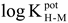Composition and characteristics of methacrylic-actaylic polymers
The composition of the copolymers synthesised can be determined from their proton FTNMR spectra
. The characteristic chemical shift of the nBA and MMA monomers remain the same even though after incorporation into the polymer network
(13). For nBA the characteristic chemical shift is at 1.4 ppm (- CH
2 – proton) whilst for MMA, 3.6 ppm (-COCH
3 – proton). Because of that, the FTNMR spectrum can be used to determine the composition of MMA and nBA in the polymer. After the monomer peak is identified, the fraction of the monomer in the copolymer may be calculated by using the peak intensity (I) according to Equation (1) below
(17).
For copolymer MB28, the monomer composition that calculated based on proton FTNMR spectra was closely resembled to the monomer compositions used in the feed before polymerisation (
Table 2). Although the reactivity of the monomer MMA, r
1 is approximately four times higher than that of nBA, r
2(18), the large amount of nBA in the feed dominated the polymerisation reaction. Hence, the composition of the copolymer produced was higher in nBA then MMA. The yield of the copolymer after purification was 74 %.
Table 2.
The monomer and copolymer composition of MB28.
Table 2.
The monomer and copolymer composition of MB28.
| Monomer | Composition from feed (mol fraction) | Composition from FTNMR (mol fraction) |
|---|
| MMA | 0.20 | 0.30 |
| nBA | 0.80 | 0.70 |
The MB28 copolymer has a soft but elastic physical characteristic. This is in agreement with the glass transition temperatures (T
g) of –30.9 °C obtained from DSC study. For a functional hydrogen ion sensor, the ionophore tridodecylamine must be ‘active’ after entrapped in a polymer matrix. The ability of the ionophore to selectively detect the presence hydrogen ions depends on the complexation processes that occurring at the membrane-solution interface
(19). It is generally known that for a ionophore to be active, the matrix materials must be highly flexible to allow movement of the ionophore for conformation changes during ion complexation processes
(20). For the methacrylic-acrylic types of copolymers, it has been established that the very low T
g, i.e. below -20°C is essential for the functioning of the ionophore without resort to incorporation of a plasticiser to reduce the T
g(13). Therefore, the copolymer MB28 has satisfied the requirement of a non-plasticised membrane for ion sensor as the copolymer demonstrated T
g value well below that of –20 °C.
Response of hydrogen ion sensor and effects of KTClPB
For a non-plasticised methacrylic-acrylic membrane containing only the tridodecylamine ionophore, no clear response to changes in pH was observed (
Fig. 1, sensor E1). However, if the hydrogen ionophore is added together with 17.1 mole percent of a lipophilic salt, KTClPB, the sensor (E2) gave satisfactory response slope and selectivity towards hydrogen ions in the absence of interference cations (
Fig. 2). A good response was also obtained even in the presence of 0.1M of sodium ions (
Fig. 1).
The lipophilic anions or anionic sites play an important role in inducing good response of an ion sensor. Lipophilic anionic sites in ion sensor membrane is beneficial in lowering the electrical membrane resistance, reduce the activation barrier for the cation-exchange reaction at the membrane/solution interface, improve response time and also selectivity towards the primary ions
(21). The absence of anionic sites in the methacrylic-acrylic type of membranes have been known and was considered as one of the factor affecting the performance of ion sensors based on these polymer membranes
(14). Thus incorporation of anionic sites such as KTClPB is essential for a functional ion sensor based on methacrylic-acrylic membranes. Nevertheless, a membrane containing only KTClPB will also response to cations, including hydrogen ions in a sub-Nerntian manner, e.g. sensor E3 (
Table 2) with a small linear response range. But unlike the hydrogen ionophore, cationic response from KTClPB is not selective. The hydrogen ion sensor E2 although also contained KTClPB but the response is not attributed to the lipophilic anion but the hydrogen ionophore. This is confirmed by the response of E2 in the presence of 0.1 M Ca
2+ or Mg
2+ ions where the slope remained near to the theoretical value of 59.6 mV/decade for a single charge ion instead of 29.8 mV/decade for a doubly charge ion (
Table 2).
Figure 1.
The response of hydrogen ion sensor of non-plasticised methacrylic-acrylic membrane to various concentrations of hydrogen ion (0.05 M TrisHCl buffer) in 0.1 M sodium ion. E1: membrane contained only a hydrogen ionophore, E2: Membrane contained both hydrogen ionophore and the lipophilic salt, KTClPB.
Figure 1.
The response of hydrogen ion sensor of non-plasticised methacrylic-acrylic membrane to various concentrations of hydrogen ion (0.05 M TrisHCl buffer) in 0.1 M sodium ion. E1: membrane contained only a hydrogen ionophore, E2: Membrane contained both hydrogen ionophore and the lipophilic salt, KTClPB.
Figure 2.
The potentiometric response of sensor E2 with a non-plasticised methacrylic-acrylic membrane containing hydrogen ionophore and KTClPB.
Figure 2.
The potentiometric response of sensor E2 with a non-plasticised methacrylic-acrylic membrane containing hydrogen ionophore and KTClPB.
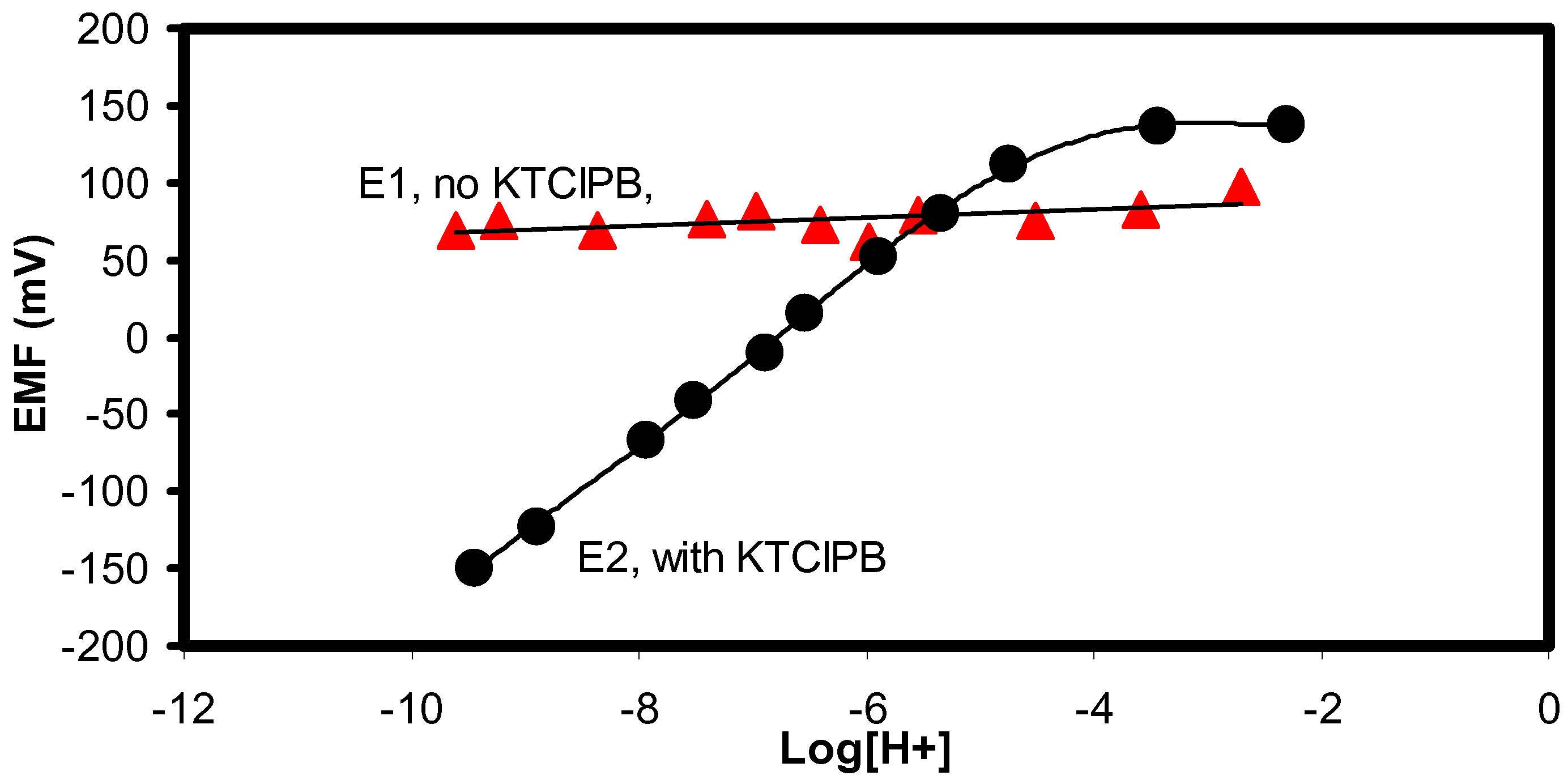
The slope of sensor E2 as shown in
Fig 2 is 56.7 mV/decade and very close to the theoretical Nernstian value. The linear pH response range was large and ranged from pH 3.4 to 9.8. In terms of selectivity towards other interfering cations, the logarithmic selectivity coefficients calculated from fixed interference studies (0.1 M interference cations) and based on the lowest hydrogen ion concentration were estimated to range from –9.15 to –9.73 (
Table 2). Hence, the presence of 0.1 M of some common interference cations did not exert large effect on the response slope and values between 55.8 to 56.9 mV/decade were obtained. However, the linear range is slightly diminished to a pH range of 5.23 to 9.56 (
Table 2,
Figure 3).
Figure 3.
The presence of 0.1 M of various cations in the solution did not interfere with the pH response of electrode E2 (From
Figure 3).
Figure 3.
The presence of 0.1 M of various cations in the solution did not interfere with the pH response of electrode E2 (From
Figure 3).
Table 2.
The performance of hydrogen ion sensors with non-plasticised MB28 methacrylic-acrylic membranes.
Table 2.
The performance of hydrogen ion sensors with non-plasticised MB28 methacrylic-acrylic membranes.
| Cations (MZ+) | E2
Slope
(mV/decade)
(0.1 M
cations) | E2
Linear
range
(- log [H+])
(0.1 M
cations) | E2
**Estimated
selectivity
coefficient
( ![Sensors 02 00339 i002]() ) ) | E2
Slope
(mV/decade)
(1.0 M
cations) | E2
Linear range
(-log [H+])
(1.0 M
cations) | E3
Slope
(mV/decade) | E3
Linear range
(-log [MZ+]) |
|---|
| NH4+ | 56.3 | 5.23–8.15 | < -9.15 | 33.6 | 4.88–7.05 | 51.5 | 1.0 – 3.0 |
| Li+ | 56.7 | 5.47–9.26 | < -9.26 | 42.5 | 4.31–7.15 | 50.7 | 1.0 – 3.0 |
| Na+ | 56.6 | 5.35–9.44 | < -9.44 | 36.2 | 4.23–7.18 | 51.3 | 1.0 – 3.0 |
| K+ | 56.9 | 5.39–9.56 | < -9.56 | 39.7 | 4.44–7.26 | 51.5 | 1.0 – 4.0 |
| Ca2+ | 55.8 | 5.65–9.23 | < -9.73 | 40.9 | 4.56–7.32 | 28.1 | 1.0 – 4.0 |
| Mg2+ | 56.8 | 5.33–8.98 | < -9.48 | 32.6 | 4.35–7.07 | 32.6 | 1.0 – 4.0 |
Although the sensor contained a membrane that was not plasticised, its analytical performance is very close to the performance of other plasticised membranes for pH sensors reported in the literature (
Table 3). This non-plasticised based membrane sensor for hydrogen ion also showed constant response after been kept unused for three months where the response slopes only varied from 56.0 to 56.6 mV/decade and the linear range remained at pH 4.3 to 9.6.
Table 3.
Comparison of performance of non-plasticised membrane pH sensor with plasticised membrane pH selective electrodes.
Table 3.
Comparison of performance of non-plasticised membrane pH sensor with plasticised membrane pH selective electrodes.
| | Methacrylic-acrylic (Solid state sensor)+ | PVC
(ion-selective electrode)(22) | PVC
(solid state sensor)(23) | Tecoflex
(ion-selective electrode)(7) |
|---|
| Plasticiser | None | DOS* | DOS | DOS |
| | | | | |
| Slope (mV/decade) | 56.7 | 57.8 | 58.0 | 58.6 |
Selectivity
coefficient, ![Sensors 02 00339 i002]()
(0.1 M cations) | |
| Na+ | < -9.44 | -10.4 ** | -11.9 | -9.1 |
| K+ | < -9.56 | -9.8** | -10.8 | -9.3 |
| Ca 2+ | < -9.73 | -11.1** | | -9.1 |
| | | | | |
| * DOS: Dioctyl sebacate | ** 1.0 M cations | + This work | | |
When the concentrations of the interference cations were increased to 10 folds, the slopes of the hydrogen ion response reduced to 33-45 mV/decade (
Table 2) indicating deterioration of sensor performance. There is also a decrease in the linear response range of the sensor but this occurred at low hydrogen ion concentrations. The sub-Nernstian behaviour of a cation selective sensor is normally caused by permeation of anions
(24), especially if the sample activities are low compared to the anion activities. In this study, when the interference cation concentration was increased, the anion concentration was also increased, therefore anion permeation might account for the reduction in response slopes. This anion permeation behaviour can be removed by the introduction of lipophilic anion such as KTClPB into the membrane. For sensor E2, the low level of KTClPB incoporated into the methacrylic-acrylic membrane is probably insufficient to hinder anion permeation, particularly when the sample anions are high.


 )
)