Microwave Bone Imaging: A Preliminary Investigation on Numerical Bone Phantoms for Bone Health Monitoring
Abstract
1. Introduction
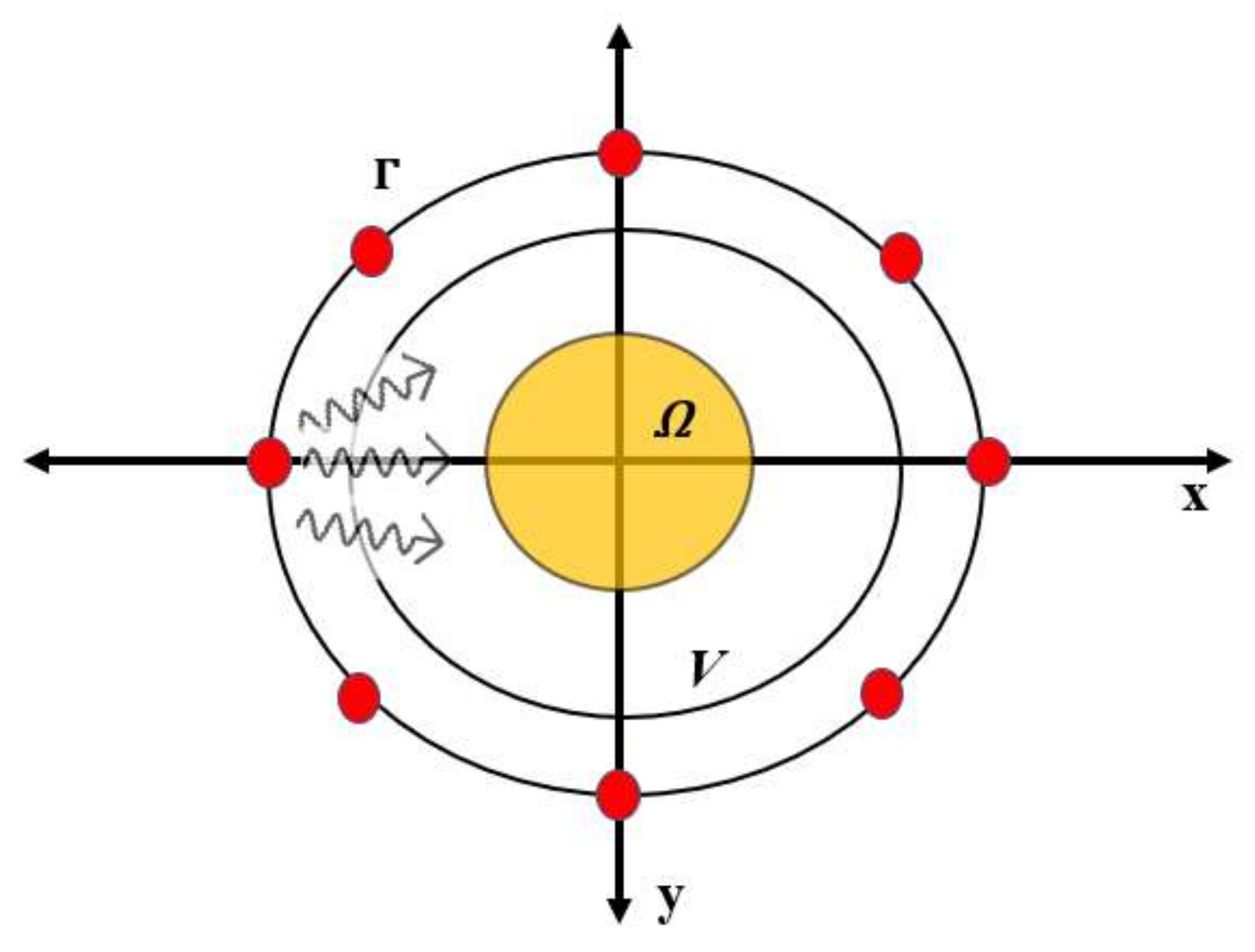
2. Mathematical Formulation
2.1. DBIM Formulation
2.2. IMATCS Algorithm
| Algorithm 1-IMATCS |
| 1: The measurement matrix updates at each DBIM iteration 2: Input: The measurement matrix The residual measurement data vector 3: Output: The contrast function 4: Method: -IMATCS for j = 1: itrmax Thresh end for End method 5: Update the contrast function based on 6: Return to 1 |
2.3. Parameter Selection of IMATCS Algorithm
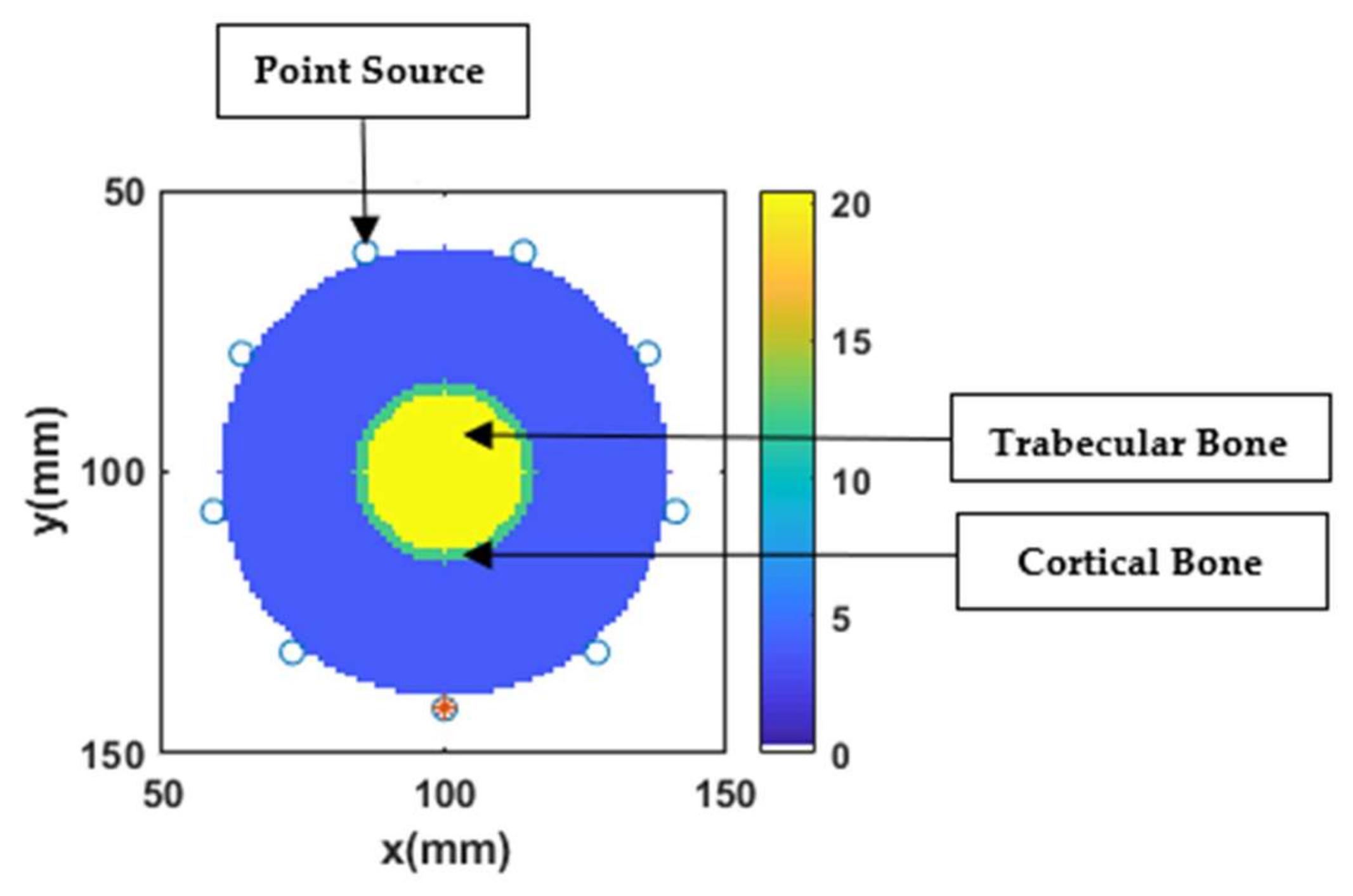
2.4. Numerical Bone Phantoms
3. Results and Discussion
3.1. Simulation Testbed
3.2. Performance Metrics
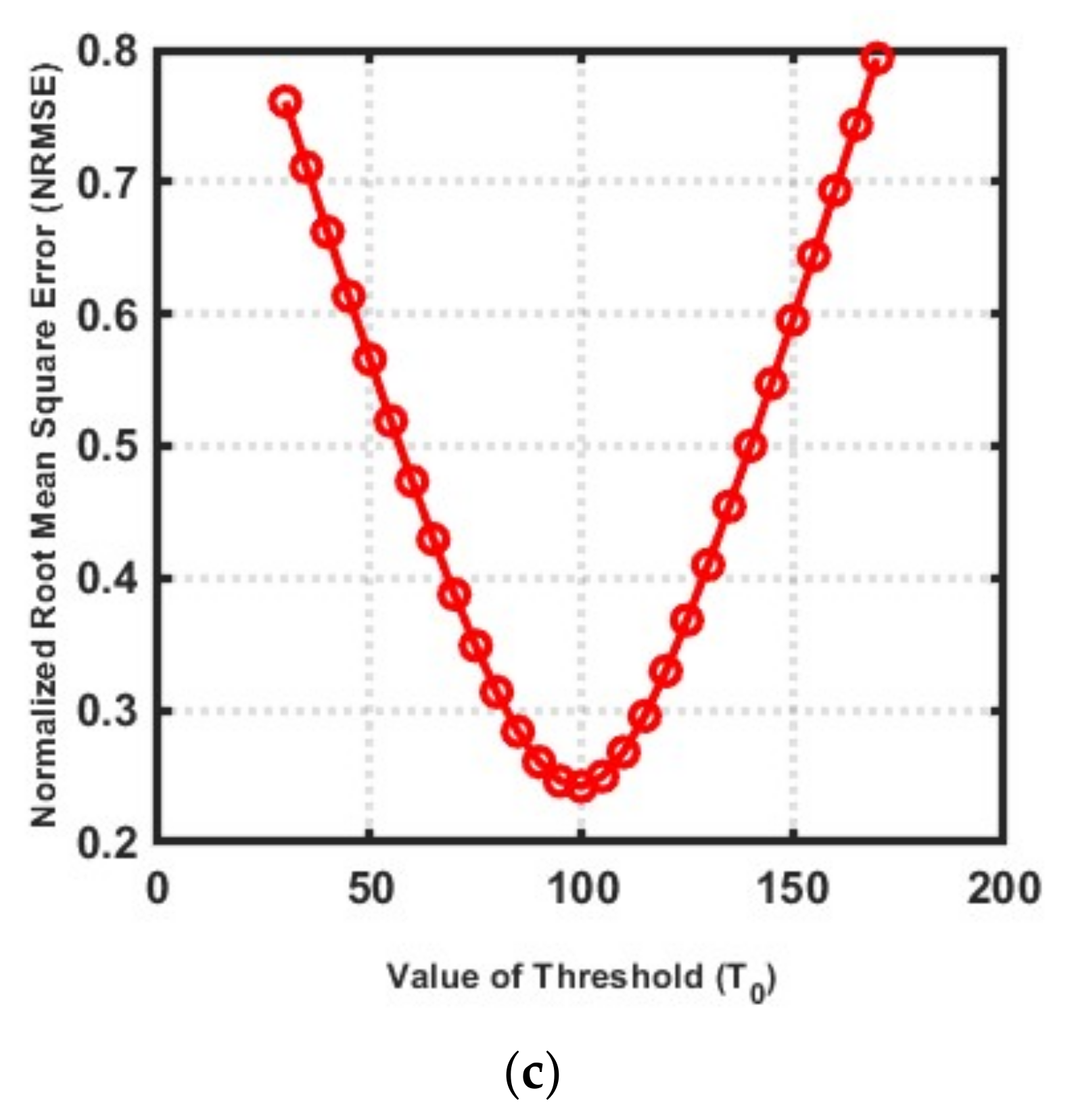
3.3. Choice of Number of IMATCS iterations, Number of DBIM iterations, and Threshold ()
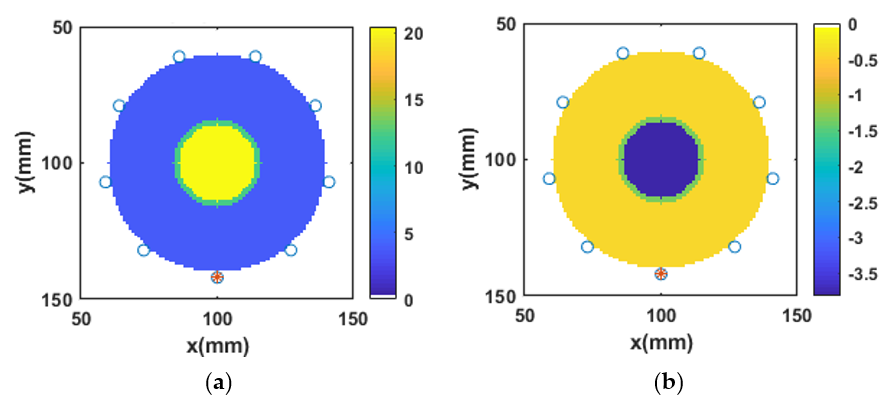
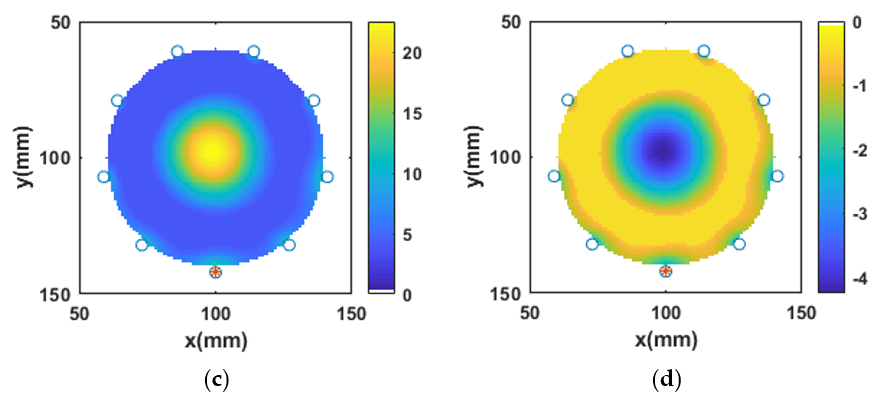
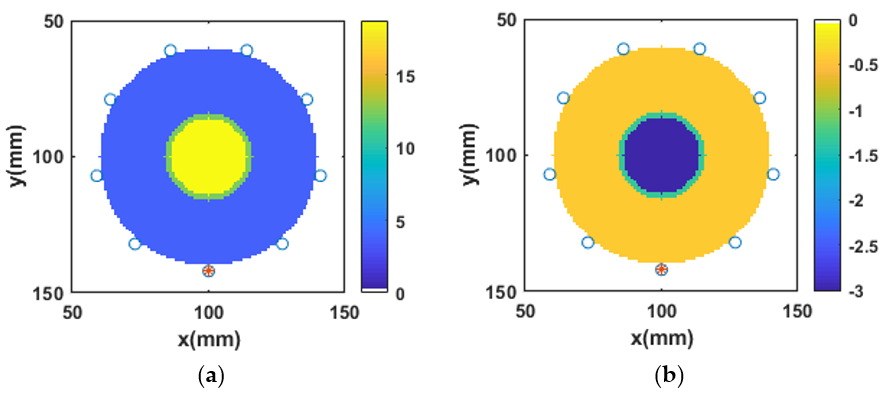
3.4. Reconstruction of Numerical Bone Phantom 1 (P1)
3.5. Reconstruction of Numerical Bone Phantom 2, 3, and 4 (P2, P3, P4)
3.6. Reconstruction of Numerical Bone Phantom 5, 6, and 7 (P5, P6, P7)
3.7. Robustness of -IMATCS for Reconstruction of Bone Phantoms P1, P2, P3, P4, P5, P6, and P7
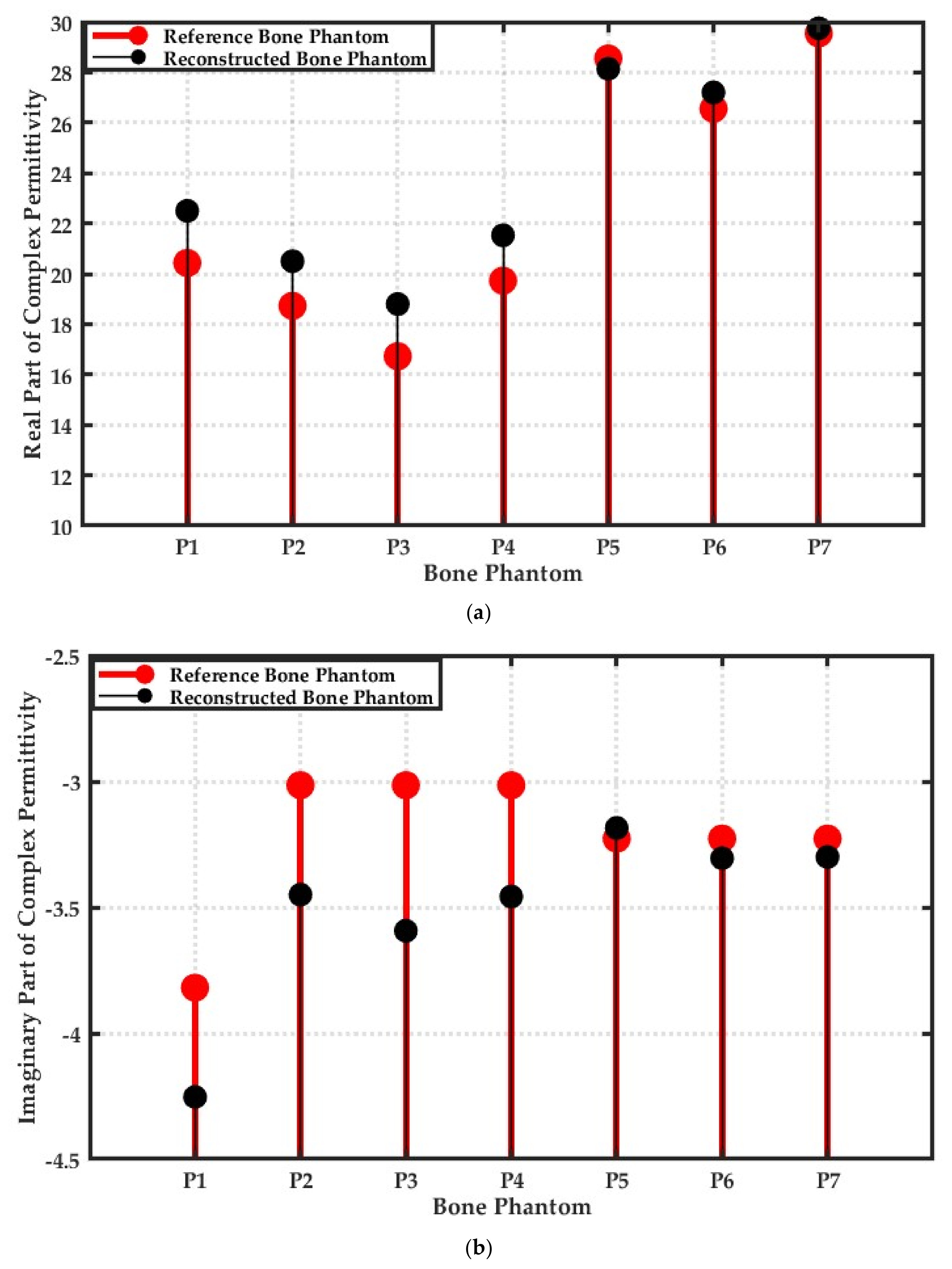
3.8. Relative Error between Reference and Reconstructed Numerical Bone Phantoms
3.9. Impact of Signal-to-Noise Ratio (SNR) on Reconstructed Numerical Bone Phantoms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scapaticci, R.; Kosmas, P.; Crocco, L. Wavelet-based regularization for robust microwave imaging in medical applications. IEEE Trans. Biomed. Eng. 2015, 62, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Elahi, M.A.; Shahzad, A.; Porter, E.; McDermott, B.; O’Halloran, M. Dielectric properties of bones for the monitoring of osteoporosis. Med. Biol. Eng. Comput. 2018. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.A.; O’Loughlin, D.; Lavoie, B.R.; Glavin, M.; Jones, E.; Fear, E.C.; O’Halloran, M. Evaluation of image reconstruction algorithms for confocal microwave imaging: Application to patient data. Sensors (Switzerland) 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, D.O.; Oliveira, B.L.; Santorelli, A.; Porter, E.; Glavin, M.; Jones, E.; Popovi, M.; Halloran, M.O. Sensitivity and specificity estimation using patient-specific microwave imaging in diverse experimental breast phantoms. IEEE Trans. Med. Imaging 2019, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Coates, M.; Popović, M. An early clinical study of time-domain microwave radar for breast health monitoring. IEEE Trans. Biomed. Eng. 2016, 63, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093–6115. [Google Scholar] [CrossRef] [PubMed]
- Bourqui, J.; Fear, E.C. System for bulk dielectric permittivity estimation of breast tissues at microwave frequencies. IEEE Trans. Microw. Theory Tech. 2016, 64, 3001–3009. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Oliveira, B.L.; Adnan Elahi, M.; Glavin, M.; Jones, E.; Popović, M.; Halloran, M.O. Parameter search algorithms for microwave radar-based breast imaging: Focal quality metrics as fitness functions. Sensors (Switzerland) 2017, 17, 1–20. [Google Scholar] [CrossRef]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A feasibility study on microwave imaging for brain stroke monitoring. Prog. Electromagn. Res. 2012, 40, 305–324. [Google Scholar] [CrossRef]
- Vasquez, J.A.T.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Rodriguez-Duarte, D.O.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; et al. A prototype microwave system for 3d brain stroke imaging. Sensors (Switzerland) 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Meaney, P.M.; Goodwin, D.; Golnabi, A.H.; Zhou, T.; Pallone, M.; Geimer, S.D.; Burke, G.; Paulsen, K.D. Clinical microwave tomographic imaging of the calcaneus: A first-in-human case study of two subjects. IEEE Trans. Biomed. Eng. 2012, 59, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Shahzad, A.; Farina, L.; Parle, E.; McNamara, L.; O’Halloran, M.; Elahi, M.A. Dielectric characterization of diseased human trabecular bones at microwave frequency. Med. Eng. Phys. 2020, 78, 21–28. [Google Scholar] [CrossRef]
- Makarov, S.N.; Noetscher, G.M.; Arum, S.; Rabiner, R.; Nazarian, A. Concept of a radiofrequency device for osteopenia / osteoporosis screening. Sci. Rep. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lochmüller, E.-M.; Müller, R.; Kuhn, V.; Lill, C.A.; Eckstein, F. Can novel clinical densitometric techniques replace or improve Dxa in predicting bone strength in osteoporosis at the hip and other skeletal sites? J. Bone Miner. Res. 2003, 18, 906–912. [Google Scholar] [CrossRef]
- Amin, B.; Elahi, M.A.; Shahzad, A.; Parle, E.; McNamara, L.; Orhalloran, M. An insight into bone dielectric properties variation: A foundation for electromagnetic medical devices. In Proceedings of the EMF-Med 2018—1st EMF-Med World Conference on Biomedical Applications of Electromagnetic Fields. COST EMF-MED Final Event with 6th MCM, Split, Croatia, 10–13 September 2018; pp. 3–4. [Google Scholar] [CrossRef]
- Bourqui, J.; Sill, J.M.; Fear, E.C. A prototype system for measuring microwave frequency reflections from the breast. Int. J. Biomed. Imaging 2012, 2012. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Loughlin, D.O.; O’Halloran, M.; Porter, E.; Glavin, M.; Jones, E. Microwave Breast Imaging: Experimental tumour phantoms for the evaluation of new breast cancer diagnosis systems. Biomed. Phys. Eng. Express 2018, 4, 25036. [Google Scholar] [CrossRef]
- Shahzad, A.; O’Halloran, M.; Jones, E.; Glavin, M. A multistage selective weighting method for improved microwave breast tomography. Comput. Med. Imaging Graph. 2016, 54, 6–15. [Google Scholar] [CrossRef]
- Shahzad, A.; O’Halloran, M.; Glavin, M.; Jones, E. A novel optimized parallelization strategy to accelerate microwave tomography for breast cancer screening. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2456–2459. [Google Scholar]
- Scapaticci, R.; Bjelogrlic, M.; Vasquez, J.A.T.; Vipiana, F.; Mattes, M.; Crocco, L. Microwave technology for brain imaging and monitoring: Physical foundations, potential and limitations. In Emerging Electromagnetic Technologies for Brain Diseases Diagnostics, Monitoring and Therapy; Crocco, L., Karanasiou, I., James, M., Conceição, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 7–35. [Google Scholar]
- Takenaka, T.; Jia, H.; Tanaka, T. Microwave imaging of electrical property distributions by a forward-backward time-stepping method. J. Electromagn. Waves Appl. 2000, 14, 1609–1626. [Google Scholar] [CrossRef]
- Fang, Q.; Meaney, P.M.; Paulsen, K.D. Microwave image reconstruction of tissue property dispersion characteristics utilizing multiple-frequency information. IEEE Trans. Microw. Theory Techn. 2004, 52, 1866–1875. [Google Scholar] [CrossRef]
- Fhager, A.; Gustafsson, M.; Nordebo, S. Image reconstruction in microwave tomography using a dielectric debye model. IEEE Trans. Biomed. Eng. 2012, 59, 156–166. [Google Scholar] [CrossRef]
- Gilmore, C.; Mojabi, P.; LoVetri, J. Comparison of an enhanced distorted born iterative method and the multiplicative-regularized contrast source inversion method. IEEE Trans. Antennas Propag. 2009, 57, 2341–2351. [Google Scholar] [CrossRef]
- Amin, B.; Elahi, M.A.; Shahzad, A.; Porter, E.; O’Halloran, M. A review of the dielectric properties of the bone for low frequency medical technologies. Biomed. Phys. Eng. Express 2019, 5, 022001. [Google Scholar] [CrossRef]
- Miao, Z.; Kosmas, P. Multiple-Frequency DBIM-TwIST algorithm for microwave breast imaging. IEEE Trans. Antennas Propag. 2017, 65, 2507–2516. [Google Scholar] [CrossRef]
- Neira, L.M.; Van Veen, B.D.; Hagness, S.C. High-resolution microwave breast imaging using a 3-D inverse scattering algorithm with a variable-strength spatial prior constraint. IEEE Trans. Antennas Propag. 2017, 65, 6002–6014. [Google Scholar] [CrossRef]
- Wang, Y.M. Reconstruction of two-dimensional permittivity distribution using the distorted born iterative method. IEEE Trans. Med. Imaging 1990, 9, 218–225. [Google Scholar]
- Ambrosanio, M.; Kosmas, P.; Member, S.; Pascazio, V.; Member, S. A multithreshold iterative DBIM-based algorithm for the imaging of heterogeneous breast tissues. IEEE Trans. Biomed. Eng. 2019, 66, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Azghani, M.; Kosmas, P.; Marvasti, F. Microwave medical imaging based on sparsity and an iterative method with adaptive thresholding. IEEE Trans. Med. Imaging 2014, 34, 357–365. [Google Scholar] [CrossRef]
- Blumensath, T.; Davies, M.E. Iterative thresholding for saparse approximations. J. Fourier Anal. Appl. 2008, 14, 629–654. [Google Scholar] [CrossRef]
- Beck, A.; Teboulle, M. A fast iterative shrinkage-thresholding algorithm. Soc. Ind. Appl. Math. J. Imaging Sci. 2009, 2, 183–202. [Google Scholar] [CrossRef]
- Meaney, P.M.; Zhou, T.; Goodwin, D.; Golnabi, A.; Attardo, E.A.; Paulsen, K.D. Bone dielectric property variation as a function of mineralization at microwave frequencies. Int. J. Biomed. Imaging 2012, 2012. [Google Scholar] [CrossRef]
- Zhurbenko, V. Challenges in the design of microwave imaging systems for breast cancer detection. Adv. Electr. Comput. Eng. 2011, 11, 91–96. [Google Scholar] [CrossRef]
- Catapano, I.; Di Donato, L.; Crocco, L.; Bucci, O.M.; Morabito, A.F.; Isernia, T.; Massa, R. On quantitative microwave tomography of female breast. Prog. Electromagn. Res. 2009, 97, 75–93. [Google Scholar] [CrossRef]
- Gilmore, C.; Zakaria, A.; Pistorius, S.; Lovetri, J. Microwave imaging of human forearms: Pilot study and image enhancement. Int. J. Biomed. Imaging 2013, 2013. [Google Scholar] [CrossRef]
- Amin, B.; Kelly, D.; Shahzad, A.; O’Halloran, M.; Elahi, M.A. Microwave calcaneus phantom for bone imaging applications. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; pp. 1–5. [Google Scholar]
- Salahuddin, S.; Porter, E.; Krewer, F.; O’Halloran, M. Optimised analytical models of the dielectric properties of biological tissue. Med. Eng. Phys. 2017, 43, 103–111. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271. [Google Scholar] [CrossRef]
- Topoliński, T.; Mazurkiewicz, A.; Jung, S.; Cichański, A.; Nowicki, K. Microarchitecture parameters describe bone structure and its strength better than BMD. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef]
- Van Der Linden, J.C.; Weinans, H. Effects of microarchitecture on bone strength. Curr. Osteoporos. Rep. 2007, 5, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.D.; Kosmas, P.; Hagness, S.C.; van Veen, B.D. Three-dimensional microwave imaging of realistic numerical breast phantoms via a multiple-frequency inverse scattering technique. Med. Phys. 2010, 37, 4210–4226. [Google Scholar] [CrossRef]
- Lazebnik, M.; Okoniewski, M.; Booske, J.H.; Hagness, S.C. Highly accurate debye models for normal and malignant breast tissue dielectric properties at microwave frequencies. IEEE Microw. Wirel. Compon. Lett. 2007, 17, 822–824. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed]





| PL | OBTL | IBTL |
|---|---|---|
| P1 | Cortical Bone | Trabecular Bone |
| P2 | Cortical Bone | Osteoporotic Bone Mean |
| P3 | Cortical Bone | Osteoporotic Bone Lower Bound |
| P4 | Cortical Bone | Osteoporotic Bone Upper Bound |
| P5 | Cortical Bone | Osteoarthritis Bone Mean |
| P6 | Cortical Bone | Osteoarthritis Bone Lower Bound |
| P7 | Cortical Bone | Osteoarthritis Bone Upper Bound |
| Tissue | |||||
|---|---|---|---|---|---|
| Cortical Bone | 8.75 | 4 | 0.01 | 12.39 | 0.0736 |
| Trabecular Bone | 14 | 7 | 0.1 | 20.43 | 0.2125 |
| Osteoporotic Bone Mean | 16 | 3 | 0.12 | 18.73 | 0.1677 |
| Osteoporotic Bone Lower Bound | 14 | 3 | 0.12 | 16.73 | 0.1677 |
| Osteoporotic Bone Upper Bound | 17 | 3 | 0.12 | 19.73 | 0.1677 |
| Osteoarthritis Bone Mean | 24 | 5 | 0.1 | 28.55 | 0.1795 |
| Osteoarthritis Bone Lower Bound | 22 | 5 | 0.1 | 26.55 | 0.1795 |
| Osteoarthritis Bone Upper Bound | 25 | 5 | 0.1 | 29.55 | 0.1795 |
| Phantom | NRMSE | |
|---|---|---|
| P1 | 0.212 | 0.253 |
| P2 | 0.239 | 0.228 |
| P3 | 0.249 | 0.228 |
| P4 | 0.226 | 0.222 |
| P5 | 0.246 | 0.252 |
| P6 | 0.228 | 0.242 |
| P7 | 0.242 | 0.245 |
| Phantom | SSIM | |
|---|---|---|
| P1 | 0.973 | 0.995 |
| P2 | 0.968 | 0.997 |
| P3 | 0.959 | 0.997 |
| P4 | 0.971 | 0.997 |
| P5 | 0.959 | 0.993 |
| P6 | 0.966 | 0.994 |
| P7 | 0.953 | 0.992 |
| SNR (dB) | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
| 20 | 0.224 | 0.239 | 0.249 | 0.228 | 0.247 | 0.228 | 0.244 |
| 30 | 0.220 | 0.238 | 0.249 | 0.226 | 0.245 | 0.229 | 0.243 |
| 40 | 0.220 | 0.239 | 0.249 | 0.226 | 0.245 | 0.229 | 0.242 |
| 50 | 0.220 | 0.239 | 0.249 | 0.226 | 0.247 | 0.228 | 0.243 |
| 60 | 0.220 | 0.239 | 0.249 | 0.226 | 0.246 | 0.228 | 0.243 |
| SNR (dB) | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
| 20 | 0.256 | 0.229 | 0.228 | 0.223 | 0.253 | 0.241 | 0.247 |
| 30 | 0.254 | 0.229 | 0.229 | 0.222 | 0.252 | 0.243 | 0.246 |
| 40 | 0.253 | 0.228 | 0.228 | 0.221 | 0.252 | 0.242 | 0.245 |
| 50 | 0.253 | 0.228 | 0.228 | 0.222 | 0.252 | 0.242 | 0.245 |
| 60 | 0.253 | 0.228 | 0.228 | 0.222 | 0.252 | 0.242 | 0.245 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, B.; Shahzad, A.; O’Halloran, M.; Elahi, M.A. Microwave Bone Imaging: A Preliminary Investigation on Numerical Bone Phantoms for Bone Health Monitoring. Sensors 2020, 20, 6320. https://doi.org/10.3390/s20216320
Amin B, Shahzad A, O’Halloran M, Elahi MA. Microwave Bone Imaging: A Preliminary Investigation on Numerical Bone Phantoms for Bone Health Monitoring. Sensors. 2020; 20(21):6320. https://doi.org/10.3390/s20216320
Chicago/Turabian StyleAmin, Bilal, Atif Shahzad, Martin O’Halloran, and Muhammad Adnan Elahi. 2020. "Microwave Bone Imaging: A Preliminary Investigation on Numerical Bone Phantoms for Bone Health Monitoring" Sensors 20, no. 21: 6320. https://doi.org/10.3390/s20216320
APA StyleAmin, B., Shahzad, A., O’Halloran, M., & Elahi, M. A. (2020). Microwave Bone Imaging: A Preliminary Investigation on Numerical Bone Phantoms for Bone Health Monitoring. Sensors, 20(21), 6320. https://doi.org/10.3390/s20216320





