Analysis of Visuo Motor Control between Dominant Hand and Non-Dominant Hand for Effective Human-Robot Collaboration
Abstract
1. Introduction
2. Materials and Methods
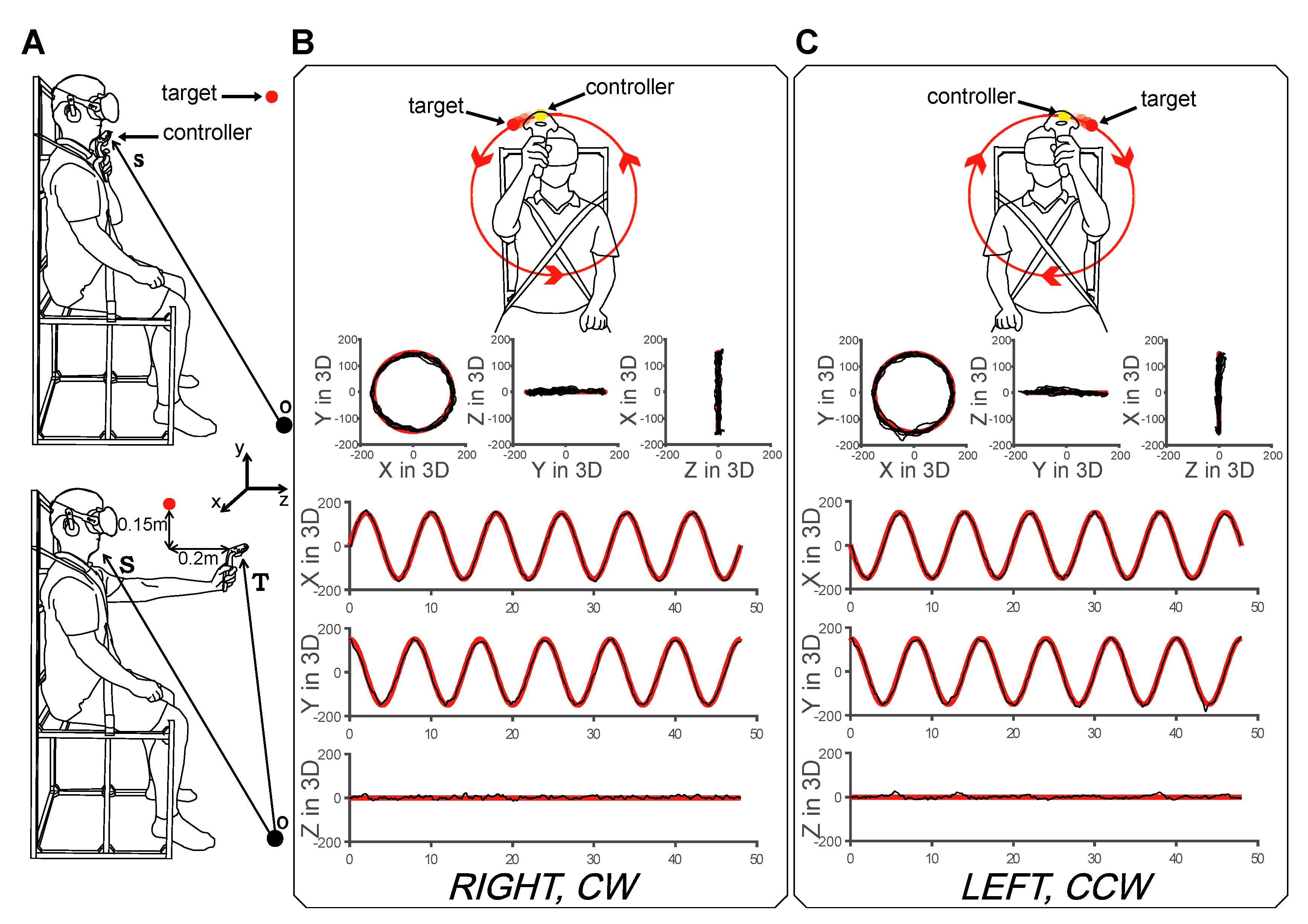
2.1. Subjects and Experimental Setup
2.2. Experimental Task
2.3. Experimental Task
2.4. Statistical Analysis
3. Results
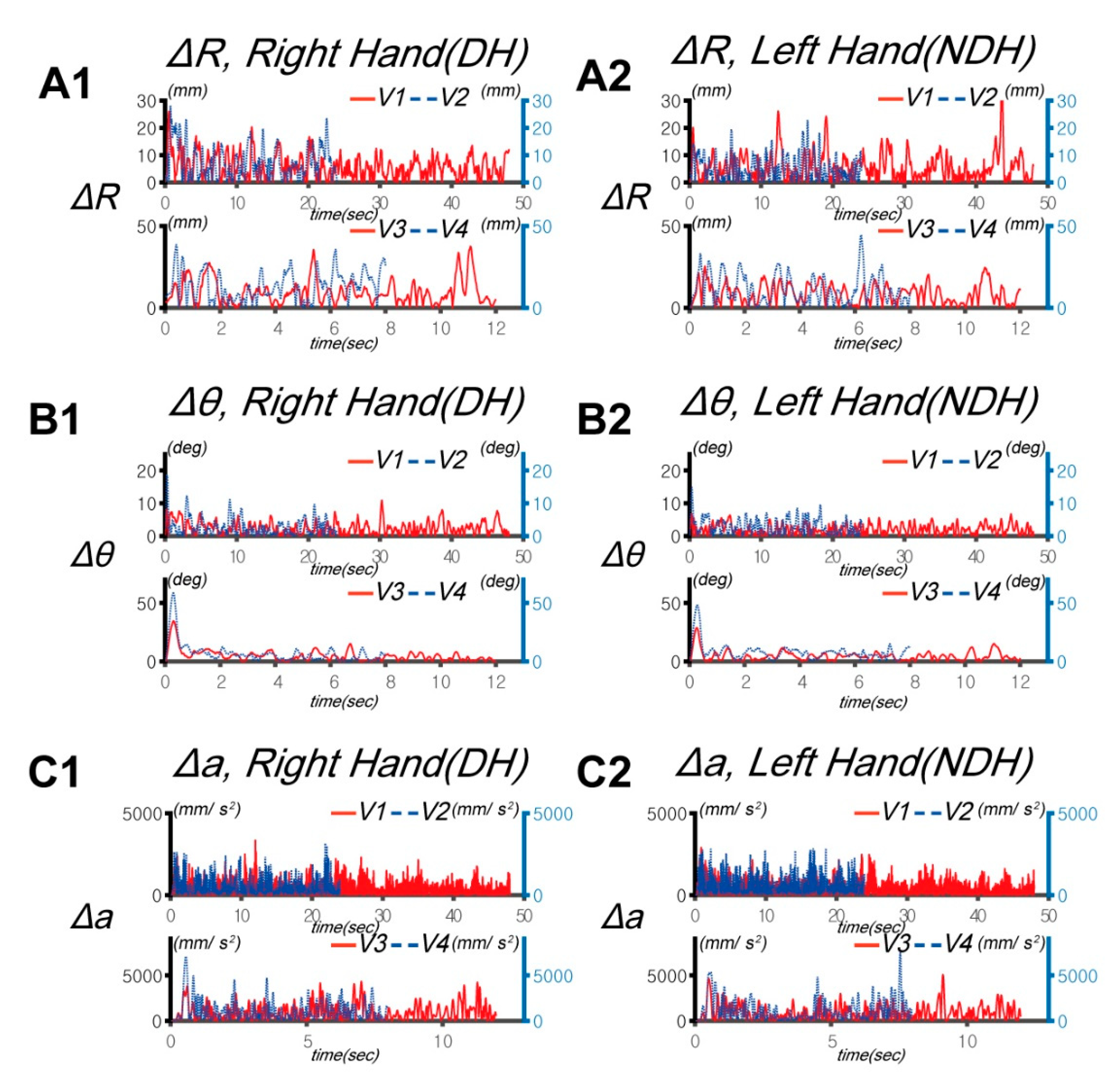
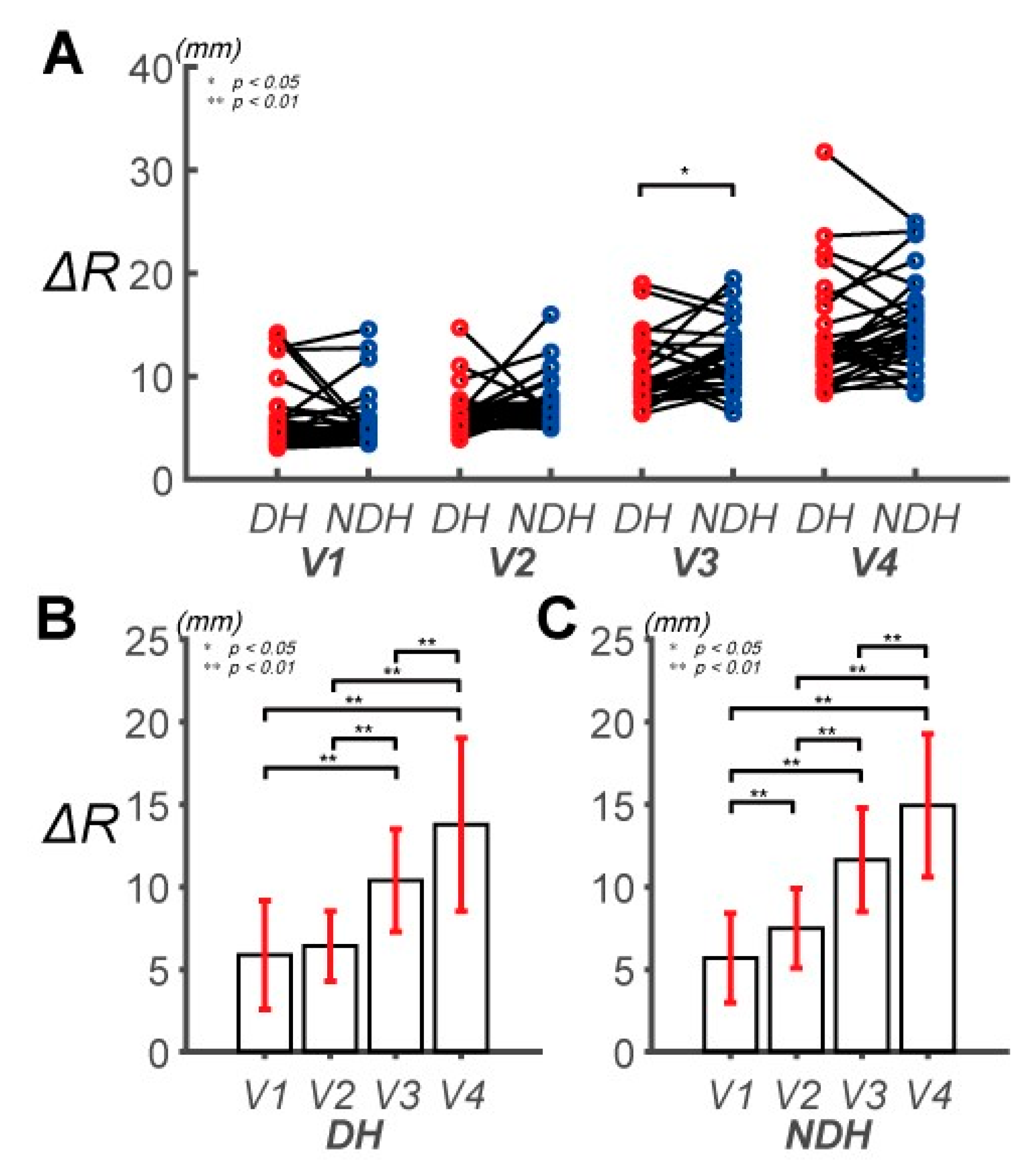
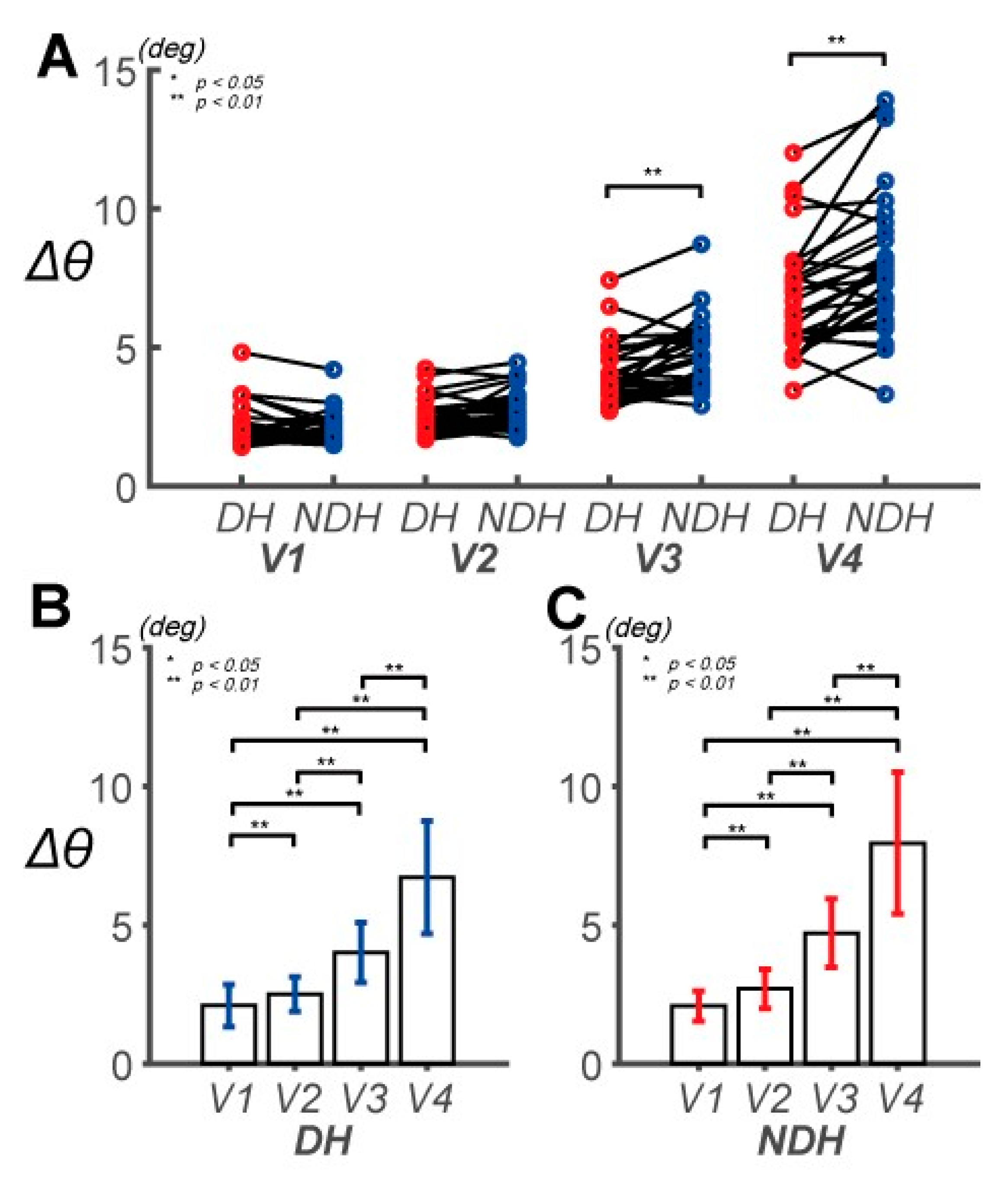
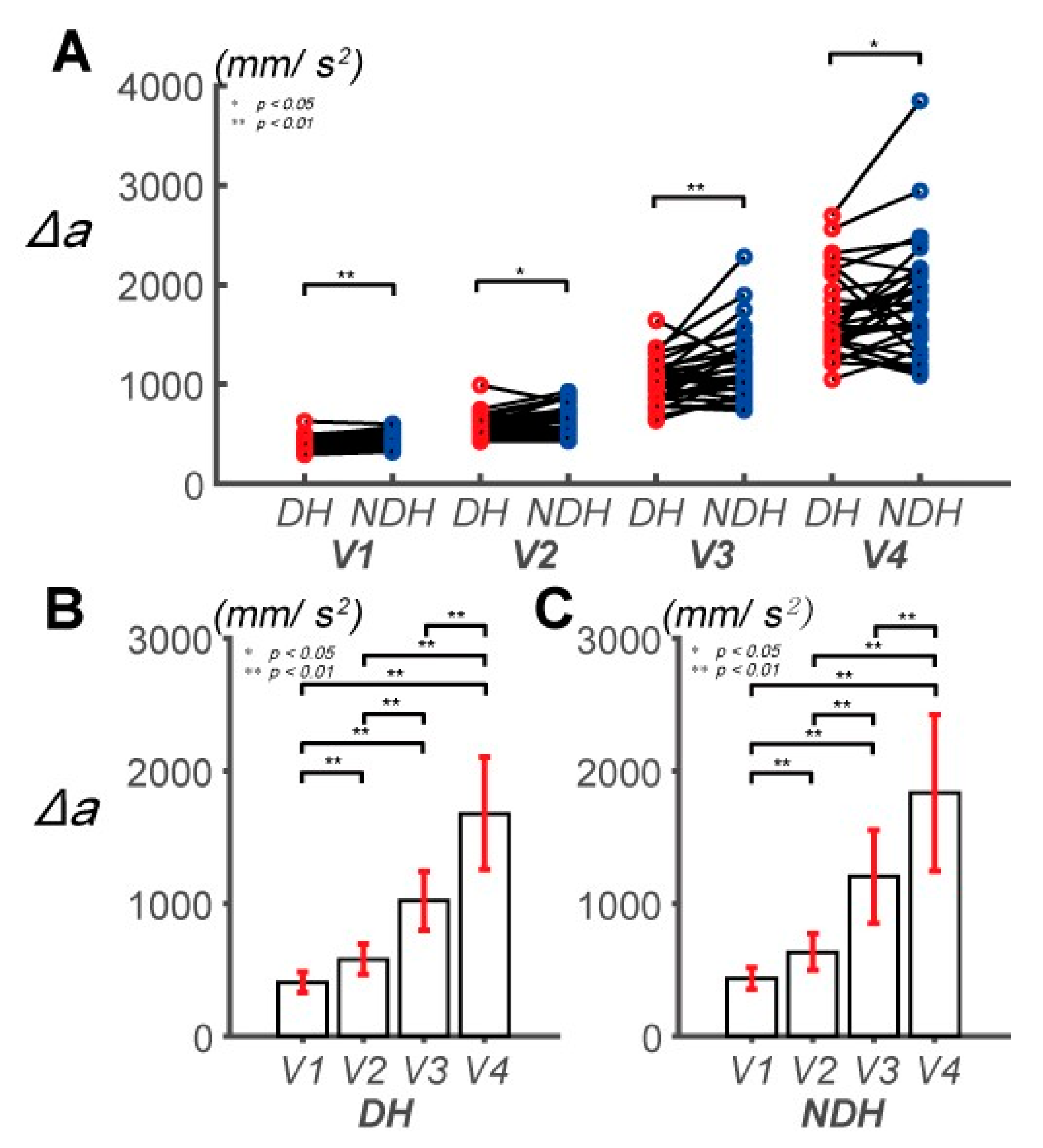
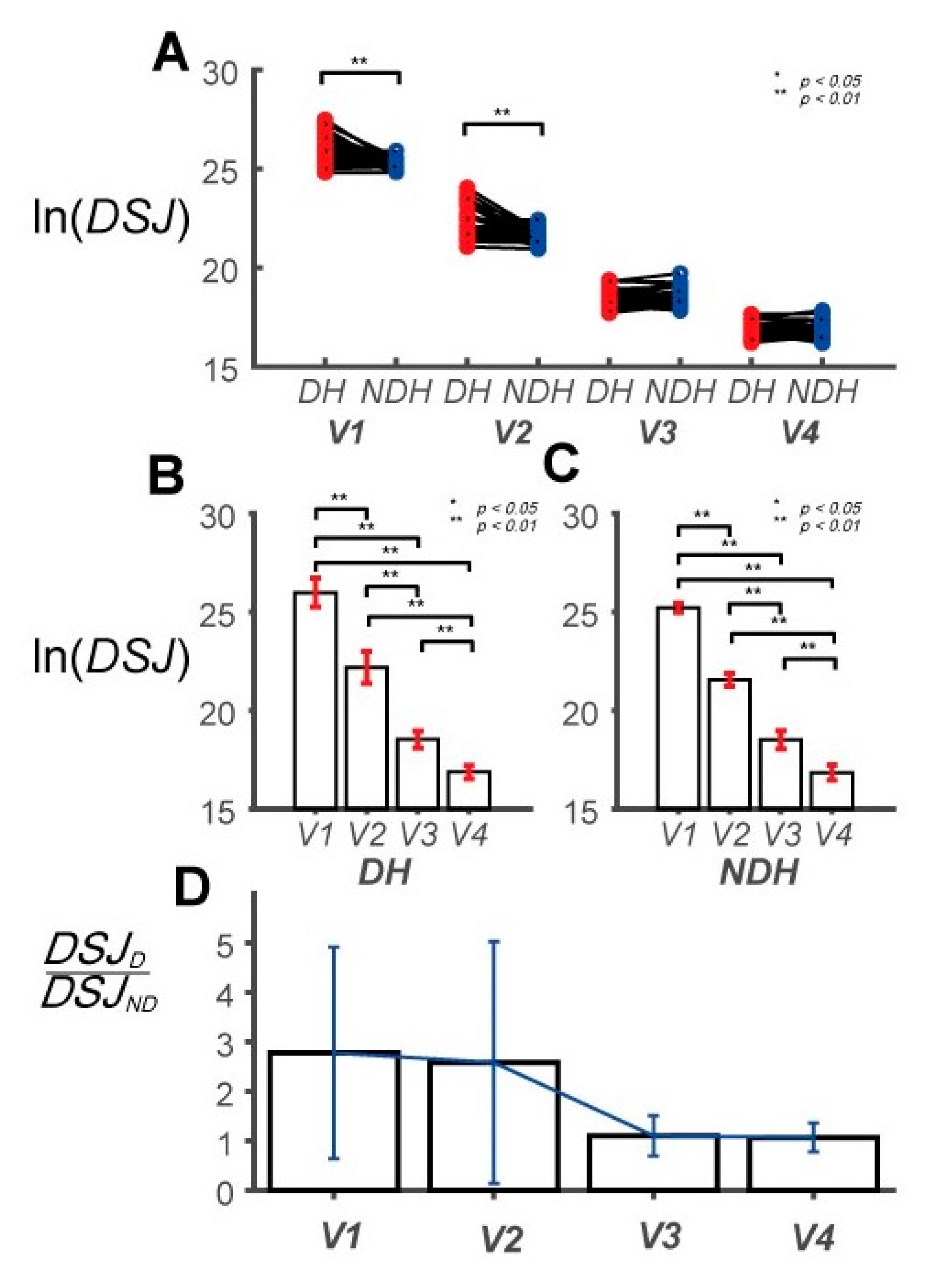
3.1. Differences in Control Outcomes between the DH and NDH in the Circular Tracking Movement
3.2. Differences in Circular Tracking Movement Based on ∆a and DSJ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berg, J.; Lottermoser, A.; Richter, C.; Reinhart, G. Human-Robot-Interaction for mobile industrial robot teams. Procedia CIRP 2019, 79, 614–619. [Google Scholar] [CrossRef]
- Ficuciello, F.; Villani, L.; Siciliano, B. Variable Impedance Control of Redundant Manipulators for Intuitive Human–Robot Physical Interaction. IEEE Trans. Robot. 2015, 31, 850–863. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L. Remote human–robot collaboration: A cyber–physical system application for hazard manufacturing environment. J. Manuf. Syst. 2020, 54, 24–34. [Google Scholar] [CrossRef]
- Mainprice, J.; Berenson, D. Human-robot collaborative manipulation planning using early prediction of human motion. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z. Computational principles of movement neuroscience. Nat. Neurosci. 2000, 3, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Yin, X. Adaptive finite-time prescribed performance control of switched nonlinear systems with unknown actuator dead-zone. Int. J. Syst. Sci. 2019, 51, 133–145. [Google Scholar] [CrossRef]
- De Santis, A.; Siciliano, B.; De Luca, A.; Bicchi, A. An atlas of physical human–robot interaction. Mech. Mach. Theory 2008, 43, 253–270. [Google Scholar] [CrossRef]
- Haddadin, S.; Albu-Schaeffer, A.; Hirzinger, G. Safety Evaluation of Physical Human-Robot Interaction via Crash-Testing. In Proceedings of the Robotics: Science and Systems III (Robotics: Science and Systems Foundation), Wessling, Germany, 27–30 June 2007. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, S.; Hu, B. A Practical Method to Improve Absolute Positioning Accuracy of Industrial Robot. J. Phys. Conf. Ser. 2019, 1453, 012121. [Google Scholar] [CrossRef]
- Wang, W.; Yu, G.; Xu, M.; Walker, D. Coordinate transformation of an industrial robot and its application in deterministic optical polishing. Opt. Eng. 2014, 53, 055102. [Google Scholar] [CrossRef]
- Bruttini, C.; Esposti, R.; Bolzoni, F.; Cavallari, P. Higher Precision in Pointing Movements of the Preferred vs. Non-Preferred Hand Is Associated with an Earlier Occurrence of Anticipatory Postural Adjustments. Front. Hum. Neurosci. 2016, 10, 365. [Google Scholar] [CrossRef]
- Roitman, A.V. Position, Direction of Movement, and Speed Tuning of Cerebellar Purkinje Cells during Circular Manual Tracking in Monkey. J. Neurosci. 2005, 25, 9244–9257. [Google Scholar] [CrossRef] [PubMed]
- Gollee, H.; Mamma, A.; Loram, I.D.; Gawthrop, P.J. Frequency-domain identification of the human controller. Biol. Cybern. 2012, 106, 359–372. [Google Scholar] [CrossRef]
- Georgopoulos, A.; Grillner, S. Visuo-motor coordination in reaching and locomotion. Science 1989, 245, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Naito, M.; Mizuno, N.; Ohshima, S. Framework for visual-feedback training based on a modified self-organizing map to imitate complex motion. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2019. [Google Scholar] [CrossRef]
- Kang, T.; He, J.; Tillery, S.I.H. Determining natural arm configuration along a reaching trajectory. Exp. Brain Res. 2005, 167, 352–361. [Google Scholar] [CrossRef]
- Hudson, T.E.; Wolfe, U.; Maloney, L.T. Speeded Reaching Movements around Invisible Obstacles. PLoS Comput. Biol. 2012, 8, e1002676. [Google Scholar] [CrossRef]
- Van den Berg, A.V. Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Exp. Brain Res. 1988, 72, 95–108. [Google Scholar] [CrossRef]
- Zajac, F.E. Muscle coordination of movement: A perspective. J. Biomech. 1993, 26, 109–124. [Google Scholar] [CrossRef]
- Wakeling, J.M.; Blake, O.M.; Chan, H.K. Muscle coordination is key to the power output and mechanical efficiency of limb movements. J. Exp. Biol. 2010, 213, 487–492. [Google Scholar] [CrossRef]
- Ishida, F.; Sawada, Y.E. Human hand moves proactively to the external stimulus: An evolutional strategy for minimizing transient error. Phys. Rev. Lett. 2004, 93, 168105. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.; Ghahramani, Z.; Jordan, M. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Miall, R.C.; Kawato, M. Internal models in the cerebellum. Trends Cogn. Sci. 1998, 2, 338–347. [Google Scholar] [CrossRef]
- Scott, S.H.; Cluff, T.; Lowrey, C.R.; Takei, T. Feedback-control during voluntary motor actions. Curr. Opin. Neurobiol. 2015, 33, 85–94. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kakei, S.; Kim, J. Motor control characteristics for circular tracking movements of human wrist. Adv. Robot. 2016, 31, 29–39. [Google Scholar] [CrossRef]
- Kambara, H.; Shin, D.; Koike, Y. A computational model for optimal muscle activity considering muscle viscoelasticity in wrist movements. J. Neurophysiol. 2013, 109, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Egger, S.W.; Remington, E.D.; Chang, C.-J.; Jazayeri, M. Internal models of sensorimotor integration regulate cortical dynamics. Nat. Neurosci. 2019, 22, 1871–1882. [Google Scholar] [CrossRef]
- Susilaradeya, D.; Xu, W.; Hall, T.M.; Galán, F.; Alter, K.; Jackson, A. Extrinsic and intrinsic dynamics in movement intermittency. eLife 2019, 8, e40145. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.; Sternad, D. Dynamic primitives of motor behavior. Biol. Cybern. 2012, 106, 727–739. [Google Scholar] [CrossRef]
- Nagaoka, M.; Tanaka, R. Contribution of kinesthesia on human visuo-motor elbow tracking movements. Neurosci. Lett. 1981, 26, 245–249. [Google Scholar] [CrossRef]
- Engel, K.C.; Soechting, J.F. Manual tracking in 2 dimensions. J. Neurophysiol. 2000, 83, 3483–3496. [Google Scholar] [CrossRef]
- Todorov, E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef]
- Sabes, P. The planning and control of reaching movements. Curr. Opin. Neurobiol. 2000, 10, 740–746. [Google Scholar] [CrossRef]
- Elliott, D.; Heath, M.; Binsted, G.; Ricker, K.L.; Roy, E.A.; Chua, R. Goal-directed aiming: Correcting a force-specification error with the right and left hands. J. Mot. Behav. 1999, 31, 309–324. [Google Scholar] [CrossRef]
- Beppu, H.; Suda, M.; Tanaka, R. Analysis of cerebellar motor disorders by visually guided elbow tracking movement. Brain 1984, 107, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Miall, R.C.; Weir, D.J.; Stein, J.F. Planning of movement parameters in a visuo-motor tracking task. Behav. Brain Res. 1988, 27, 1–8. [Google Scholar] [CrossRef]
- Miall, R.C.; Weir, D.J.; Stein, J.F. Manual tracking of visual targets by trained monkeys. Behav. Brain Res. 1986, 20, 185–201. [Google Scholar] [CrossRef]
- Hayashi, Y.; Tamura, Y.; Sase, K.; Sugawara, K.; Sawada, Y. Intermittently-visual tracking experiments reveal the roles of error-correction and predictive mechanisms in the human visual-motor control system. Trans. Soc. Instrum. Control Eng. 2010, 46, 391–400. [Google Scholar] [CrossRef]
- Roitman, A.V.; Massaquoi, S.G.; Takahashi, K.; Ebner, T.J. Kinematic analysis of manual tracking in monkeys: Characterization of movement intermittencies during a circular tracking task. J. Neurophysiol. 2004, 91, 901–911. [Google Scholar] [CrossRef]
- Doeringer, J.A.; Hogan, N. Intermittency in preplanned elbow movements persists in the absence of visual feedback. J. Neurophysiol. 1998, 80, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.M.; Ward, K.L.; Amazeen, E.L. Manual coordination with intermittent targets: Velocity information for prospective control. Acta Psychol. 2014, 149, 24–31. [Google Scholar] [CrossRef]
- Miall, R.C.; Weir, D.J.; Stein, J.F. Intermittency in human manual tracking tasks. J. Mot. Behav. 1993, 25, 53–63. [Google Scholar] [CrossRef]
- Roitman, A.V.; Pasalar, S.; Ebner, T.J. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp. Brain Res. 2008, 192, 241–251. [Google Scholar] [CrossRef]
- Loram, I.D.; Gawthrop, P.J.; Lakie, M. The frequency of human, manual adjustments in balancing an inverted pendulum is constrained by intrinsic physiological factors. J. Physiol. 2006, 577, 417–432. [Google Scholar] [CrossRef]
- Churchland, M.M.; Cunningham, J.P.; Kaufman, M.T.; Foster, J.D.; Nuyujukian, P.; Ryu, S.I.; Shenoy, K.V. Neural population dynamics during reaching. Nature 2012, 487, 51–56. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaguchi, Y. Periodic change in phase relationship between target and hand motion during visuo-manual tracking task: Behavioral evidence for intermittent control. Hum. Mov. Sci. 2014, 33, 211–226. [Google Scholar] [CrossRef]
- Wiberg, A.; Ng, M.; Al Omran, Y.; Alfaro-Almagro, F.; McCarthy, P.; Marchini, J.; Bennett, D.L.; Smith, S.; Douaud, G.; Furniss, D. Handedness, language areas and neuropsychiatric diseases: Insights from brain imaging and genetics. Brain 2019, 142, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Brasselet, R.; Zoia, S.; Bulgheroni, M.; Castiello, U. The origin of human handedness and its role in pre-birth motor control. Sci. Rep. 2017, 7, 16804. [Google Scholar] [CrossRef] [PubMed]
- Flowers, K. Handedness and controlled movement. Br. J. Psychol. 1975, 66, 39–52. [Google Scholar] [CrossRef]
- Todor, J.I.; Kyprie, P.M.; Price, H.L. Lateral asymmetries in arm, wrist and finger movements. Cortex 1982, 18, 515–523. [Google Scholar] [CrossRef]
- Hoffmann, E.R. Movement time of right- and left-handers using their preferred and non-preferred hands. Int. J. Ind. Ergon. 1997, 19, 49–57. [Google Scholar] [CrossRef]
- Simon, J.R.; De Crow, T.W.; Lincoln, R.S.; Smith, K.U. Effects of handedness on tracking accuracy. Mot. Ski. Res. Exch. 1952, 4, 53–57. [Google Scholar] [CrossRef]
- Mathew, J.; Sarlegna, F.R.; Bernier, P.-M.; Danion, F.R. Handedness matters for motor control but not for prediction. eNeuro 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Li, L.; Lee, J. Characteristic of motor control in 3d circular tracking movements during monocular vision. BioMed Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Yanagihara, N.; Li, L.; Kim, J. Development of a quantitative evaluation system for visuo-motor control in 3D VR space. Sci. Rep. 2018, 8, 13439. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Melendez-Calderon, A.; Roby-Brami, A.; Burdet, E. On the analysis of movement smoothness. J. Neuroeng. Rehabil. 2015, 12, 112. [Google Scholar] [CrossRef]
- Richardson, M.J.E.; Flash, T. Comparing smooth arm movements with the two-thirds power law and the related segmented-control hypothesis. J. Neurosci. 2002, 22, 8201–8211. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.H.; Patton, J.L.; Mussa-Ivaldi, F.A. Minimum jerk reaching movements of human arm with mechanical constraints at endpoint. Int. J. Comput. Syst. Signal 2006, 7, 41–50. [Google Scholar]
- Flash, T.; Hogan, N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985, 5, 1688–1703. [Google Scholar] [CrossRef]
- Takada, K.; Yashiro, K.; Takagi, M. Reliability and sensitivity of jerk-cost measurement for evaluating irregularity of chewing jaw movements. Physiol. Meas. 2006, 27, 609–622. [Google Scholar] [CrossRef]
- Yashiro, K.; Nakamura, T.; Mizumori, T.; Yatani, H.; Takada, K. Clinical validity of measuring jerk-cost of jaw movement during speech: Effect of mouthguard design on smoothness of jaw movements. In Proceedings of the SICE 2004 Annual Conference, Sapporo, Japan, 4–6 August 2004; Volume 1, pp. 93–96. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Tokuda, K.; Lee, B.; Shiihara, Y.; Takahashi, K.; Wada, N.; Shirakura, K.; Watanabe, H. Muscle activation patterns in acceleration-based phases during reach-to-grasp movement. J. Phys. Ther. Sci. 2016, 28, 3105–3111. [Google Scholar] [CrossRef]
- Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Biomechanics and muscle coordination of human walking. Gait Posture 2002, 16, 215–232. [Google Scholar] [CrossRef]
- Bagesteiro, L.B.; Sainburg, R.L. Handedness: Dominant arm advantages in control of limb dynamics. J. Neurophysiol. 2002, 88, 2408–2421. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Wang, J.; Liu, F.; Long, Z.; Wang, Y.; Iturria-Medina, Y.; Zhang, J.; Yu, C.; Chen, H. Handedness- and Hemisphere-Related Differences in Small-World Brain Networks: A Diffusion Tensor Imaging Tractography Study. Brain Connect. 2014, 4, 145–156. [Google Scholar] [CrossRef]
- Oguz, O.S.; Zhou, Z.; Glasauer, S.; Wollherr, D. An inverse optimal control approach to explain human arm reaching control based on multiple internal models. Sci. Rep. 2018, 8, 5583. [Google Scholar] [CrossRef]
- Miranda, J.G.V.; Daneault, J.F.; Vergara-Diaz, G.; Quixadá, A.P.; de Lemos Fonseca, M.; Vieira, J.P.B.C.; dos Santos, V.S.; da Figueiredo, T.C.; Pinto, E.B.; Peña, N.; et al. Complex upper-limb movements are generated by combining motor primitives that scale with the movement size. Sci. Rep. 2018, 8, 12918. [Google Scholar] [CrossRef]
- Cho, J.; Park, K.-S.; Kim, M.; Park, S.-H. Handedness and asymmetry of motor skill learning in right-handers. J. Clin. Neurol. 2006, 2, 113. [Google Scholar] [CrossRef][Green Version]
- Engelbrecht, S.E. Minimum principles in motor control. J. Math. Psychol. 2001, 45, 497–542. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Choi, W.; Lee, G.; Park, W.; Kim, J. Analysis of Visuo Motor Control between Dominant Hand and Non-Dominant Hand for Effective Human-Robot Collaboration. Sensors 2020, 20, 6368. https://doi.org/10.3390/s20216368
Jo H, Choi W, Lee G, Park W, Kim J. Analysis of Visuo Motor Control between Dominant Hand and Non-Dominant Hand for Effective Human-Robot Collaboration. Sensors. 2020; 20(21):6368. https://doi.org/10.3390/s20216368
Chicago/Turabian StyleJo, Hanjin, Woong Choi, Geonhui Lee, Wookhyun Park, and Jaehyo Kim. 2020. "Analysis of Visuo Motor Control between Dominant Hand and Non-Dominant Hand for Effective Human-Robot Collaboration" Sensors 20, no. 21: 6368. https://doi.org/10.3390/s20216368
APA StyleJo, H., Choi, W., Lee, G., Park, W., & Kim, J. (2020). Analysis of Visuo Motor Control between Dominant Hand and Non-Dominant Hand for Effective Human-Robot Collaboration. Sensors, 20(21), 6368. https://doi.org/10.3390/s20216368






