A Prototype of an Electromagnetic Induction Sensor for Non-Destructive Estimation of the Presence of Corrosive Chemicals Ensuing Concrete Corrosion
Abstract
1. Introduction
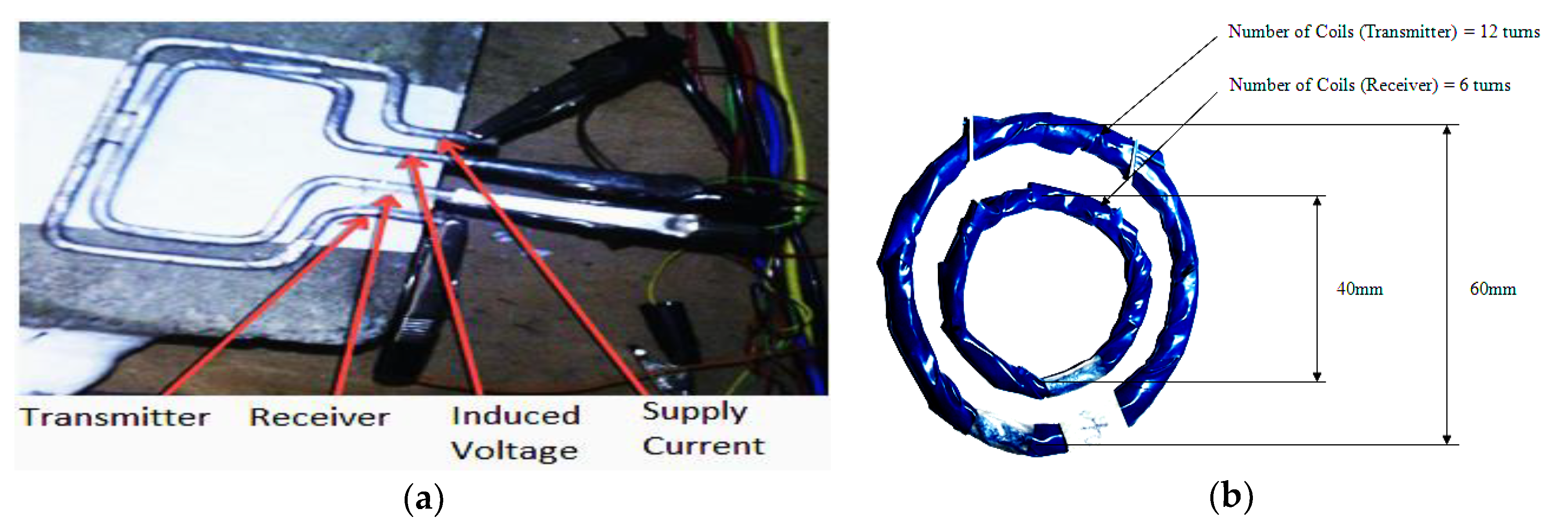
2. EMI Sensor Principles
3. Sensor Design
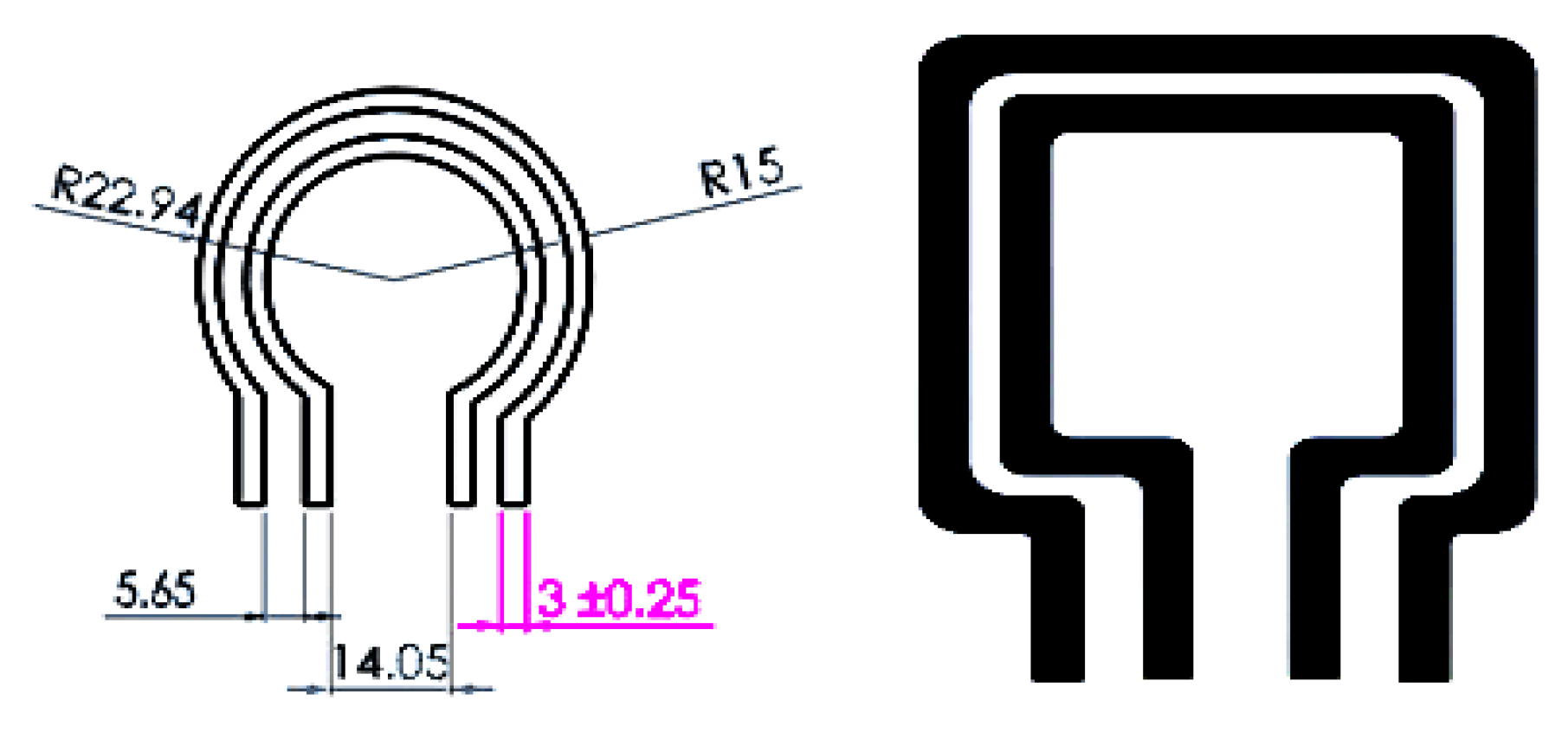
3.1. SLC
3.2. MLC
4. Experimental Design and Tests
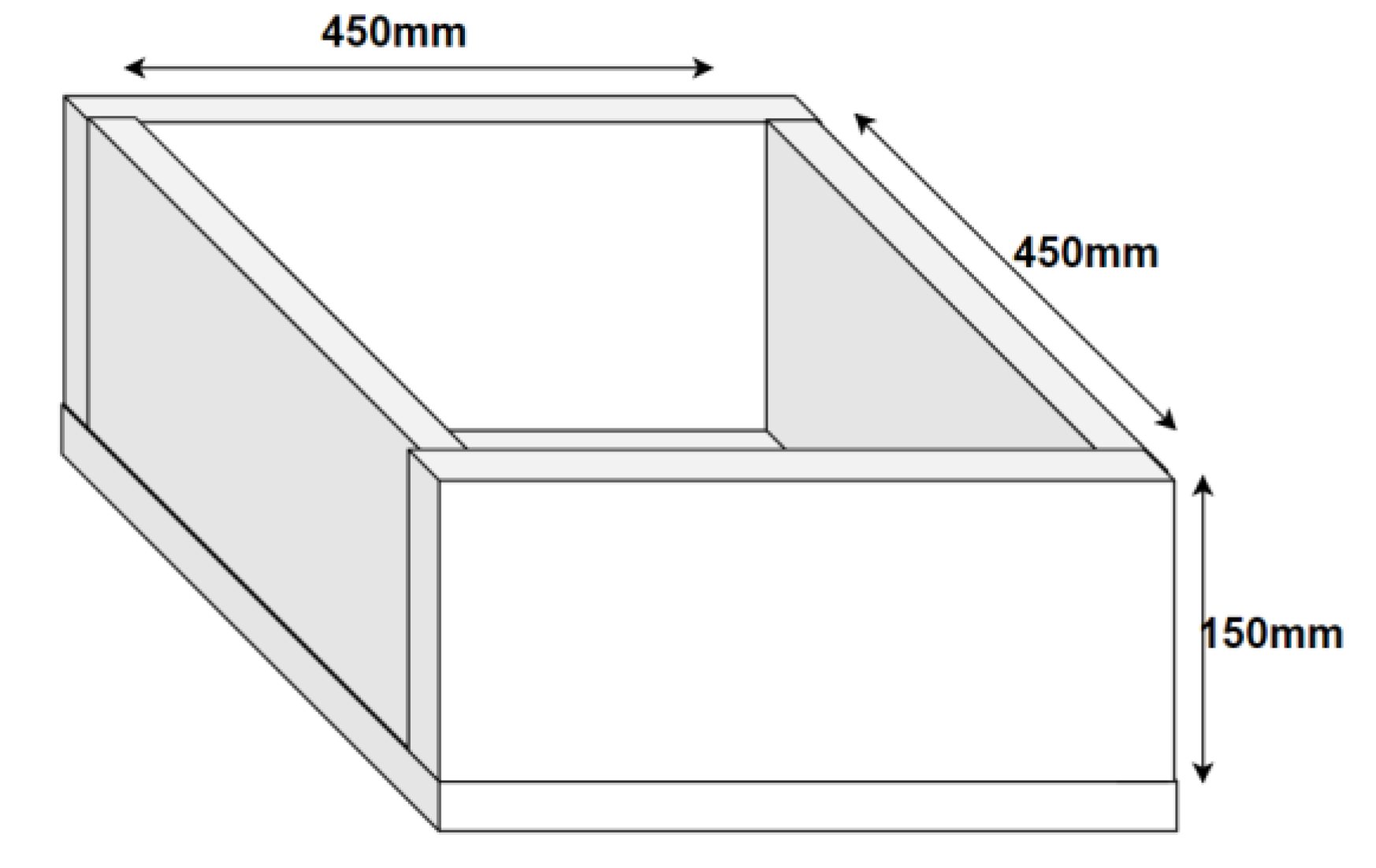
4.1. Materials
4.2. Testing Methods
5. Results and Discussion
5.1. Coplanar-Placed Sensors
5.2. Coil Placed on Opposite Surfaces
6. Conclusions
7. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, H.W.; Saraswathy, V. Corrosion Monitoring of Reinforced Concrete Structures-A. Int. J. Electrochem. Sci. 2007, 2, 1–28. [Google Scholar]
- Revie, R.W. Corrosion and Corrosion Control, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Broomfield, J.P. Corrosion of Steel in Concrete: Understanding, Investigation and Repair, 2nd ed.; E&FN Spon: London, UK, 2007; p. 277. [Google Scholar]
- Baroghel-Bouny, V.; Belin, P.; Maultzsch, M.; Henry, D. AgNO3 spray tests—Advantages, weaknesses, and various applications to quantify chloride ingress into concrete, Part 1: Non-steady-state diffusion tests in laboratory and exposure to natural conditions. Mater. Struct. 2007, 40, 759–781. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, D.; Zhang, L.; Wang, B. Detection of Defects in Reinforced Concrete Structures Using Ultrasonic Nondestructive Evaluation with Piezoceramic Transducers and the Time Reversal Method. Sensors 2018, 18, 4176. [Google Scholar] [CrossRef] [PubMed]
- Montemor, H.; Simoes, A.; Ferreira, M. Chloride induced corrosion on reinforcing steel: From the fundamentals to the monitoring techniques. Cem. Concr. Compos. 2003, 2, 491–502. [Google Scholar] [CrossRef]
- Rostam, S. Service life design of concrete structures—A challenge to designers as well as to owners. Asian J. Civ. Eng. (Build. Hous.) 2005, 6, 423–445. [Google Scholar]
- De Alcantara, N.; da Silva, F.; Guimarães, M.; Pereira, M. Corrosion assessment of steel bars used in reinforced concrete structures by means of eddy current testing. Sensors 2016, 16, 15. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Li, J.; Lim, K.S.; Nazal, N.A.M.; Ahmad, H. A Recent Progress of Steel Bar Corrosion Diagnostic Techniques in RC Structures. Sensors 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Femenias, Y.; Angst, U.; Moro, F.; Elsener, B. Development of a novel methodology to assess the corrosion threshold in concrete based on simultaneous monitoring of pH and free chloride concentration. Sensors 2018, 18, 3101. [Google Scholar] [CrossRef]
- Plooy, R.D.; Villain, S.G.; Lopes, P.; Ihamouten, A.; Dérobert, X.; Thauvin, B. Electromagnetic non-destructive evaluation techniques for the monitoring of water and chloride ingress into concrete: A comparative study. Mater. Struct. 2015, 48, 369–386. [Google Scholar] [CrossRef]
- Moreira, R.R.; Soares, T.F.; Ribeiro, J. Electrochemical investigation of corrosion on AISI 316 stainless steel and AISI 1010 carbon steel: Study of the behaviour of imidazole and benzimidazole as corrosion inhibitors. Adv. Chem. Eng. Sci. 2014, 4, 503. [Google Scholar] [CrossRef]
- Leckie, H.P.; Uhlig, H.H. Environmental factors affecting the critical potential for pitting in 18-8 stainless steel. J. Electrochem. Soc. 1966, 113, 1262–1267. [Google Scholar] [CrossRef]
- Kilic, G. Integrated health assessment strategy using NDT for reinforced concrete bridges. NDT&E Int. 2014, 61, 80–94. [Google Scholar]
- Loto, R.T.; Busari, A. Influence of White Aluminum Dross on the Corrosion Resistance of Reinforcement Carbon Steel in Simulated Concrete Pore Solution. J. Bio-Tribo-Corros. 2019, 5, 19. [Google Scholar] [CrossRef]
- Sangwook, L.; Kalos, N. Non-destructive testing methods in the US for bridge inspection and maintenance. KSCE J. Civ. Eng. 2014, 18, 1322–1331. [Google Scholar]
- Mamun, K.; Islam, F.M.; Deo, R.; Chand, A.; Cakacaka, A.; Prasad, K. An Emi Sensor for Non-Destructive Corrosion Estimation in Concrete. Available online: http://repository.usp.ac.fj/id/eprint/10940 (accessed on 9 March 2019).
- Ryan, T.W.; Hartle, R.A.; Mann, J.E.; Danovich, L.J. Bridge Inspector’s Reference Manual; Report No. FHWA NHI 03-001; Federal Highway Administration National Highway Institute: Arlington, VA, USA, 2006.
- Parmar, D.S.; Sharp, S.R. Acoustic emission for non-destructive testing of bridges and other transportation infrastructure. In Beyond the Crossroads: A National Conference on Transportation Infrastructure & Regulatory Policy; University of Denver: Denver, CO, USA, 2009. [Google Scholar]
- Sassine, E.; Laurens, S.; François, R.; Ringot, E. A critical discussion on rebar electrical continuity and usual interpretation thresholds in the field of half-cell potential measurements in steel reinforced concrete. Mater. Struct. 2018, 51, 93. [Google Scholar] [CrossRef]
- Celaya, M.; Nazarian, S.; Rao, C.; Von Quintus, H. Delamination detection of HMA airport pavements with NDT devices. In Proceedings of the 2010 FAA Worldwide Airport Technology Transfer Conference, Atlantic City, NJ, USA, 20–22 April 2010. [Google Scholar]
- Huston, D.; Hu, J.Q.; Maser, K.; Weedon, W.; Adam, C. GIMA Ground penetrating radar system for monitoring concrete bridge decks. J. Appl. Geophys. 2000, 43, 139–146. [Google Scholar] [CrossRef]
- Scott, M.; Rezaizadeh, A.; Delahaza, A.; Santos, C.G.; Moore, M.; Graybeal, B.; Washer, G. A comparison of nondestructive evaluation methods for bridge deck assessment. NDT&E Int. 2003, 36, 245–255. [Google Scholar]
- Yaman, I.O.; Inci, G.; Yesiller, N.; Aktan, H.M. Ultrasonic pulse velocity in concrete using direct and indirect transmission. ACI Mater. J. 2001, 98, 450–452. [Google Scholar]
- Liu, L.P.; Yeh, L.P. Spectral tomography of concrete structures based on impact echo depth spectra. NDT&E Int. 2011, 44, 692–702. [Google Scholar]
- Stimolo, M. Passive infrared thermography as inspection and observation tool in bridge and road construction. In Proceedings of the International Symposium on Non-Destructive Testing in Civil Engineering 2003, Berlin, Germany, 16–19 September 2003. [Google Scholar]
- Szymanik, B.; Frankowski, P.K.; Tomasz, C.; Robinson, C.; Chelliah, A.J. Detection and Inspection of Steel Bars in Reinforced Concrete Structures Using Active Infrared Thermography with Microwave Excitation and Eddy Current Sensors. Sensors 2016, 16, 234. [Google Scholar] [CrossRef]
- Hellier, C. Handbook of Nondestructive Evaluation; McGrawHill: Columbus, OH, USA, 2001. [Google Scholar]
- Naqvi, A.A.; Nagadi, M.M.; Al-Amoudi, B.S.O. Nondestructive evaluation of concrete samples using PGNAA technique. J. Radioanal. Nucl. Chem. 2007, 271, 151–154. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Zhao, T.; Lehmann, E.H.; Vontobel, P. Neutron radiography, A powerful method to determine time-dependent moisture distributions in concrete. Nucl. Eng. Des. 2011, 241, 4758–4766. [Google Scholar] [CrossRef]
- Wang, X.; Chung, D.D.L. Short carbon fiber reinforced epoxy coating as a piezoresistive strain sensor for cement mortar. Sens. Actuators A Phys. 1998, 71, 208–212. [Google Scholar] [CrossRef]
- Uemoto, T. Maintenance of concrete structure and application of non-destructive inspection in Japan. In Proceedings of the International Symposium Non-Destructive Testing in Civil Engineering, Tokyo, Japan, 25–27 April 2000. [Google Scholar]
- Rehman, S.K.; Ibrahim, Z.; Memon, S.A.; Jameel, M. Nondestructive test methods for concrete bridges: A review. Constr. Build. Mater. 2016, 107, 58–86. [Google Scholar] [CrossRef]
- Oleksandr, M.; Vincent, F. High-Resolution Microwave Near-Field Surface Imaging Using Resonance Probes. IEEE Trans. Instrum. Meas. 2016, 65, 189–200. [Google Scholar]
- Al-Mattarneh, H. Determination of chloride content in concrete using near-and far-field microwave non-destructive methods. Corros. Sci. 2016, 105, 133–140. [Google Scholar] [CrossRef]
- Bois, K.J.; Benally, A.D.; Zoughi, R. Near-field microwave non-invasive determination of NaCl in mortar. IEE Proc. Sci. Meas. Technol. 2001, 148, 178–182. [Google Scholar] [CrossRef]
- Dérobert, X.; Iaquinta, J.; Klysz, G.; Balayssac, J. Use of capacitive and GPR techniques for the non-destructive evaluation of cover concrete. NDT&E Int. 2008, 41, 44–52. [Google Scholar]
- Robert, A. Dielectric permittivity of concrete between 50 MHz and 1 GHz and GPR measurements for building materials evaluation. J. Appl. Geophys. 1998, 40, 89–94. [Google Scholar] [CrossRef]
- Scott, W.R. Broadband electromagnetic induction sensor for detecting buried landmines. In Proceedings of the 2007 IEEE International Geoscience and Remote Sensing Symposium, Barcelona, Spain, 23–28 July 2007; pp. 22–25. [Google Scholar]
- Pajewski, L.; Benedetto, A.; Derobert, X.; Giannopoulos, A.; Loizos, A.; Manacorda, G.; Marciniak, M.; Plati, C.; Schettini, G.; Trinks, I. Applications of Ground Penetrating Radar in civil engineering—COST action TU1208. In Proceedings of the 2013 7th International Workshop on Advanced Ground Penetrating Radar, Nantes, France, 2–5 July 2013; pp. 1–6. [Google Scholar]
- Nabighian, M.N.; Macnae, J.C. Time domain electromagnetic prospecting methods. Electromagn. Methods Appl. Geophys. 1991, 2, 427–509. [Google Scholar]
- Yao, J.; Tjuatja, S.; Huang, H. Real time vibratory strain sensing using passive wireless antenna sensor. IEEE Sens. J. 2015, 15, 8, 4338–4345. [Google Scholar] [CrossRef]
- Cheng, H.; Ebadi, S.; Ren, X.; Gong, X. Wireless passive high temperature sensor based on multifunctional reflective patch antenna up to 1050 degrees centigrade. Sens. Actuators A Phys. 2015, 222, 204–211. [Google Scholar] [CrossRef]
- Girbau, D.; Ramos, A.; Lazaro, A.; Rima, S.; Villarino, R. Passive wireless temperature sensor based on time-coded UWB chipless RFID tags. IEEE Trans. Microw. Theory Tech. 2012, 60, 3623–3632. [Google Scholar] [CrossRef]
- Sanders, J.W.; Yao, J.; Huang, H. Microstrip patch antenna temperature sensor. IEEE Sens. J. 2015, 15, 5312–5319. [Google Scholar] [CrossRef]
- García-Martín, J.; Gómez-Gil, J.; Vázquez-Sánchez, E. Non-destructive techniques based on eddy current testing. Sensors 2011, 11, 2525–2565. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Deo, R.N.; Kumar, A.; Mamun, K. Rebar corrosion due to chlorides in synergy with sodium, potassium, and magnesium. Constr. Build. Mater. 2018, 165, 533–540. [Google Scholar] [CrossRef]




| No. | Time Interval (s) | Dry Block (V) | Distilled Water (V) | NaCl (V) | ||||
|---|---|---|---|---|---|---|---|---|
| COP | OPP | COP | OPP | COP | OPP | |||
| 1 | 0–53,333 | Min | −0.778 | −0.053 | −1.138 | −0.053 | −0.761 | −0.044 |
| Max | 1.147 | 0.058 | 0.793 | 0.073 | 1.213 | 0.044 | ||
| Pk–Pk | 1.925 | 0.111 | 1.931 | 0.126 | 1.974 | 0.088 | ||
| 2 | 53,334–10,666 | Min | −0.767 | −0.057 | −1.14 | −0.054 | −0.758 | −0.052 |
| Max | 1.14 | 0.058 | 0.795 | 0.08 | 1.228 | 0.066 | ||
| Pk–Pk | 1.907 | 0.115 | 1.935 | 0.134 | 1.986 | 0.118 | ||
| 3 | 10,667–16,000 | Min | −0.774 | −0.056 | −1.128 | −0.053 | −0.753 | −0.053 |
| Max | 1.14 | 0.059 | 0.785 | 0.074 | 1.247 | 0.066 | ||
| Pk–Pk | 1.914 | 0.115 | 1.913 | 0.127 | 2 | 0.119 | ||
| Pk–Pk Average | 1.915 | 0.113 | 1.926 | 0.129 | 1.986 | 0.1185 | ||
| Time (10−4 s) | Dry Block (s × 10−4) | Distilled Water (s × 10−4) | NaCl (s × 10−4) | |||
|---|---|---|---|---|---|---|
| cop | opp | cop | opp | cop | opp | |
| Min 1 | 30,086 | 36,601 | 25,913 | 18,458 | 8569 | 32 |
| Max 1 | 30,346 | 32,165 | 24,565 | 20,166 | 8741 | 324 |
| Min 2 | 80,026 | 94,077 | 73,813 | 68,790 | 74,469 | 69,924 |
| Max 2 | 81,198 | 86,201 | 72,425 | 71,586 | 74,329 | 67,376 |
| Min 3 | 130,939 | 148,118 | 121,758 | 119,935 | 139,934 | 140,901 |
| Max 3 | 131,063 | 142,722 | 120,458 | 122,555 | 139,618 | 143,985 |
| Max2 − Max1 | 50,852 | 54,036 | 47,860 | 51,420 | 65,588 | 76,611 |
| Max3 − Max1 | 49,865 | 56,521 | 48,033 | 50,969 | 65,289 | 76,609 |
| Max (Diff) | 50,358 | 55,278 | 47,946 | 51,194 | 65,438 | 76,610 |
| Min2 − Min1 | 49,940 | 57,476 | 47,900 | 50,332 | 65,900 | 70,975 |
| Min3 − Min2 | 50,913 | 54,041 | 47,945 | 51,145 | 65,465 | 70,977 |
| Min (Diff) | 50,426 | 55,758 | 47,922 | 50,738 | 65,682 | 70,976 |
| Average Time Difference | 50,392 | 55,518 | 47,934 | 50,966 | 65,560 | 73,793 |
| Samples | Dry Block | Distilled Water | NaCl | Opposite |
| Dry Block | - | 0.45 s | 1.83 s | |
| Distilled Water | 0.25 s | - | 2.20 s | |
| NaCl | 1.80 s | 1.50 s | - | |
| Coplanar | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, K.A.; Deo, R.N.; Islam, F.R.; Pota, H.R.; Chand, A.A.; Prasad, K.A.; Cakacaka, A. A Prototype of an Electromagnetic Induction Sensor for Non-Destructive Estimation of the Presence of Corrosive Chemicals Ensuing Concrete Corrosion. Sensors 2019, 19, 1959. https://doi.org/10.3390/s19091959
Mamun KA, Deo RN, Islam FR, Pota HR, Chand AA, Prasad KA, Cakacaka A. A Prototype of an Electromagnetic Induction Sensor for Non-Destructive Estimation of the Presence of Corrosive Chemicals Ensuing Concrete Corrosion. Sensors. 2019; 19(9):1959. https://doi.org/10.3390/s19091959
Chicago/Turabian StyleMamun, Kabir A., Ravin N. Deo, F. R. Islam, Hemanshu R. Pota, Aneesh A. Chand, Kushal A. Prasad, and Aisake Cakacaka. 2019. "A Prototype of an Electromagnetic Induction Sensor for Non-Destructive Estimation of the Presence of Corrosive Chemicals Ensuing Concrete Corrosion" Sensors 19, no. 9: 1959. https://doi.org/10.3390/s19091959
APA StyleMamun, K. A., Deo, R. N., Islam, F. R., Pota, H. R., Chand, A. A., Prasad, K. A., & Cakacaka, A. (2019). A Prototype of an Electromagnetic Induction Sensor for Non-Destructive Estimation of the Presence of Corrosive Chemicals Ensuing Concrete Corrosion. Sensors, 19(9), 1959. https://doi.org/10.3390/s19091959






