Quality Assessment during Incubation Using Image Processing
Abstract
:1. Introduction
2. Materials and Methods
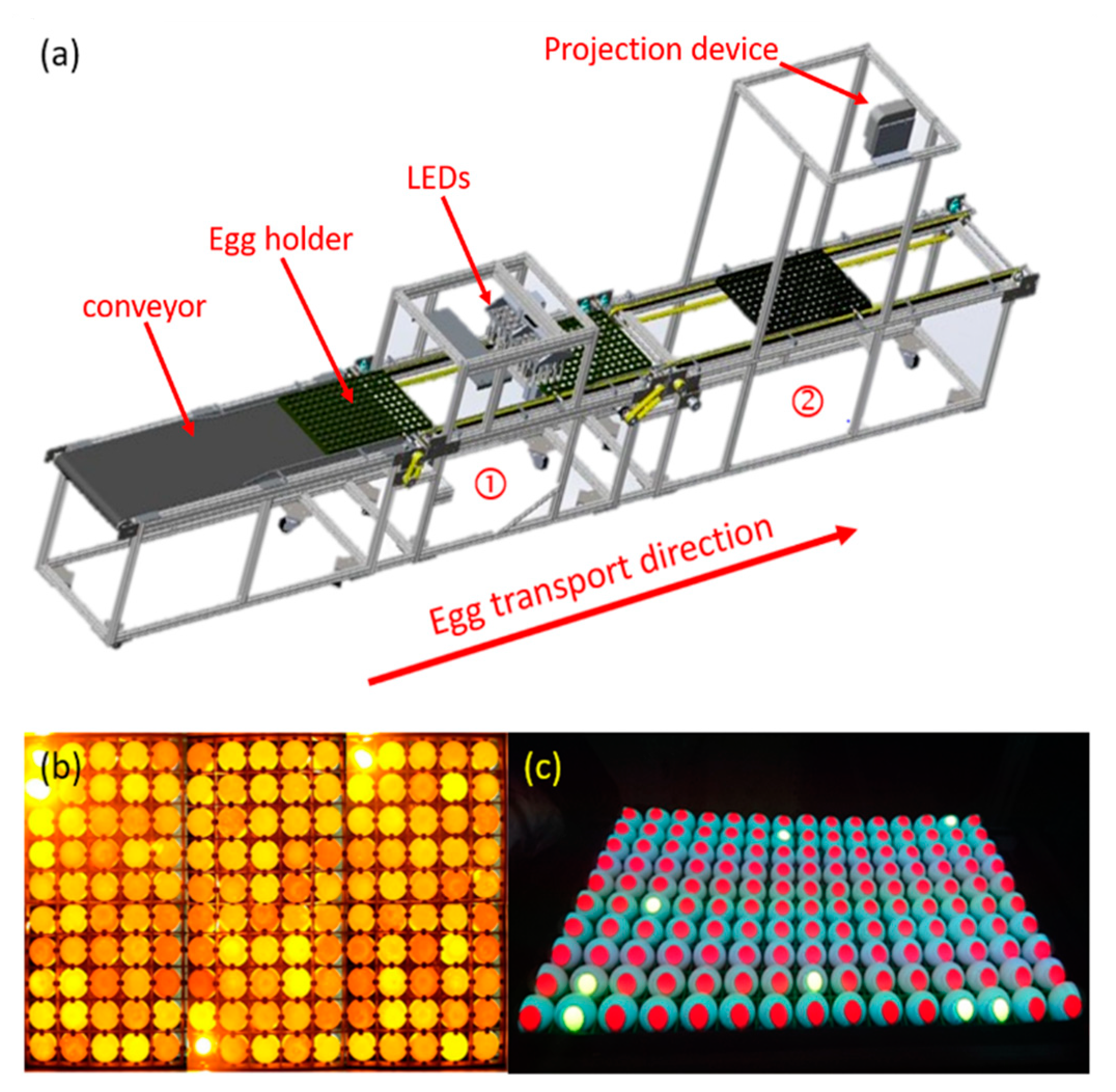
2.1. Sample and Experimental Equipment
2.2. Experimental Apparatus and Measuring Methods
2.2.1. Experiment 1
2.2.2. Experiment 2
2.2.3. Digital Image Processing
2.2.4. Statistical Analysis
3. Results and Discussion
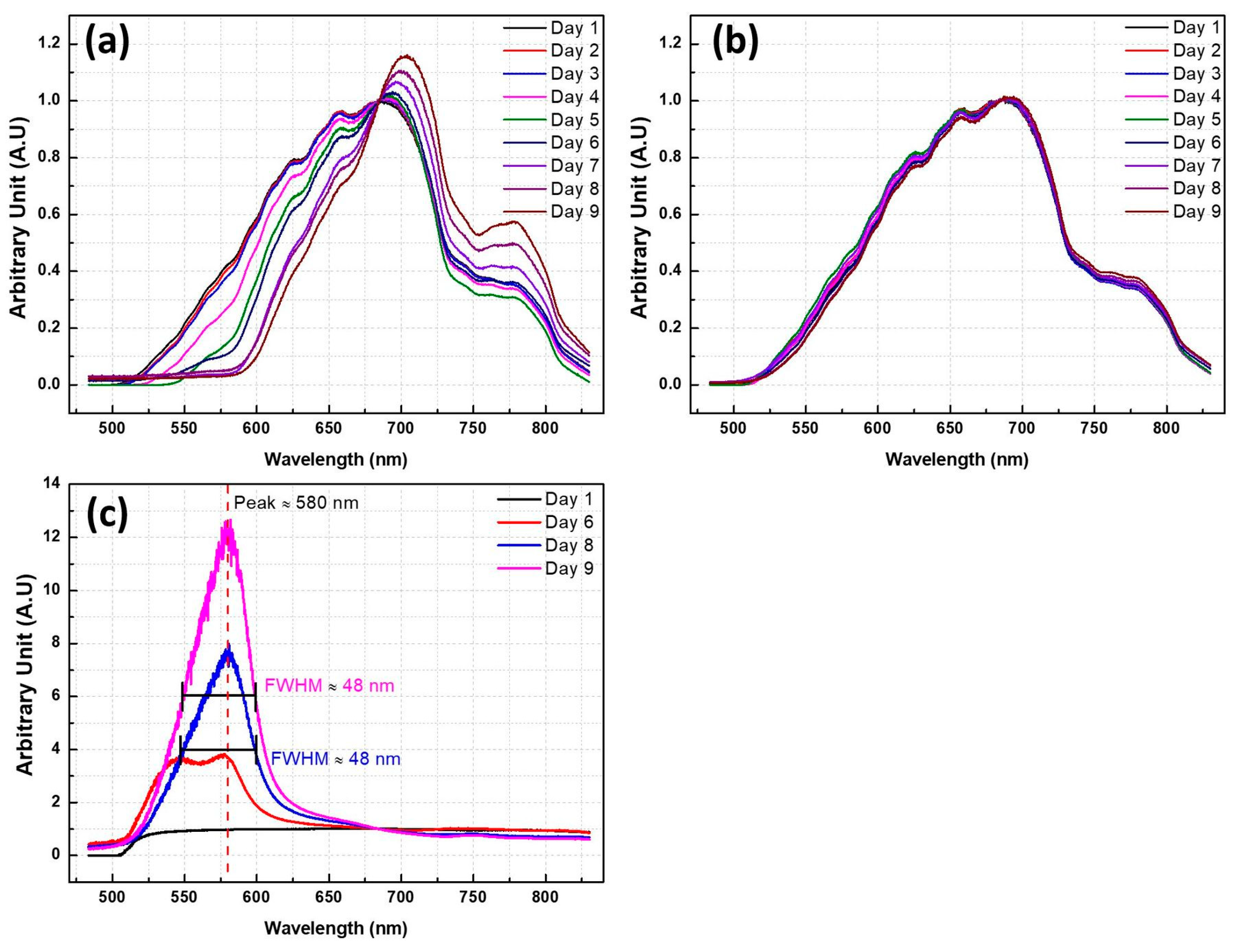
3.1. Experiment 1
3.2. Experiment 2
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajão, D.S.; Pérez, D.R. Universal Vaccines and Vaccine Platforms to Protect against Influenza Viruses in Humans and Agriculture. Front. Microbiol. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef]
- Kimura, K.; Nakano, K.; Ohashi, S.; Takizawa, K.; Nakano, T. LED measurement for development of a non-destructive detector of unsuitable chicken eggs in influenza vaccine production. Biosyst. Eng. 2015, 134, 68–73. [Google Scholar] [CrossRef]
- Liu, L.; Ngadi, M.O. Detecting Fertility and Early Embryo Development of Chicken Eggs Using Near-Infrared Hyperspectral Imaging. Food Bioprocess. Technol. 2013, 6, 2503–2513. [Google Scholar] [CrossRef]
- Coucke, P.M.; Room, G.M.; Decuypere, E.M.; de Baerdemaeker, J.G. Monitoring embryo development in chicken eggs using acoustic resonance analysis. Biotechnol. Prog. 1997, 13, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Farajzadeh, N. A Machine Vision System for Detecting Fertile Eggs in the Incubation Industry. Int. J. Comput. Intell. Syst. 2016, 9, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Liu, J.; Huang, K.; Kuang, J.; Liu, D. Evaluation of moisture content in processed apple chips using NIRS and wavelength selection techniques. Infrared Phys. Technol. 2019, 98, 305–310. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Zhao, C.; Zhang, B. A comparative study for the quantitative determination of soluble solids content, pH and firmness of pears by Vis/NIR spectroscopy. J. Food Eng. 2013, 116, 324–332. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, Y. Nondestructive measurement of internal quality in pear using genetic algorithms and FT-NIR spectroscopy. J. Food Eng. 2008, 84, 206–213. [Google Scholar] [CrossRef]
- Giunchi, A.; Berardinelli, A.; Ragni, L.; Fabbri, A.; Silaghi, F.A. Non-destructive freshness assessment of shell eggs using FT-NIR spectroscopy. J. Food Eng. 2008, 89, 142–148. [Google Scholar] [CrossRef]
- Abdel-Nour, N.; Ngadi, M.; Prasher, S.; Karimi, Y. Prediction of Egg Freshness and Albumen Quality Using Visible/Near Infrared Spectroscopy. Food Bioprocess. Technol. 2011, 4, 731–736. [Google Scholar] [CrossRef]
- Bamelis, F.R.; Tona, K.; De Baerdemaeker, J.G.; Decuypere, E.M. Detection of early embryonic development in chicken eggs using visible light transmission. Br. Poultry Sci. 2002, 43, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Dong, X.; Li, Y.; Peng, Y.; Chao, K.; Gao, C.; Tang, X. Identification of unfertilized duck eggs before hatching using visible/near infrared transmittance spectroscopy. Comput. Electron. Agric. 2019, 157, 471–478. [Google Scholar] [CrossRef]
- Smith, D.P. Fertility and Embryo Development of Broiler Hatching Eggs Evaluated with a Hyperspectral Imaging and Predictive Modeling System. Int. J. Poultry Sci. 2008, 7, 1001. [Google Scholar]
- Zhu, Z.H.; Liu, T.; Xie, D.J.; Wang, Q.H.; Ma, M.H. Nondestructive detection of infertile hatching eggs based on spectral and imaging information. Int. J. Agric. Biol. Eng. 2015, 8, 69–76. [Google Scholar]
- Ghaderi, M.; Banakar, A.; Masoudi, A.A. Using dielectric properties and intelligent methods in separating of hatching eggs during incubation. Measurement 2018, 114, 191–194. [Google Scholar] [CrossRef]
- Kemps, B.J.; De Ketelaere, B.; Bamelis, F.R.; Decuypere, E.M.; De Baerdemaeker, J.G. Vibration analysis on incubating eggs and its relation to embryonic development. Biotechnol. Prog. 2003, 19, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-W.; Jung, S.-Y.; Lai, C.-C.; Tsai, S.-Y.; Jeng, C.-C. Transmission spectral analysis models for the assessment of white-shell eggs and brown-shell eggs freshness. J. Supercomput. 2020, 76, 1680–1694. [Google Scholar] [CrossRef]
- Li, T.; Jean, J. Digital Signal Processing; Academic Press: Cambridge, MA, USA, 2013; p. 896. [Google Scholar] [CrossRef]
- Husted, J.A.; Cook, R.J.; Farewell, V.T.; Gladman, D.D. Methods for assessing responsiveness: A critical review and recommendations. J. Clin. Epidemiol. 2000, 53, 459–468. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Hanson, R.K. The Development of a Brief Actuarial Risk Scale for Sexual Offense Recidivism; Department of the Solicitor General of Canada: Ottawa, ON, Canada, 1997.
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef] [PubMed]


| Real State | ||||
|---|---|---|---|---|
| True | False | |||
| Predict | True | True Positive (TP) | False Positive (FP) | |
| False | False Negative (FN) | True Negative (TN) | ||
| ROC | Day 7 | Day 8 | Day 9 | Day 10 | |
|---|---|---|---|---|---|
| Unfertilized eggs | Grayscale values | 190.5 | 188.0 | 182.5 | 181.0 |
| AUC | 0.97 | 0.99 | 0.99 | 0.99 | |
| Dead-in-shell embryos | Grayscale values | 122.0 | 107.5 | 74.0 | 65.0 |
| AUC | 0.83 | 0.95 | 0.99 | 0.99 |
| Day 7 | Real State | Grayscale Values: 190.5 | AUC: 0.97 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 9 | 1 | 10 | 98.0 | 90.0 |
| FALSE | 2 | 138 | 140 | Sensitivity (%) | Specificity (%) | |
| Total | 11 | 139 | 150 | 81.8 | 99.3 | |
| Day 8 | Real State | Grayscale Values: 188.0 | AUC: 0.99 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 10 | 0 | 10 | 99.3 | 100.0 |
| FALSE | 1 | 139 | 140 | Sensitivity (%) | Specificity (%) | |
| Total | 11 | 139 | 150 | 90.9 | 100.0 | |
| Day 9 | Real State | Grayscale Values: 182.5 | AUC: 0.99 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 11 | 0 | 11 | 100.0 | 100.0 |
| FALSE | 0 | 139 | 139 | Sensitivity (%) | Specificity (%) | |
| Total | 11 | 139 | 150 | 100.0 | 100.0 | |
| Day 10 | Real State | Grayscale Values: 181.0 | AUC: 0.99 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 11 | 0 | 11 | 100.0 | 100.0 |
| FALSE | 0 | 139 | 139 | Sensitivity (%) | Specificity (%) | |
| Total | 11 | 139 | 150 | 100.0 | 100.0 | |
| Day 7 | Real State | Grayscale Values: 122.0 | AUC: 0.83 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 4 | 8 | 12 | 92.1 | 33.3 |
| FALSE | 3 | 124 | 127 | Sensitivity (%) | Specificity (%) | |
| Total | 7 | 132 | 139 | 57.1 | 93.9 | |
| Day 8 | Real State | Grayscale Values: 107.5 | AUC: 0.95 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 5 | 1 | 6 | 97.8 | 83.3 |
| FALSE | 2 | 131 | 133 | Sensitivity (%) | Specificity (%) | |
| Total | 7 | 132 | 139 | 71.4 | 99.2 | |
| Day 9 | Real State | Grayscale Values: 74.0 | AUC: 0.99 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 7 | 0 | 7 | 100.0 | 100.0 |
| FALSE | 0 | 132 | 132 | Sensitivity (%) | Specificity (%) | |
| Total | 7 | 132 | 139 | 100.0 | 100.0 | |
| Day 10 | Real State | Grayscale Values: 65.0 | AUC: 0.99 | |||
| TRUE | FALSE | Total | Accuracy (%) | Precision (%) | ||
| Predict | TRUE | 6 | 0 | 6 | 99.3 | 100.0 |
| FALSE | 1 | 132 | 133 | Sensitivity (%) | Specificity (%) | |
| Total | 7 | 132 | 139 | 85.7 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-Y.; Li, C.-H.; Jeng, C.-C.; Cheng, C.-W. Quality Assessment during Incubation Using Image Processing. Sensors 2020, 20, 5951. https://doi.org/10.3390/s20205951
Tsai S-Y, Li C-H, Jeng C-C, Cheng C-W. Quality Assessment during Incubation Using Image Processing. Sensors. 2020; 20(20):5951. https://doi.org/10.3390/s20205951
Chicago/Turabian StyleTsai, Sheng-Yu, Cheng-Han Li, Chien-Chung Jeng, and Ching-Wei Cheng. 2020. "Quality Assessment during Incubation Using Image Processing" Sensors 20, no. 20: 5951. https://doi.org/10.3390/s20205951
APA StyleTsai, S.-Y., Li, C.-H., Jeng, C.-C., & Cheng, C.-W. (2020). Quality Assessment during Incubation Using Image Processing. Sensors, 20(20), 5951. https://doi.org/10.3390/s20205951





