Measurement of Early Disease Blueberries Based on Vis/NIR Hyperspectral Imaging System
Abstract
1. Introduction
2. Materials and Methods
2.1. Blueberry Samples and Evaluation of Early Disease
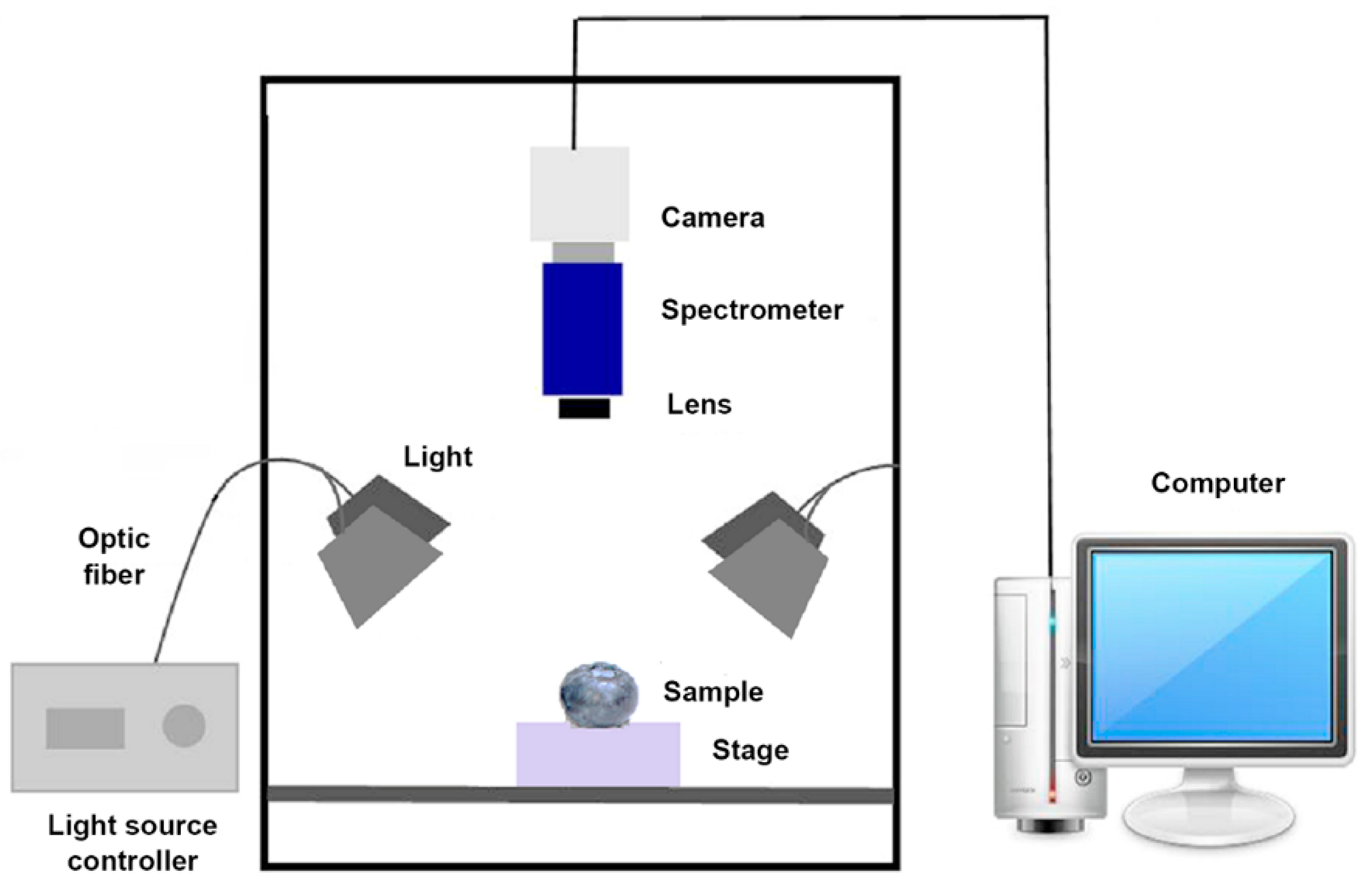
2.2. Hyperspectral Imaging Acquisition
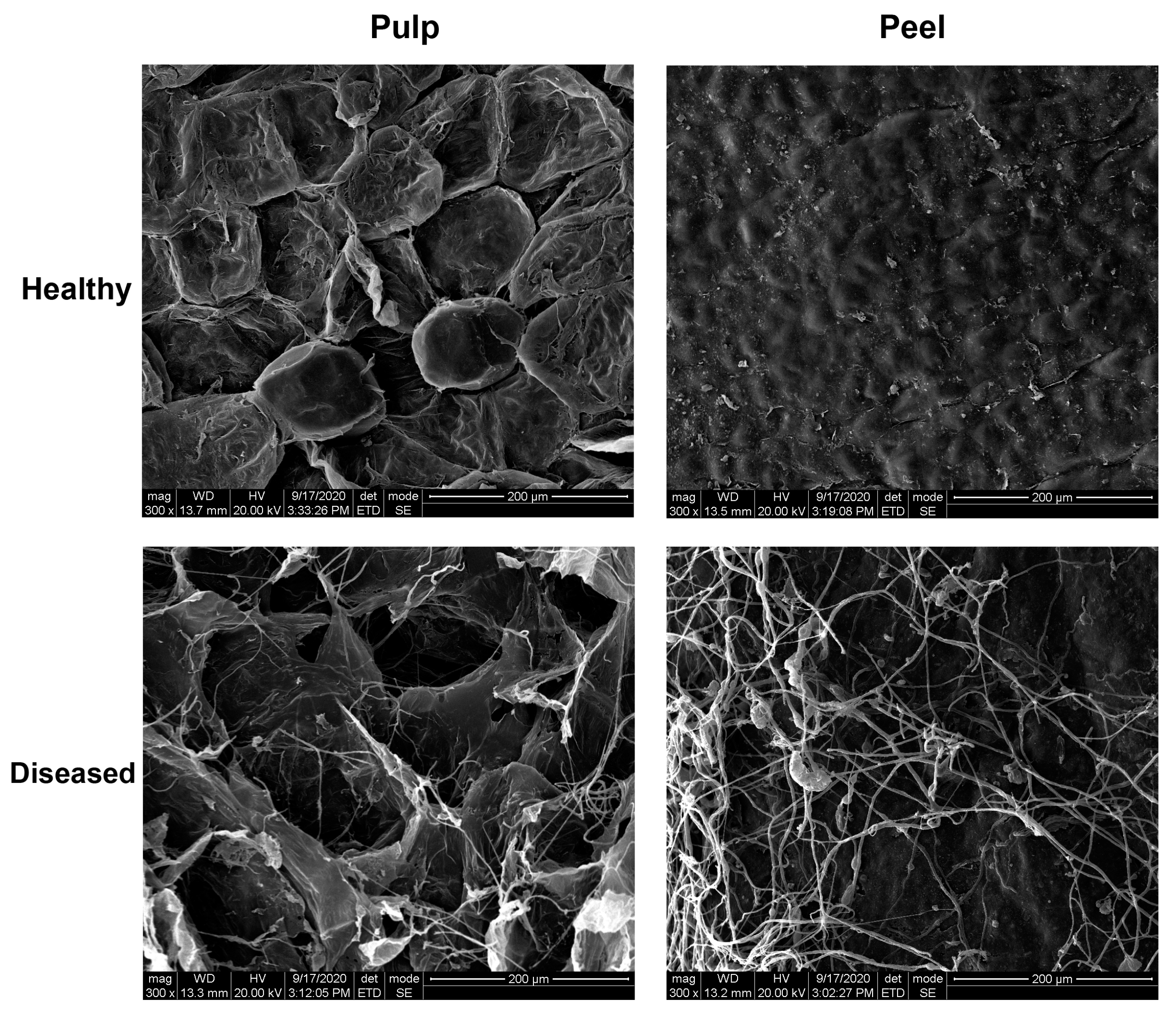
2.3. Microstructural Analysis
2.4. Data Analysis and Chemometrics
3. Results and Discussion
3.1. Disease Evaluation and SEM Observation
3.2. Spectral Correlation Analysis and Effective Spectral Range Selection
3.3. Spectral Features Related to Early Disease
3.4. Discrimination Models for Early Disease Blueberry Detection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, Y.; Li, C.; Yang, F. Fully convolutional networks for blueberry bruising and calyx segmentation using hyperspectral transmittance imaging. Biosyst. Eng. 2020, 192, 159–175. [Google Scholar] [CrossRef]
- Yu, P.; Li, C.; Takeda, F.; Krewer, G.; Glen, R.; Hamrita, T. Measurement of mechanical impacts created by rotary, slapper, and sway blueberry mechanical harvesters. Comput. Electron. Agric. 2014, 101, 84–92. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Lu, Y.; Tu, K.; Pan, L. Detection of early decay in peaches by structured-illumination reflectance imaging. Postharvest Biol. Technol. 2019, 151, 68–78. [Google Scholar] [CrossRef]
- Sugiyama, T.; Sugiyama, J.; Tsuta, M.; Fujita, K.; Shibata, M.; Kokawa, M.; Araki, T.; Nabetani, H.; Sagara, Y. NIR spectral imaging with discriminant analysis for detecting foreign materials among blueberries. J. Food Eng. 2010, 101, 244–252. [Google Scholar] [CrossRef]
- Kuzy, J.; Jiang, Y.; Li, C. Blueberry bruise detection by pulsed thermographic imaging. Postharvest Biol. Technol. 2018, 136, 166–177. [Google Scholar] [CrossRef]
- Du, Z.; Zeng, X.; Li, X.; Ding, X.; Cao, J.; Jiang, W. Recent advances in imaging techniques for bruise detection in fruits and vegetables. Trends Food Sci. Technol. 2020, 99, 133–141. [Google Scholar] [CrossRef]
- Yang, C.; Lee, W.S.; Gader, P.D. Hyperspectral band selection for detecting different blueberry fruit maturity stages. Comput. Electron. Agric. 2014, 109, 23–31. [Google Scholar] [CrossRef]
- Hu, M.; Dong, Q.; Liu, B. Classification and characterization of blueberry mechanical damage with time evolution using reflectance, transmittance and interactance imaging spectroscopy. Comput. Electron. Agric. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Leivavalenzuela, G.A.; Lu, R.; Aguilera, J.M. Prediction of firmness and soluble solids content of blueberries using hyperspectral reflectance imagings. J. Food Eng. 2013, 115, 91–98. [Google Scholar] [CrossRef]
- Qiao, S.; Tian, Y.; Gu, W.; He, K.; Yao, P.; Song, S.; Wang, J.; Wang, H.; Zhang, F. Research on simultaneous detection of SSC and FI of blueberry based on hyperspectral imaging combined MS-SPA. Eng. Agric. Environ. Food 2019, 12, 540–547. [Google Scholar] [CrossRef]
- Leivavalenzuela, G.A.; Lu, R.; Aguilera, J.M. Assessment of internal quality of blueberries using hyperspectral transmittance and reflectance images with whole spectra or selected wavelengths. Innov. Food Sci. Emerg. Technol. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Prediction of Firmness Parameters of Tomatoes by Portable Visible and Near-Infrared Spectroscopy. J. Food Eng. 2018, 222, 185–198. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Qi, C.; Chen, K. Measurement of Tomato Quality Attributes Based on Wavelength Ratio and Near-Infrared Spectroscopy. Spectrosc. Spectr. Anal. 2018, 38, 2362–2368. [Google Scholar]
- Huang, Y.; Lu, R.; Qi, C.; Chen, K. Tomato Maturity Classification Based on Spatially Resolved Spectra. Spectrosc. Spectr. Anal. 2018, 38, 2183–2188. [Google Scholar]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Bai, W.; Yoshimura, N.; Takayanagi, M. Quantitative analysis of ingredients of blueberry fruits by near infrared spectroscopy. J. Near Infrared Spectrosc. 2014, 22, 357. [Google Scholar] [CrossRef]
- Sinelli, N.; Casiraghi, E.; Barzaghi, S.; Brambilla, A.; Giovanelli, G. Near infrared (NIR) spectroscopy as a tool for monitoring blueberry osmo–air dehydration process. Food Res. Int. 2011, 44, 1427–1433. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Noferini, M.; Rombolá, A.D. Non-destructive Assessment of Highbush Blueberry Fruit Maturity Parameters and Anthocyanins by Using a Visible/Near Infrared (vis/NIR) Spectroscopy Device: A Preliminary Approach. J. Soil Sci. Plant Nutr. 2016, 16, 174–186. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.; Jiang, X.; Ru, Y.; Wang, J.; Zhou, H. Application of hyperspectral imaging for detecting and visualizing leaf lard adulteration in minced pork. Infrared Phys. Technol. 2020, 110, 103467. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Pan, L.; Abbas, A.; Wang, X. Authentication of the geographic origin of Yangshan region peaches based on hyperspectral imaging. Postharvest Biol. Technol. 2021, 171, 111320. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, X.; Ru, Y.; Chen, Q.; Xu, L.; Zhou, H. Sweetness detection and grading of peaches and nectarines by combining short- and long-wave fourier-transform near-infrared spectroscopy. Anal. Lett. 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Assessment of Tomato Soluble Solids Content and pH by Spatially-Resolved and Conventional Vis/NIR Spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Sun, Y.; Zhou, H.; Chen, K. Identification of Apple Varieties Using a Multichannel Hyperspectral Imaging System. Sensors 2020, 20, 5120. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Liu, Y.; Yang, Y.-T.; Zhang, Z.-W.; Chen, K.-J. Assessment of Tomato Color by Spatially Resolved and Conventional Vis/NIR Spectroscopies. Spectrosc. Spectr. Anal. 2019, 39, 3585–3591. [Google Scholar]
- Huang, Y.; Lu, R.; Chen, K. Detection of internal defect of apples by a multichannel Vis/NIR spectroscopic system. Postharvest Biol. Technol. 2020, 161, 111065. [Google Scholar] [CrossRef]
- Pan, T.T.; Erkinbaev, C.; Sun, D.W.; Paliwal, J.; Pu, H. Pathogenetic process monitoring and early detection of pear black spot disease caused by Alternaria alternata using hyperspectral imaging. Postharvest Biol. Technol. 2019, 154, 96–104. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Cybulska, J.; Szymańska-Chargot, M.; Siedliska, A.; Zdunek, A.; Nosalewicz, A.; Baranowski, P.; Kurenda, A. Early detection of fungal infection of stored apple fruit with optical sensors—Comparison of biospeckle, hyperspectral imaging and chlorophyll fluorescence. Food Control. 2018, 85, 327–338. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Tian, X.; Wang, C.; Fan, S.; Zhao, C. Fast detection and visualization of early decay in citrus using Vis-NIR hyperspectral imaging. Comput. Electron. Agric. 2016, 127, 582–592. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Ding, P.; Jia, M. Fault Diagnosis of Rolling-Element Bearing Using Multiscale Pattern Gradient Spectrum Entropy Coupled with Laplacian Score. Complexity 2020, 2020, 1–29. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Jia, M. A Fault Diagnosis Approach for Rolling Bearing Integrated SGMD, IMSDE and Multiclass Relevance Vector Machine. Sensors 2020, 20, 4352. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Y.; Huang, D.; Jia, M. A new approach to health condition identification of rolling bearing using hierarchical dispersion entropy and improved Laplacian score. Struct. Health Monit. 2020. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Zeng, W.; Lu, X. An improved method for single image super-resolution based on deep learning. Signal. Image Video Process. 2018, 13, 557–565. [Google Scholar] [CrossRef]
- Noviyanto, A.; Abdulla, W.H. Honey Botanical Origin Classification using Hyperspectral Imaging and Machine Learning. J. Food Eng. 2020, 265, 109684. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Li, Z.; Li, H.; Li, Z.; Xu, X.; Song, X.; Zhang, Y.; Duan, D.; Zhao, C. Progress of hyperspectral data processing and modelling for cereal crop nitrogen monitoring. Comput. Electron. Agric. 2020, 172, 105321. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xiao, H.; Gu, X.; Pan, L.; Tu, K. Hyperspectral imaging detection of decayed honey peaches based on their chlorophyll content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef]
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early detection of Fusarium infection in wheat using hyper-spectral imaging. Comput. Electron. Agric. 2011, 75, 304–312. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Pan, L.; Wang, X.; Tu, K. Assessment of the optical properties of peaches with fungal infection using spatially-resolved diffuse reflectance technique and their relationships with tissue structural and biochemical properties. Food Chem. 2020, 321, 126704. [Google Scholar] [CrossRef]
- Lu, R. Light Scattering Technology for Food Property, Quality and Safety Assessment; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Qiao, S.; Wang, Q.; Zhang, J.; Pei, Z. Detection and Classification of Early Decay on Blueberry Based on Improved Deep Residual 3D Convolutional Neural Network in Hyperspectral Images. Sci. Program. 2020, 2020, 8895875. [Google Scholar] [CrossRef]
- Yu, J.; Changying, L.; Fumiomi, T. Nondestructive Detection and Quantification of Blueberry Bruising using Near-infrared (NIR) Hyperspectral Reflectance Imaging. Sci. Rep. 2016, 6, 35679. [Google Scholar]
- Fan, S.; Li, C.; Huang, W.; Chen, L. Detection of blueberry internal bruising over time using NIR hyperspectral reflectance imaging with optimum wavelengths. Postharvest Biol. Technol. 2017, 134, 55–66. [Google Scholar] [CrossRef]



| Spectral Type | Spectral Preprocessing | Total Accuracy | ||
|---|---|---|---|---|
| Training Set | Test Set | |||
| Calibration | Cross-Validation | |||
| Full (400–1000 nm) | None | 0.935 | 0.910 | 0.875 |
| Autoscale | 0.970 | 0.950 | 0.910 | |
| Log(1/R) | 0.960 | 0.960 | 0.915 | |
| Selected (685–1000 nm) | None | 0.985 | 0.970 | 0.980 |
| Autoscale | 1.000 | 0.975 | 0.995 | |
| Log(1/R) | 0.995 | 0.985 | 0.980 | |
| Spectral Type | Spectral Preprocessing | Training Set | Test Set | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calibration | Cross-Validation | |||||||||
| H | D | Accuracy | H | D | Accuracy | H | D | Accuracy | ||
| Full | None | 99 | 8 | 0.952 | 95 | 9 | 0.913 | 86 | 15 | 0.896 |
| 5 | 88 | 0.917 | 9 | 87 | 0.906 | 10 | 89 | 0.856 | ||
| Autoscale | 101 | 3 | 0.971 | 99 | 5 | 0.952 | 92 | 14 | 0.958 | |
| 3 | 93 | 0.969 | 5 | 91 | 0.948 | 4 | 90 | 0.865 | ||
| Log(1/R) | 101 | 5 | 0.971 | 101 | 5 | 0.971 | 92 | 13 | 0.958 | |
| 3 | 91 | 0.948 | 3 | 91 | 0.948 | 4 | 91 | 0.875 | ||
| Selected | None | 102 | 1 | 0.981 | 100 | 2 | 0.962 | 93 | 1 | 0.969 |
| 2 | 95 | 0.990 | 4 | 94 | 0.979 | 3 | 103 | 0.990 | ||
| Autoscale | 104 | 0 | 1.000 | 101 | 2 | 0.971 | 96 | 1 | 1.000 | |
| 0 | 96 | 1.000 | 3 | 94 | 0.979 | 0 | 103 | 0.990 | ||
| Log(1/R) | 103 | 0 | 0.990 | 102 | 1 | 0.981 | 94 | 2 | 0.979 | |
| 1 | 96 | 1.000 | 2 | 95 | 0.990 | 2 | 102 | 0.981 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, D.; Liu, Y.; Zhou, H.; Sun, Y. Measurement of Early Disease Blueberries Based on Vis/NIR Hyperspectral Imaging System. Sensors 2020, 20, 5783. https://doi.org/10.3390/s20205783
Huang Y, Wang D, Liu Y, Zhou H, Sun Y. Measurement of Early Disease Blueberries Based on Vis/NIR Hyperspectral Imaging System. Sensors. 2020; 20(20):5783. https://doi.org/10.3390/s20205783
Chicago/Turabian StyleHuang, Yuping, Dezhen Wang, Ying Liu, Haiyan Zhou, and Ye Sun. 2020. "Measurement of Early Disease Blueberries Based on Vis/NIR Hyperspectral Imaging System" Sensors 20, no. 20: 5783. https://doi.org/10.3390/s20205783
APA StyleHuang, Y., Wang, D., Liu, Y., Zhou, H., & Sun, Y. (2020). Measurement of Early Disease Blueberries Based on Vis/NIR Hyperspectral Imaging System. Sensors, 20(20), 5783. https://doi.org/10.3390/s20205783





