Polystyrene Based Silver Selective Electrodes
Abstract
:Introduction
Experimental
Reagents
Preparation of Me6(14) diene.2HClO4

Apparatus
Membrane preparation
Determination of Functional Properties of Polystyrene based Membranes
Porosity
Electrolyte Absorption
Water Content
Swelling
Potential Measurements
Results and Discussion
Membrane Characteristics
| Membrane No.3 | Water content per gram of wet membrane
g(H2O)/g(w.mem.);g/g | Porosity | Swelling | Amount of electrolyte absorbed per gram of wet membrane (moles) |
|---|---|---|---|---|
| Me6(14)diene. 2HClO4 | 0.0254 | 0.0320 | 0.204 | 4.6 × 10-2 |
| Membrane No. | Composition in ratio (w/w) | Working concentration range, M | Slope, mV/decade[Ag+] | Respon se time, s | |
|---|---|---|---|---|---|
| Me6(14)diene. 2HClO4 (ionophore) | Polystyrene (Binder) | ||||
| 1 | 8 | 1 | 5.0 × 10-5 – 1.0 × 10-1 | 48 | 30 |
| 2 | 12 | 1 | 1.0 × 10-5 – 1.0 × 10-1 | 53 | 20 |
| 3 | 15 | 1 | 5.0 × 10-6 – 1.0 × 10-1 | 53 | 15 |
| 4 | 20 | 1 | 5.0 × 10-5 – 1.0 × 10-1 | 40 | 20 |
Reference Solution
Response and Lifetime
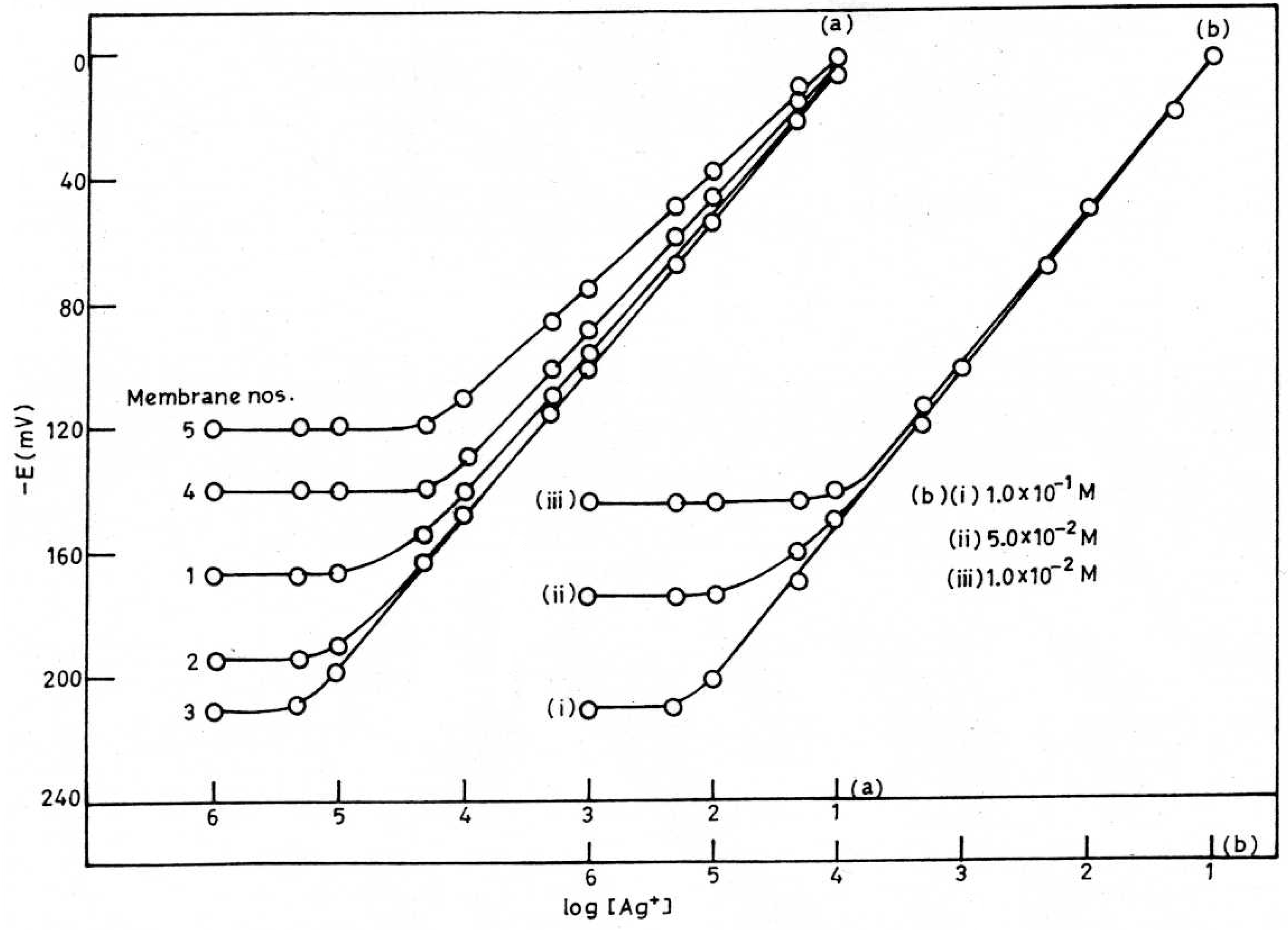
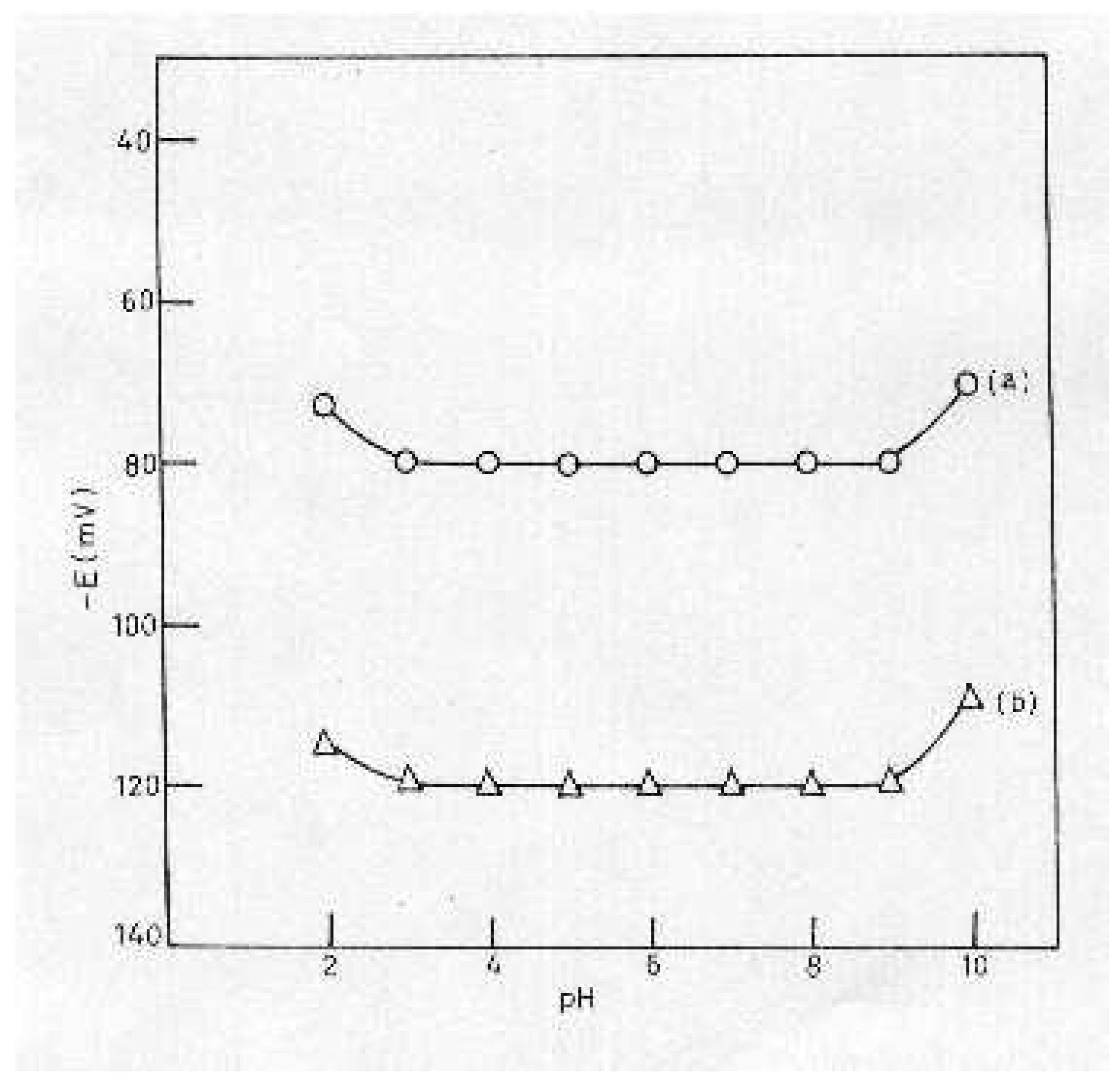
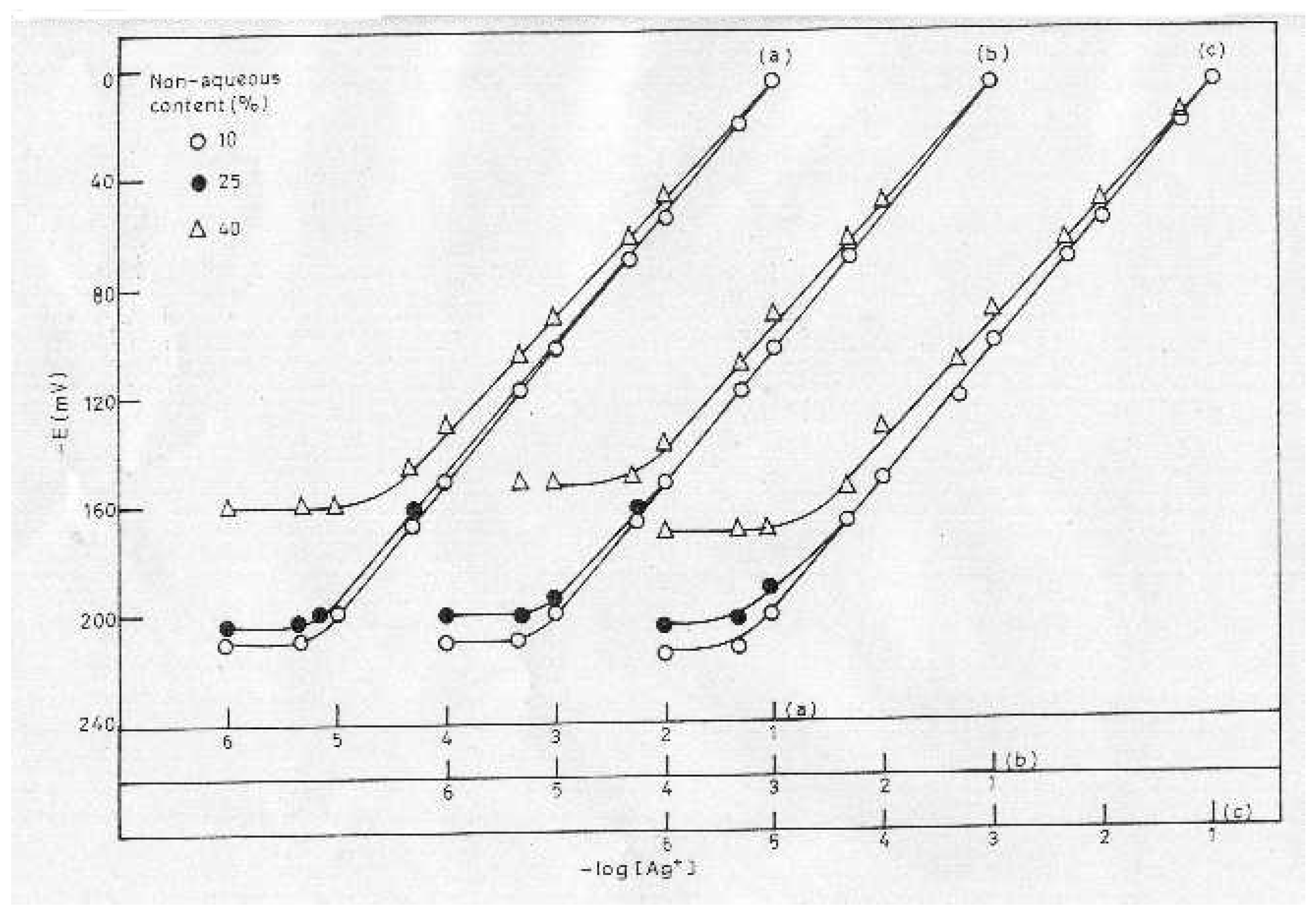
pH and Solvent Effect
Potentiometric Selectivity
 ) were evaluated by modified form of the fixed interference method [16] as suggested by Sa’ez de Viteri and Diamond at 1.0 × 10-2 M interfering ion concentration and varying concentration of Ag+ solution (Table 3). The selectivity pattern indicates sufficiently low (~ 10-3) values for monovalent cations and quite low (~ 10-4) for bivalent and trivalent ions. As such, these cations are not expected to interfere even at this higher concentration level (1.0 ×10-2 M) of the interfering ions. Heavy metals such as Cu2+ , Cd2+, Hg2+ and Pb2+ ( normal interferents) also do not disturb the functioning of the membrane sensor at all.
) were evaluated by modified form of the fixed interference method [16] as suggested by Sa’ez de Viteri and Diamond at 1.0 × 10-2 M interfering ion concentration and varying concentration of Ag+ solution (Table 3). The selectivity pattern indicates sufficiently low (~ 10-3) values for monovalent cations and quite low (~ 10-4) for bivalent and trivalent ions. As such, these cations are not expected to interfere even at this higher concentration level (1.0 ×10-2 M) of the interfering ions. Heavy metals such as Cu2+ , Cd2+, Hg2+ and Pb2+ ( normal interferents) also do not disturb the functioning of the membrane sensor at all.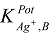 ) for Ag+ selective sensor as calculated by fixed interference method
) for Ag+ selective sensor as calculated by fixed interference method
| Interfering ion (B) | Selectivity coefficients by Fixed interference method |
|---|---|
| NH4+ | 4.46 × 10-3 |
| Na+ | 3.16 × 10-3 |
| K+ | 4.46 × 10-3 |
| Li+ | 5.0 × 10-3 |
| Mg2+ | 5.0 × 10-4 |
| Ca2+ | 3.54 × 10-4 |
| Sr2+ | 3.54 × 10-4 |
| Ba2+ | 5.0 × 10-4 |
| Cu2+ | 3.54 × 10-4 |
| Cd2+ | 2.52 × 10-4 |
| Co2+ | 3.54 × 10-4 |
| Pb2+ | 3.16 × 10-4 |
| Hg2+ | 4.46 × 10-4 |
| Ni2+ | 3.54 × 10-4 |
| Al3+ | 1.16× 10-4 |
| Cr3+ | 2.32 × 10-4 |
| Fe3+ | 1.32 × 10-4 |
Analytical Application

Conclusion
Acknowledgement
References
- Umezawa, Y. CRC Handbook of Ion Selective Electrodes: Selectivity Coefficients; CRC Press: Boca Raton, Ann Arbor, Boston, 1990. [Google Scholar]
- Casabo, J.; Teixidor, F.; Escriche, L.; Vinas, C.; Perez- Jimenez, C. Adv. Mater. 1995, 7, 238.
- Lai, M.T.; Shia, J.S. Analyst. 1986, 111, 891.
- Oue, M.; Kimura, K.; Akama, K.; Tanaka, M.; Shono, T. Chem. Lett. 1988, 3, 409.
- Xi, Z.; Li, J.; Yu, L.; Zhang, D.; Yang, J.; Luo, S.; Wu, B.; Cun, L. Huaxue Xuebao 1986, 44, 951.
- Malinowska, E.; Brazozka, Z.; Kasiura, K.; Egberink, R.J.M.; Reinhoudt, D.N. Anal. Chi. Acta. 1994, 298, 245.
- Chen, L.X.; Zeng, X.S.; He, X.M.; Zhang, Z.Z. Frensenius’ J Anal. Chem. 2000, 367, 535. [CrossRef]
- Bates, M.; Cardwell, T.J.; Cattrall, R.W.; Deady, L.W.; Gregorio, C.G. Talanta 1995, 42, 999. [PubMed]
- Chung, S.; Kim, W.; Park, S.B.; Kim, O.Y.; Lee, S.S. Talanta 1997, 44, 1087.
- Katsu, T.; Xu, D.H. Anal. Lett. 1998, 31, 1979.
- Michaelis, L. Proc. Intern. Congr. Plant Sci. Itlaco 1929, 2, 1139.
- Hale, D.K.; McCanley, M.J. Trans. Faraday. Soc. 1961, 57, 135.
- Gregor, H.P.; Jacobson, H.; Shir, R.C. J. Phy. Chem. 1957, 61, 141.
- Kawabe, M.; Tanagita, M.; Shinohara, M.; Takamatsu, T. Bull. Chem. Soc. Jpn. 1962, 21, 157.
- Wyllie, M.R.J.; Kannan, S.L. J. Phy. Chem. 1954, 58, 73.
- Lakshminarayaniah, N.; Subrahmanayam, V. J. Phy. Chem. 1968, 72, 4314.
- Marco, R.D.; Cattrall, R.W.W.; Leisengang, J. Anal. Chem. 1990, 62, 2339.
- Mizutani, V.; Nishimura, M. J. Appl. Polymer Sci. 1970, 14, 1847.
- Srivastava, S.K.; Gupta, V.K.; Dwivedi, M.K.; Jain, S. Anal. Proc. 1995, 32, 21.
- Sample Availability: Available from the authors.
2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gupta, V.K.; Antonijevic, M.V.; Chandra, S.; Agarwal, S. Polystyrene Based Silver Selective Electrodes. Sensors 2002, 2, 233-243. https://doi.org/10.3390/s20600233
Gupta VK, Antonijevic MV, Chandra S, Agarwal S. Polystyrene Based Silver Selective Electrodes. Sensors. 2002; 2(6):233-243. https://doi.org/10.3390/s20600233
Chicago/Turabian StyleGupta, Vinod Kumar, Milan V Antonijevic, Sudeshna Chandra, and Shiva Agarwal. 2002. "Polystyrene Based Silver Selective Electrodes" Sensors 2, no. 6: 233-243. https://doi.org/10.3390/s20600233
APA StyleGupta, V. K., Antonijevic, M. V., Chandra, S., & Agarwal, S. (2002). Polystyrene Based Silver Selective Electrodes. Sensors, 2(6), 233-243. https://doi.org/10.3390/s20600233




