Indirect Sensing of Lower Aliphatic Ester Using Atomic Gold Decorated Polyaniline Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Compounds
2.2. Chemicals and Materials
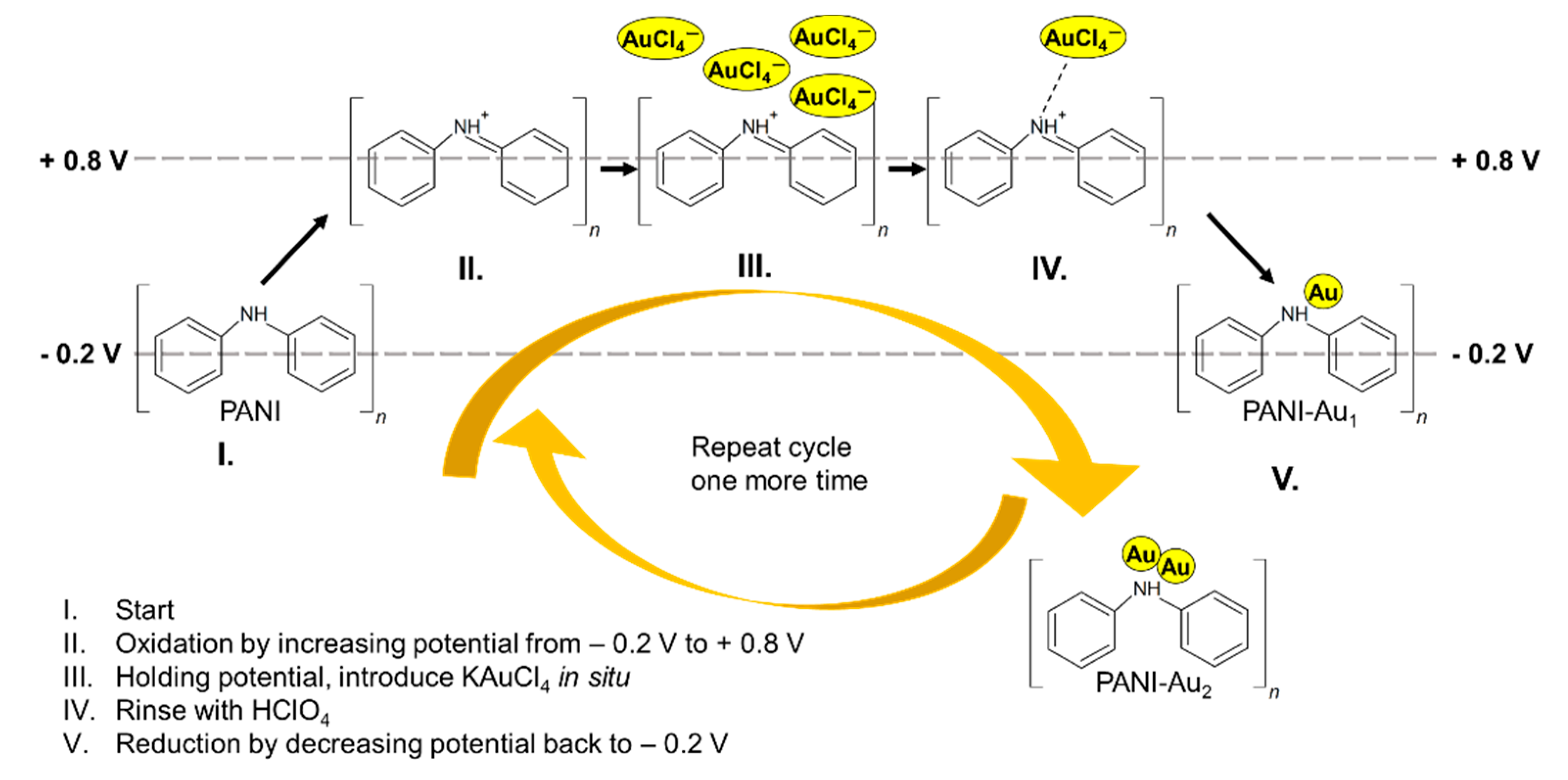
2.3. Synthesis of PANI and PANI-Au2 Nanocomposite
2.4. Instrumentation and Electrochemical Setup
3. Results
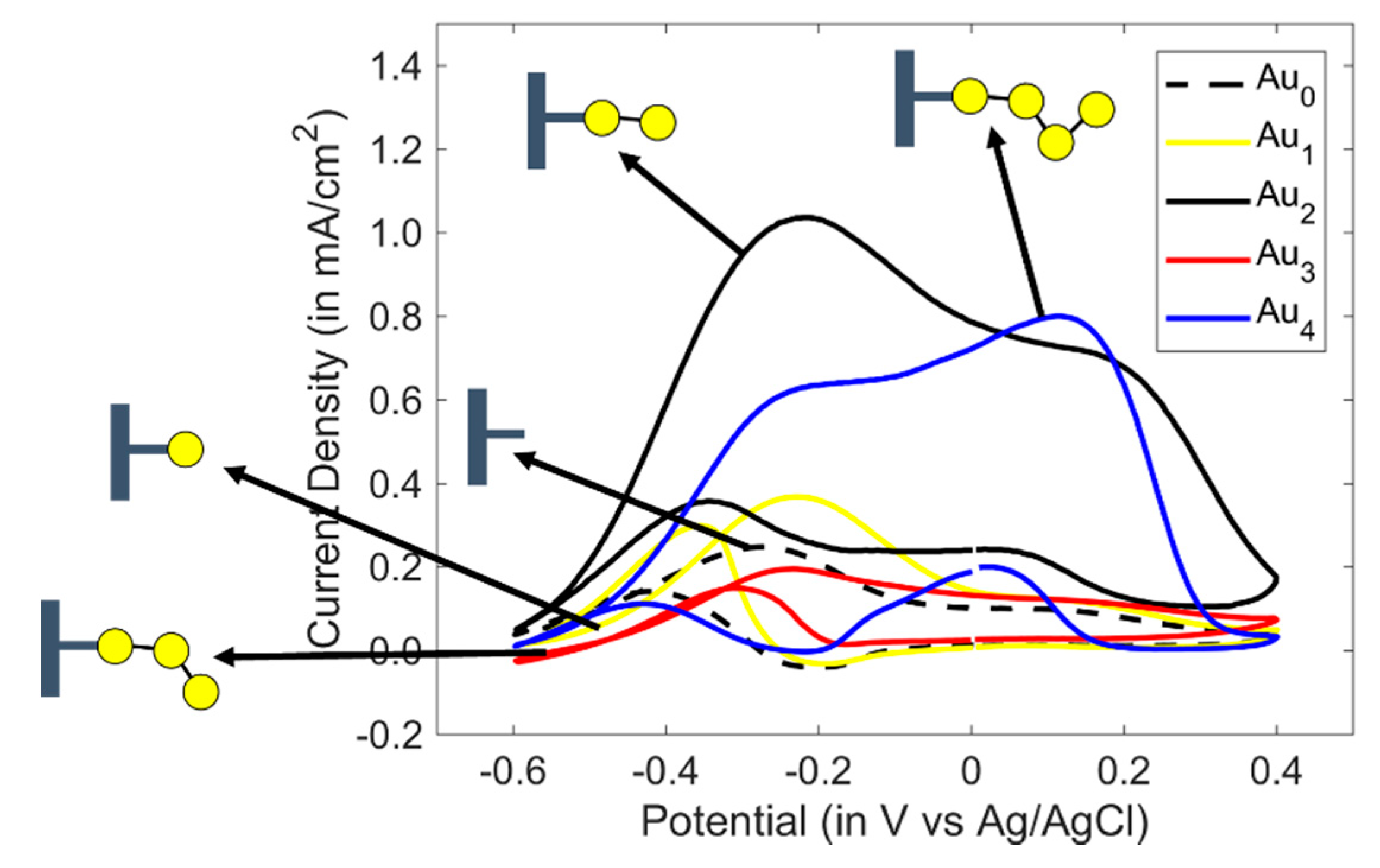
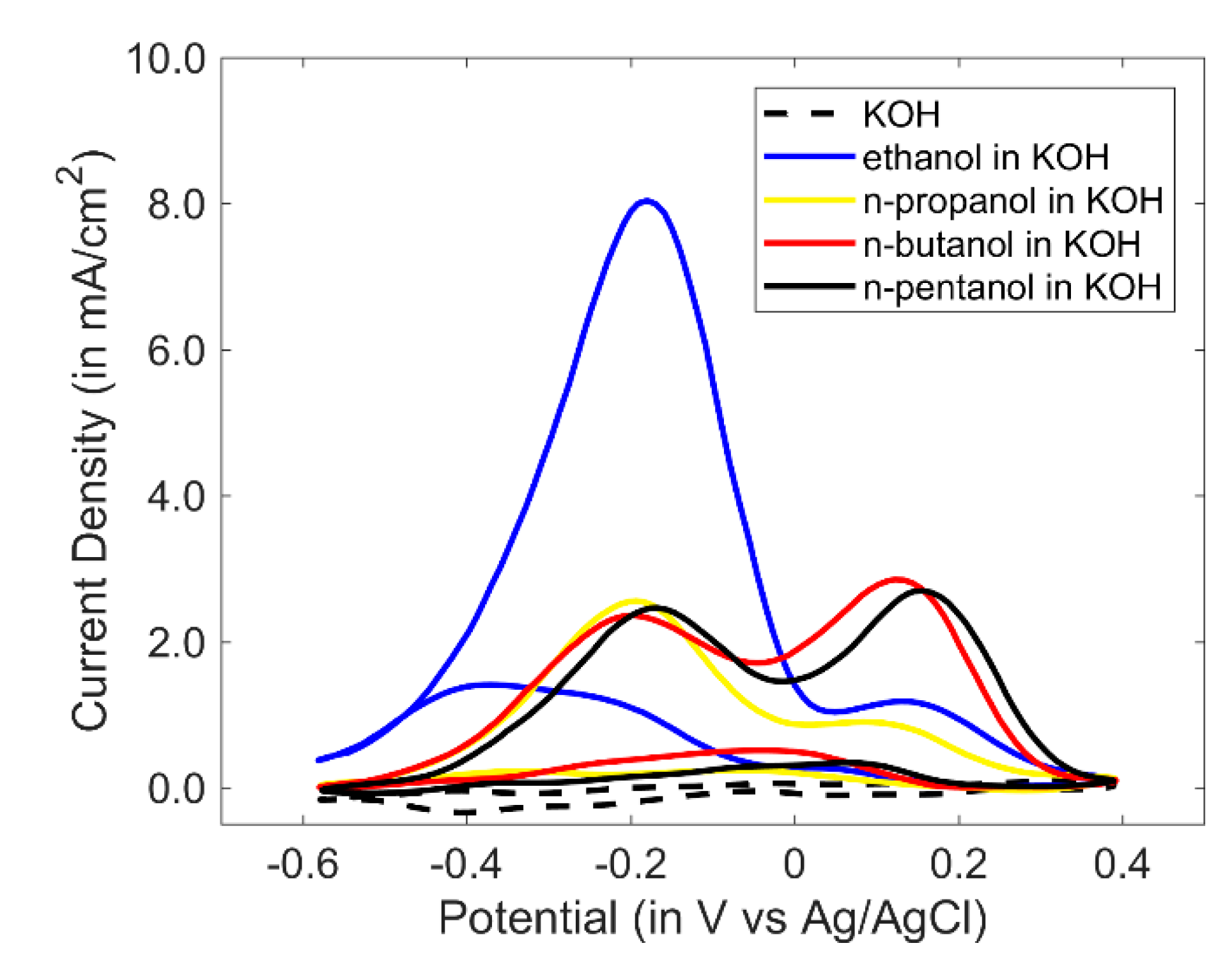
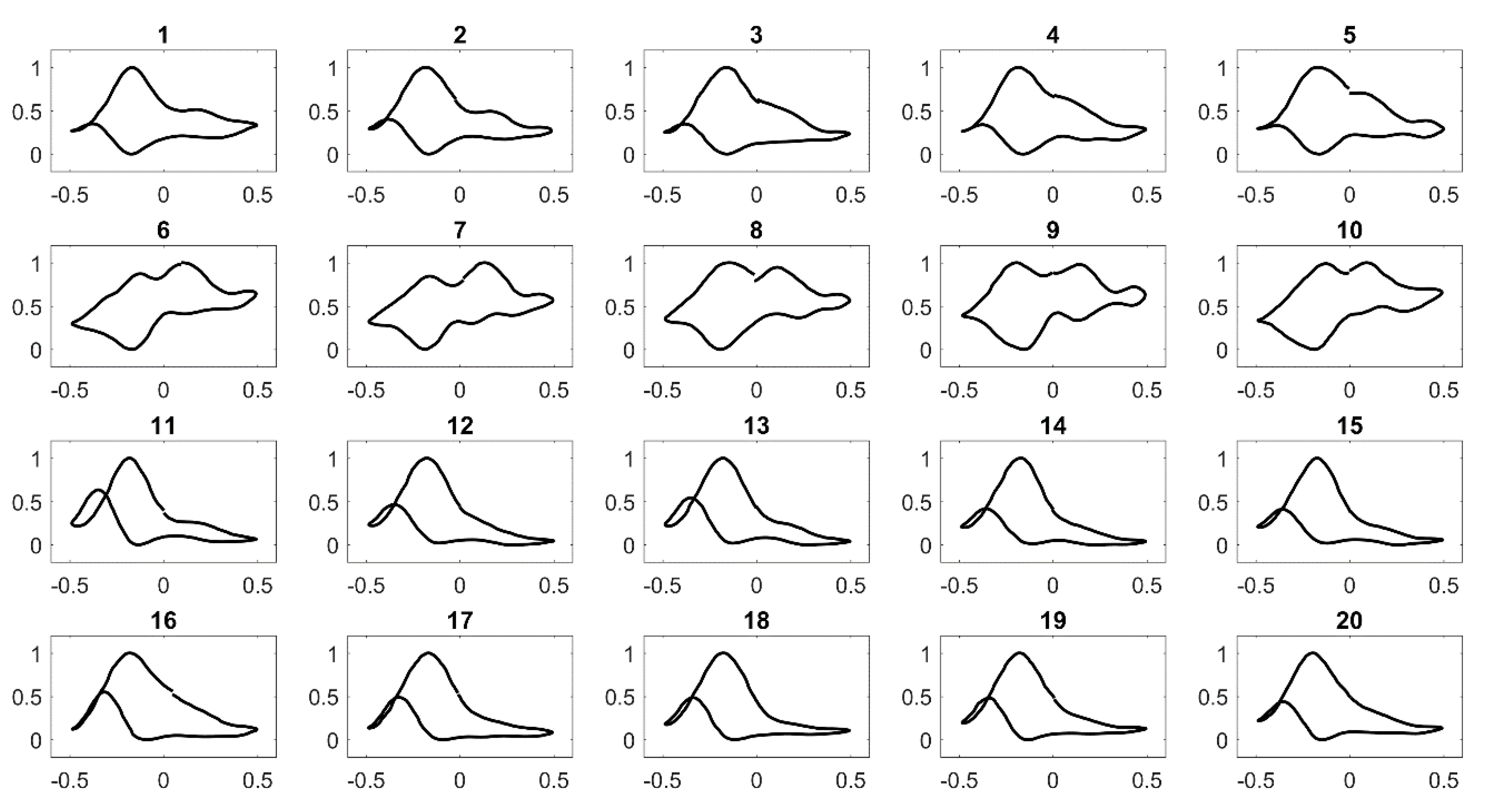
3.1. Study of Cyclic Voltammetry of PANI/Au2 with Linear Alcohols
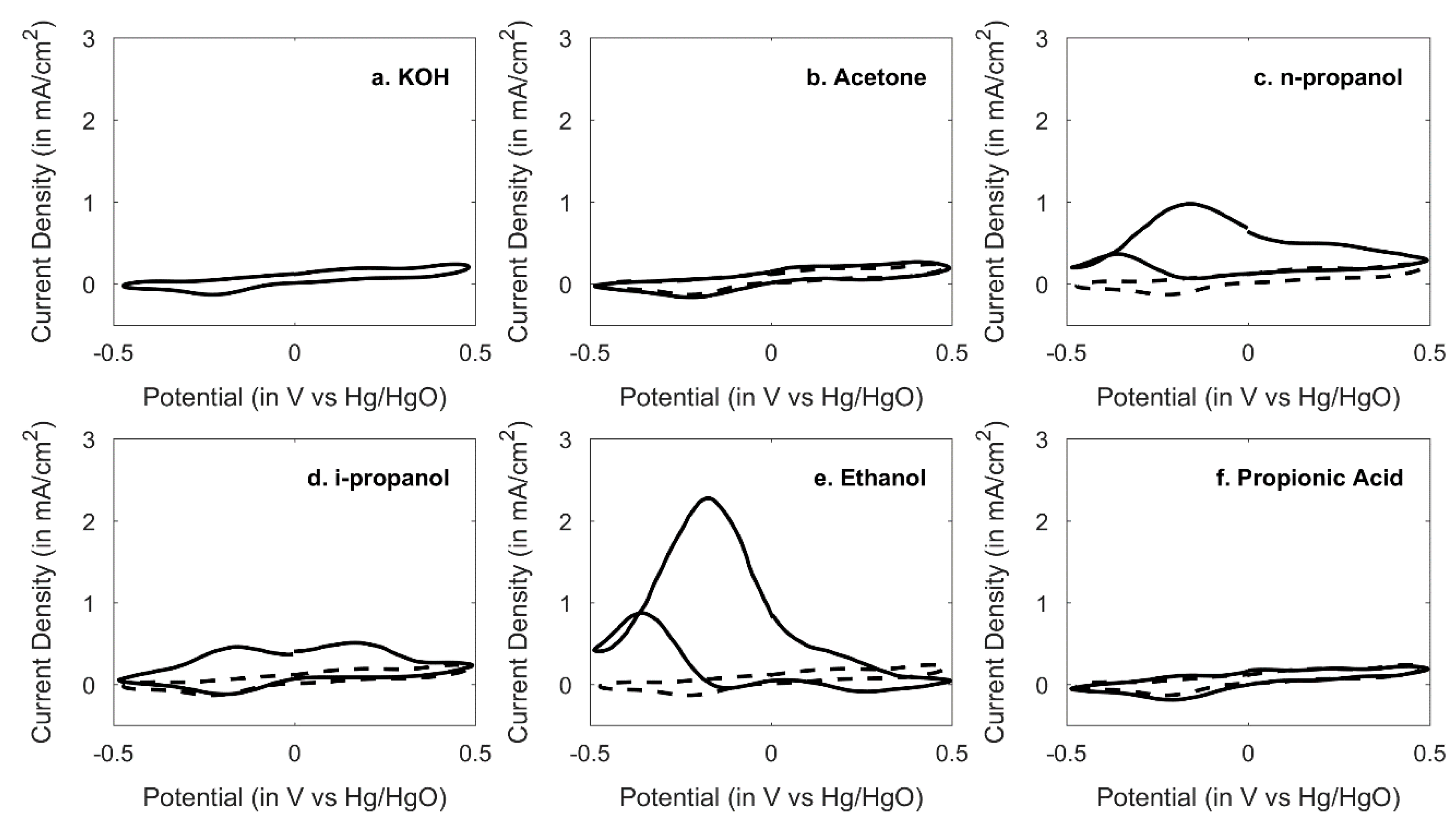
3.2. Survey of Sensor Response across Functional Groups in 1 M KOH Alkaline Medium Electrolyte
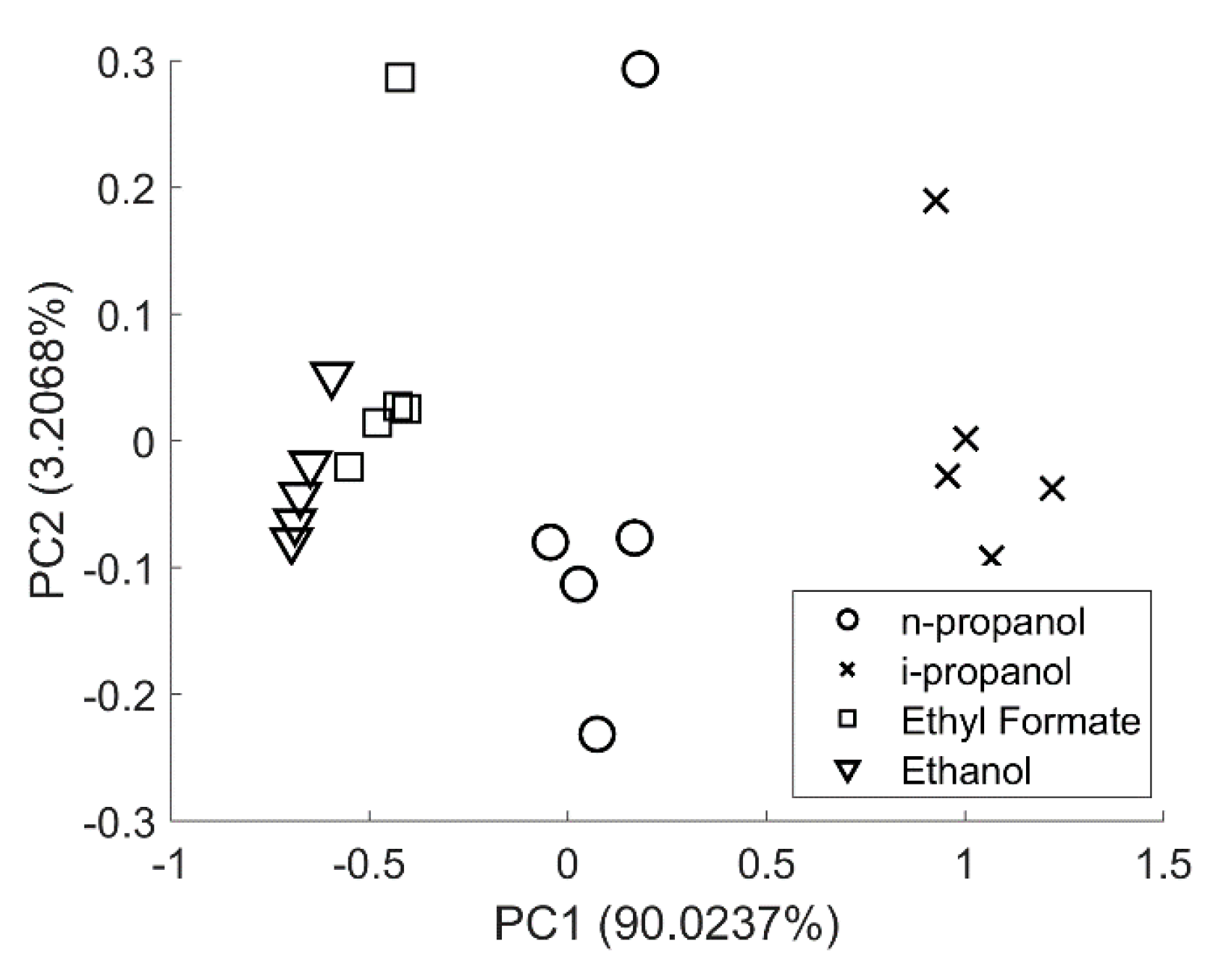
3.3. Principal Component Analysis
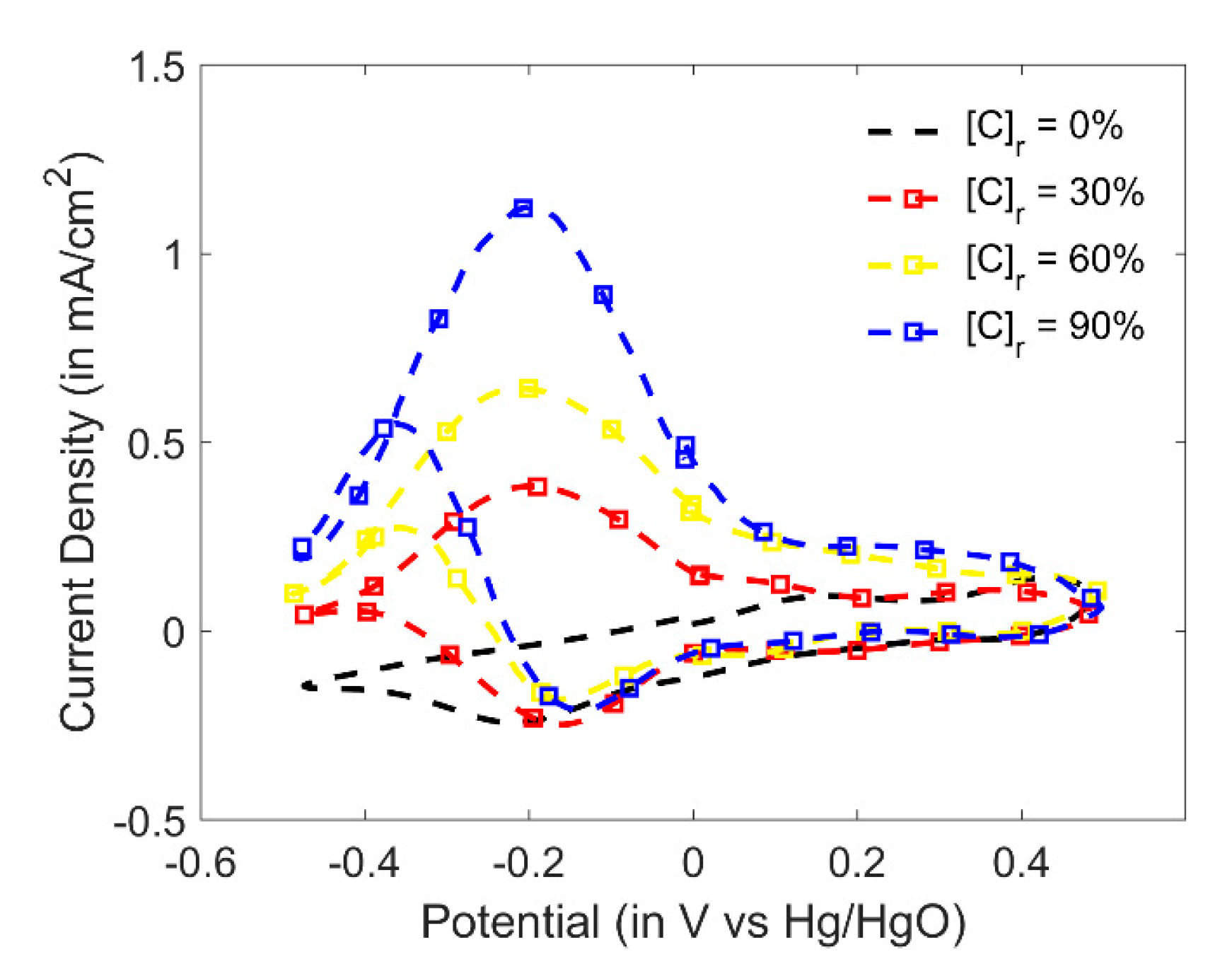
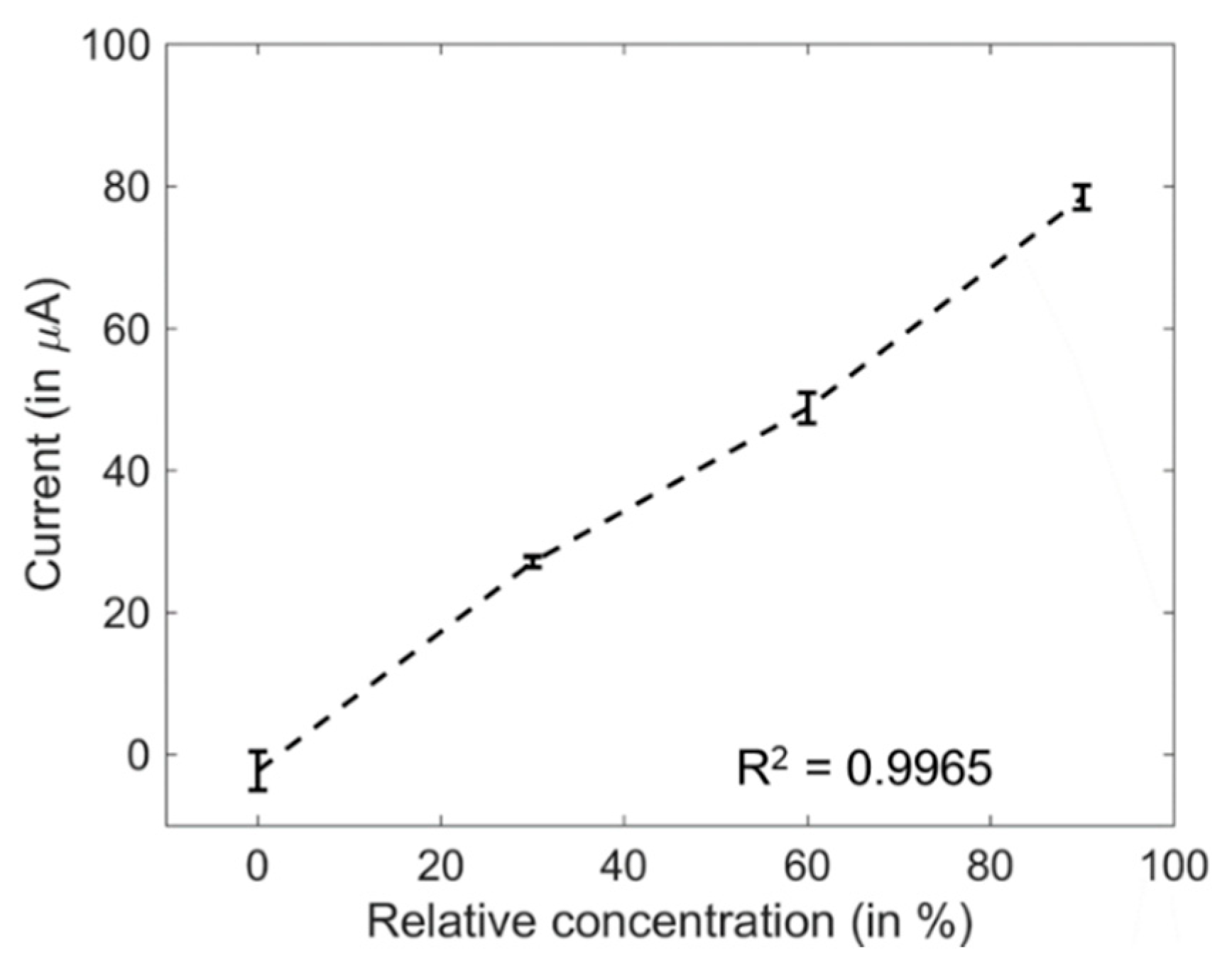
3.4. Indirect Sensing of Ethyl Formate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Al-Akraa, I.M. Efficient Electro-Oxidation of Formic Acid at Pd-MnOx Binary Nanocatalyst: Optimization of Deposition Strategy. Int. J. Hydrog. Energy 2017, 42, 4660–4666. [Google Scholar] [CrossRef]
- Hyodo, T.; Takamori, M.; Goto, T.; Ueda, T.; Shimizu, Y. Potentiometric CO Sensors Using Anion-Conducting Polymer Electrolyte: Effects of the Kinds of Noble Metal-Loaded Metal Oxides as Sensing-Electrode Materials on CO-Sensing Properties. Sens. Actuators B Chem. 2019, 287, 42–52. [Google Scholar] [CrossRef]
- Smith, J.A.; Josowicz, M.; Janata, J. Polyaniline-Gold Nanocomposite System. J. Electrochem. Soc. 2003, 150, E384. [Google Scholar] [CrossRef]
- Burke, L.D.; O’Leary, W.A. A Study of Isopropanol Oxidation at Platinised Platinum Electrodes in Acid Solution. Royal Irish Academy 1989, 89B, 389–398. [Google Scholar]
- Janata, J. Electrochemical Formation of Au Clusters in Polyaniline. Chem. Mater. 1999, 11, 2989–2994. [Google Scholar] [CrossRef]
- Liu, C.; Hayashi, K.; Toko, K. Au Nanoparticles Decorated Polyaniline Nanofiber Sensor for Detecting Volatile Sulfur Compounds in Expired Breath. Sens. Actuators B Chem. 2012, 161, 504–509. [Google Scholar] [CrossRef]
- Diaz, A.F.; Logan, J.A. Electroactive Polyaniline Films. J. Electroanal. Chem. 1980, 111, 111–114. [Google Scholar] [CrossRef]
- Kroutil, J.; Laposa, A.; Voves, J.; Davydova, M.; Nahlik, J.; Kulha, P.; Husak, M. Performance Evaluation of Low-Cost Flexible Gas Sensor Array with Nanocomposite Polyaniline Films. IEEE Sens. J. 2018, 18, 3759–3766. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline Doped with Atomic Gold. J. Electrochem. Soc. 2011, 158, E147. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J.; Engelhard, M.H. Electrochemically Controlled Atom by Atom Deposition of Gold to Polyaniline. J. Electrochem. Soc. 2010, 157, P83. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Odd-Even Pattern Observed in Polyaniline/(Au0–Au8) Composites. J. Electrochem. Soc. 2012, 159, 40–43. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.A.; Chiu, W.T.; Sone, M.; Nakamoto, T. Design and Development of Fuel Cell Type Gas Sensor with Atomic Au Decorated PANI/Pt Composite. In Proceedings of the IEEE Sensors, New Delhi, India, 28–31 October 2018. [Google Scholar] [CrossRef]
- Schwartz, I.T.; Jonke, A.P.; Josowicz, M.; Janata, J. Effect of Structured Atomic Gold on Electrooxidation of Alcohols in Alkaline Medium. Catal. Lett. 2013, 143, 777–782. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.A.; Chiu, W.T.; Chang, T.F.M.; Sone, M.; Nakamoto, T.; Josowicz, M.; Janata, J. Design and Development of Amperometric Gas Sensor with Atomic Au–Polyaniline/Pt Composite. IEEE Sens. J. 2020. In Press. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.A.; Chiu, W.T.; Chang, T.F.M.; Sone, M.; Nakamoto, T. Atomic Gold Decorated Polyaniline Sensor for Gaseous Detection. In Proceedings of the IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar] [CrossRef]
- An, L.; Chen, R. Direct Formate Fuel Cells: A Review. J. Power Sources 2016, 320, 127–139. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakamoto, T.; Moriizumi, T. Prediction of Quartz Crystal Microbalance Gas Sensor Responses Using a Computational Chemistry Method. Sens. Actuators B Chem. 1999, 61, 6–11. [Google Scholar] [CrossRef]
- Häkkinen, H.; Landman, U. Gold Clusters and Their Anions. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 62, R2287–R2290. [Google Scholar] [CrossRef]
- Schwartz, I.; Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline-Supported Atomic Gold Electrodes: Comparison with Macro Electrodes. Catal. Lett. 2012, 142, 1344–1351. [Google Scholar] [CrossRef]
- Nakamoto, T.; Minh, H.P.D. Improvement of Olfactory Display Using Solenoid Valves. In Proceedings of the IEEE Virtual Reality Conference, Charlotte, NC, USA, 10–14 March 2007; pp. 179–186. [Google Scholar] [CrossRef]
- Nakamoto, T. Odor Handling and Delivery Systems. Handb. Mach. Olfaction 2004, 55–78. [Google Scholar] [CrossRef]
- Humphreys, H.M.; Hammett, L.P. Rate Measurements on Fast Reactions in the Stirred Flow Reactor; the Alkaline Hydrolysis of Methyl and Ethyl Formate. J. Am. Chem. Soc. 1956, 78, 521–524. [Google Scholar] [CrossRef]
- Janata, J.; Nakamoto, T. Vision of New Olfactory Sensing Array. IEEE J. Trans. Electr. Electron. Eng. 2016, 11, 261–267. [Google Scholar] [CrossRef][Green Version]
- Stetter, J.R.; Li, J. Amperometric Gas Sensors—A Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T. Olfactory Display and Odor Recorder. Essent. Mach. Olfaction Tast. 2016, 247–314. [Google Scholar] [CrossRef]
| S. No. | Target Compound | Chemical Formula | Functional Group | Odor |
|---|---|---|---|---|
| 1. | acetone | C3H6O | ketone | pungent, cucumber-like |
| 2. | normal-propanol | C₃H₈O | alcohol | mild, alcohol-like |
| 3. | iso-propanol | C₃H₈O | alcohol | strong, alcohol-like |
| 4. | ethanol | C2H6O | alcohol | strong, alcohol-like |
| 5. | propionic acid | C3H6O2 | carboxylic acid | pungent, rancid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, P.; Chien, Y.-A.; Chang, T.-F.M.; Sone, M.; Nakamoto, T. Indirect Sensing of Lower Aliphatic Ester Using Atomic Gold Decorated Polyaniline Electrode. Sensors 2020, 20, 3640. https://doi.org/10.3390/s20133640
Chakraborty P, Chien Y-A, Chang T-FM, Sone M, Nakamoto T. Indirect Sensing of Lower Aliphatic Ester Using Atomic Gold Decorated Polyaniline Electrode. Sensors. 2020; 20(13):3640. https://doi.org/10.3390/s20133640
Chicago/Turabian StyleChakraborty, Parthojit, Yu-An Chien, Tso-Fu Mark Chang, Masato Sone, and Takamichi Nakamoto. 2020. "Indirect Sensing of Lower Aliphatic Ester Using Atomic Gold Decorated Polyaniline Electrode" Sensors 20, no. 13: 3640. https://doi.org/10.3390/s20133640
APA StyleChakraborty, P., Chien, Y.-A., Chang, T.-F. M., Sone, M., & Nakamoto, T. (2020). Indirect Sensing of Lower Aliphatic Ester Using Atomic Gold Decorated Polyaniline Electrode. Sensors, 20(13), 3640. https://doi.org/10.3390/s20133640







