. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa19/dl/04.pdf (accessed on 13 October 2020).
- Cerezo-Téllez, E.; Torres-Lacomba, M.; Mayoral-Del Moral, O.; Sánchez-Sánchez, B.; Dommerholt, J.; Gutiérrez-Ortega, C. Prevalence of Myofascial Pain Syndrome in Chronic Non-Specific Neck Pain: A Population-Based Cross-Sectional Descriptive Study. Pain Med. 2016, 17, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.T.; Hug, F.; Fu, S.N. Increased Upper Trapezius Muscle Stiffness in Overhead Athletes with Rotator Cuff Tendinopathy. PLoS ONE 2016, 11, e0155187. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, M.A.; Blatter, B.M.; van der Beek, A.J. Perceived muscular tension predicts future neck-shoulder and arm-wrist-hand symptoms. Occup. Environ. Med. 2012, 69, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, J.; Hagberg, M.; Toomingas, A.; Wigaeus Tornqvist, E. Perceived muscular tension, job strain, physical exposure, and associations with neck pain among VDU users; a prospective cohort study. Occup. Environ. Med. 2004, 61, 523–528. [Google Scholar] [CrossRef]
- Horikawa, M.; Ebihara, S.; Sakai, F.; Akiyama, M. Non-invasive measurement method for hardness in muscular tissues. Med. Biol. Eng. Comput. 1993, 31, 623–627. [Google Scholar] [CrossRef]
- Komiya, H.; Maeda, J.; Takemiya, T. A New Functional Measurement of Muscle Stiffness in Humans. Adv. Exerc. Sports Physiol. 1996, 2, 31–38. [Google Scholar]
- Murayama, M.; Nosaka, K.; Yoneda, T.; Minamitani, K. Changes in hardness of the human elbow flexor muscles after eccentric exercise. Eur. J. Appl. Physiol. 2000, 82, 361–367. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Eby, S.F.; Song, P.; Zhao, H.; Brault, J.S.; Chen, S.; An, K.N. Ultrasound elastography: The new frontier in direct measurement of muscle stiffness. Arch. Phys. Med. Rehabil. 2014, 95, 2207–2219. [Google Scholar] [CrossRef]
- Hug, F.; Tucker, K.; Gennisson, J.L.; Tanter, M.; Nordez, A. Elastography for Muscle Biomechanics: Toward the Estimation of Individual Muscle Force. Exerc. Sport Sci. Rev. 2015, 43, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Nordez, A.; Hug, F. Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J. Appl. Physiol. (1985) 2010, 108, 1389–1394. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Takai, Y.; Kanehisa, H.; Shinohara, M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve 2014, 50, 103–113. [Google Scholar] [CrossRef]
- Akagi, R.; Takahashi, H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med. Sci. Sports Exerc. 2013, 45, 1348–1354. [Google Scholar] [CrossRef]
- Akagi, R.; Takahashi, H. Effect of a 5-week static stretching program on hardness of the gastrocnemius muscle. Scand. J. Med. Sci. Sports 2014, 24, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Tanaka, J.; Shikiba, T.; Takahashi, H. Muscle hardness of the triceps brachii before and after a resistance exercise session: A shear wave ultrasound elastography study. Acta Radiol. 2015, 56, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Chino, K.; Dohi, M.; Takahashi, H. Relationships between muscle size and hardness of the medial gastrocnemius at different ankle joint angles in young men. Acta Radiol. 2012, 53, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Chino, K.; Akagi, R.; Dohi, M.; Fukashiro, S.; Takahashi, H. Reliability and validity of quantifying absolute muscle hardness using ultrasound elastography. PLoS ONE 2012, 7, e45764. [Google Scholar] [CrossRef]
- Yanagisawa, O.; Niitsu, M.; Kurihara, T.; Fukubayashi, T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: A feasibility study. Clin. Radiol. 2011, 66, 815–819. [Google Scholar] [CrossRef]
- Ishikawa, H.; Muraki, T.; Sekiguchi, Y.; Ishijima, T.; Morise, S.; Yamamoto, N.; Itoi, E.; Izumi, S. Noninvasive assessment of the activity of the shoulder girdle muscles using ultrasound real-time tissue elastography. J. Electromyogr. Kinesiol. 2015, 25, 723–730. [Google Scholar] [CrossRef]
- Ishikawa, H.; Muraki, T.; Morise, S.; Sekiguchi, Y.; Yamamoto, N.; Itoi, E.; Izumi, S.I. Changes in stiffness of the dorsal scapular muscles before and after computer work: A comparison between individuals with and without neck and shoulder complaints. Eur. J. Appl. Physiol. 2017, 117, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Tsujimura, T.; Shimizu, T.; Watanabe, T.; Lau, W.Y.; Nosaka, K. Relationship between isometric contraction intensity and muscle hardness assessed by ultrasound strain elastography. Eur. J. Appl. Physiol. 2017, 117, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Kusama, S. Comparison Between Neck and Shoulder Stiffness Determined by Shear Wave Ultrasound Elastography and a Muscle Hardness Meter. Ultrasound Med. Biol. 2015, 41, 2266–2271. [Google Scholar] [CrossRef]
- Atkinson, G.; Nevill, A.M. Selected issues in the design and analysis of sport performance research. J. Sports Sci. 2001, 19, 811–827. [Google Scholar] [CrossRef]
- Xie, Y.; Thomas, L.; Hug, F.; Johnston, V.; Coombes, B.K. Quantifying cervical and axioscapular muscle stiffness using shear wave elastography. J. Electromyogr. Kinesiol. 2019, 48, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ariji, Y.; Gotoh, A.; Hiraiwa, Y.; Kise, Y.; Nakayama, M.; Nishiyama, W.; Sakuma, S.; Kurita, K.; Ariji, E. Sonographic elastography for evaluation of masseter muscle hardness. Oral Radiol. 2013, 29, 64–69. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med. Biol. 2013, 39, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Korkusuz, F.; Erden, Z. Neck Muscle Stiffness in Participants With and Without Chronic Neck Pain: A Shear-Wave Elastography Study. J. Manip. Physiol. Ther. 2018, 41, 580–588. [Google Scholar] [CrossRef]
- De Pauw, R.; Coppieters, I.; Kregel, J.; De Meulemeester, K.; Danneels, L.; Cagnie, B. Does muscle morphology change in chronic neck pain patients?—A systematic review. Man. Ther. 2016, 22, 42–49. [Google Scholar] [CrossRef]
- Simons, D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef]
- Ziaeifar, M.; Arab, A.M.; Mosallanezhad, Z.; Nourbakhsh, M.R. Dry needling versus trigger point compression of the upper trapezius: A randomized clinical trial with two-week and three-month follow-up. J. Man. Manip. Ther. 2019, 27, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sciotti, V.M.; Mittak, V.L.; DiMarco, L.; Ford, L.M.; Plezbert, J.; Santipadri, E.; Wigglesworth, J.; Ball, K. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain 2001, 93, 259–266. [Google Scholar] [CrossRef]
- Gemmell, H.; Allen, A. Relative immediate effect of ischaemic compression and activator trigger point therapy on active upper trapezius trigger points: A randomised trial. Clin. Chiropr. 2008, 11, 175–181. [Google Scholar] [CrossRef]
- Sarrafzadeh, J.; Ahmadi, A.; Yassin, M. The Effects of Pressure Release, Phonophoresis of Hydrocortisone, and Ultrasound on Upper Trapezius Latent Myofascial Trigger Point. Arch. Phys. Med. Rehabil. 2012, 93, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Hansen, K.; Mortensen, O.S.; Zebis, M.K. Prevalence and anatomical location of muscle tenderness in adults with nonspecific neck/shoulder pain. BMC Musculoskelet. Disord. 2011, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Ge, H.Y.; Arendt-Nielsen, L.; Danneskiold-Samsøe, B.; Graven-Nielsen, T. Increased trapezius pain sensitivity is not associated with increased tissue hardness. J. Pain 2010, 11, 491–499. [Google Scholar] [CrossRef]
- Horikawa, M. Effect of visual display terminal height on the trapezius muscle hardness: Quantitative evaluation by a newly developed muscle hardness meter. Appl. Ergon. 2001, 32, 473–478. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Buman, M.P.; Haskell, W.L.; McConnell, M.V.; Carstensen, L.L. Twenty-four Hours of Sleep, Sedentary Behavior, and Physical Activity with Nine Wearable Devices. Med. Sci. Sports Exerc. 2016, 48, 457–465. [Google Scholar] [CrossRef]
- Brakenridge, C.L.; Fjeldsoe, B.S.; Young, D.C.; Winkler, E.A.; Dunstan, D.W.; Straker, L.M.; Healy, G.N. Evaluating the effectiveness of organisational-level strategies with or without an activity tracker to reduce office workers’ sitting time: A cluster-randomised trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 115. [Google Scholar] [CrossRef]


| Age (years) | 20.3 ± 0.6 |
| Height (m) | 1.74 ± 0.1 |
| Weight (kg) | 71.0 ± 12.2 |
| BMI (kg/m2) | 23.4 ± 3.0 |
| Dominant hand (n) | Right, 19; left, 1 |
| Duration of smartphone use per day (hours) | 5.4 ± 2.7 |
| Duration of typing per day (hours) | 1.8 ± 1.6 |
| Single Measurements | Average Measurements | |||
|---|---|---|---|---|
| ICC (1, 1) | 95% CI | ICC (1, 5) | 95% CI | |
| Right muscle hardness meter (N) | 0.974 | 0.951−0.988 | 0.995 | 0.990−0.998 |
| Left muscle hardness meter (N) | 0.962 | 0.929−0.983 | 0.992 | 0.985−0.996 |
| Right strain ratio obtained using strain elastography | 0.942 | 0.893−0.974 | 0.988 | 0.977−0.995 |
| Left strain ratio obtained using strain elastography | 0.930 | 0.874−0.968 | 0.985 | 0.972−0.993 |
| Muscle Hardness Meter (N) | Strain Ratio | |
|---|---|---|
| Right side | 1.43 (1.26, 1.58) | 4.50 (2.79, 5.53) |
| Left side | 1.51 (1.43, 1.59) | 2.80 (1.79, 10.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, T.; Okawara, H.; Nakashima, D.; Iwabuchi, S.; Matsumoto, M.; Nakamura, M.; Nagura, T. Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography. Sensors 2020, 20, 7200. https://doi.org/10.3390/s20247200
Sawada T, Okawara H, Nakashima D, Iwabuchi S, Matsumoto M, Nakamura M, Nagura T. Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography. Sensors. 2020; 20(24):7200. https://doi.org/10.3390/s20247200
Chicago/Turabian StyleSawada, Tomonori, Hiroki Okawara, Daisuke Nakashima, Shuhei Iwabuchi, Morio Matsumoto, Masaya Nakamura, and Takeo Nagura. 2020. "Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography" Sensors 20, no. 24: 7200. https://doi.org/10.3390/s20247200
APA StyleSawada, T., Okawara, H., Nakashima, D., Iwabuchi, S., Matsumoto, M., Nakamura, M., & Nagura, T. (2020). Reliability of Trapezius Muscle Hardness Measurement: A Comparison between Portable Muscle Hardness Meter and Ultrasound Strain Elastography. Sensors, 20(24), 7200. https://doi.org/10.3390/s20247200





