Detection of Congestive Heart Failure Based on LSTM-Based Deep Network via Short-Term RR Intervals
Abstract
1. Introduction
2. Materials and Methods
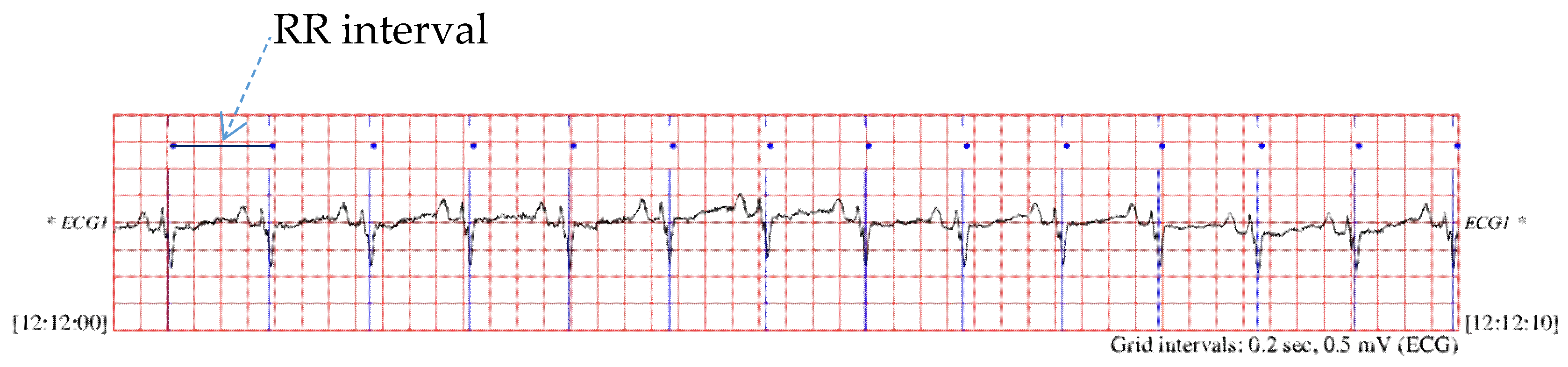
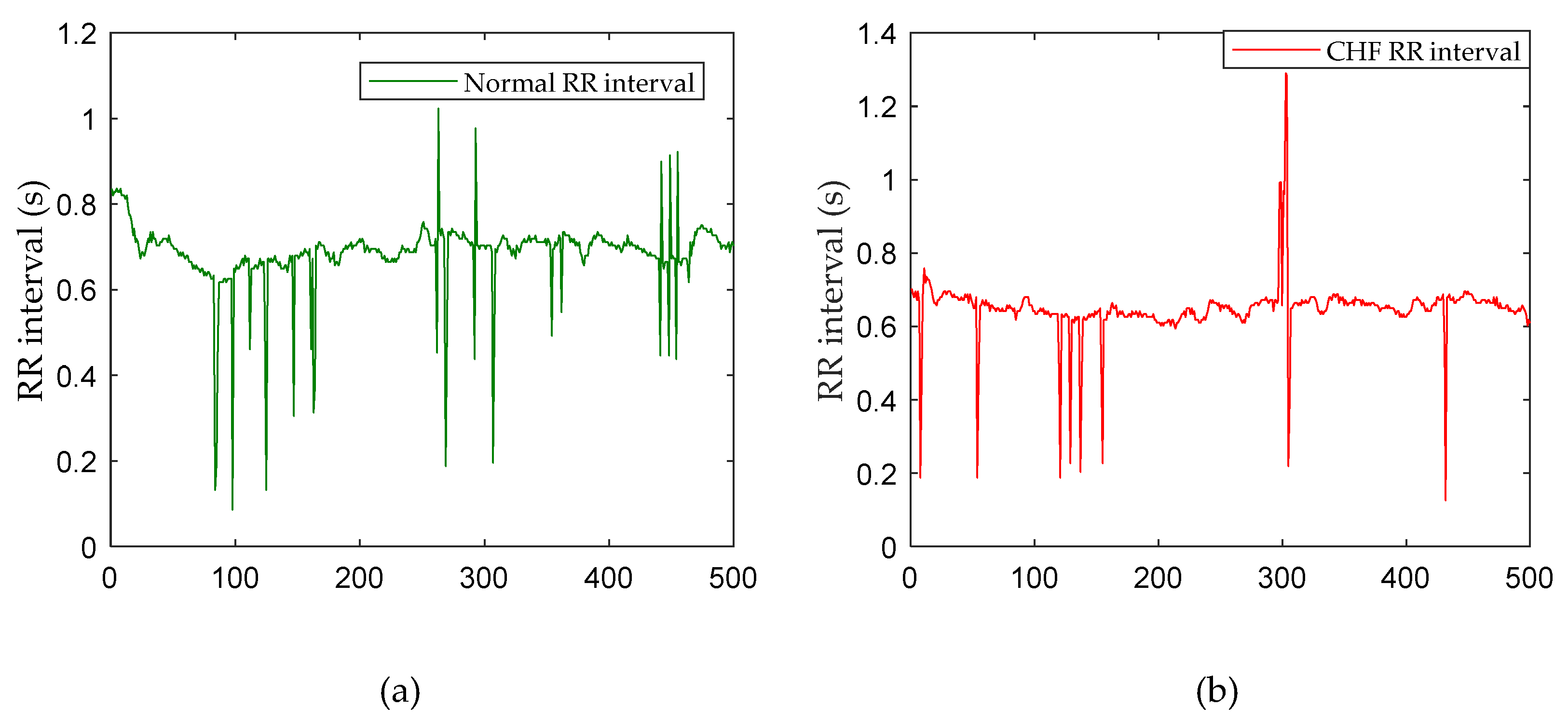
2.1. Data
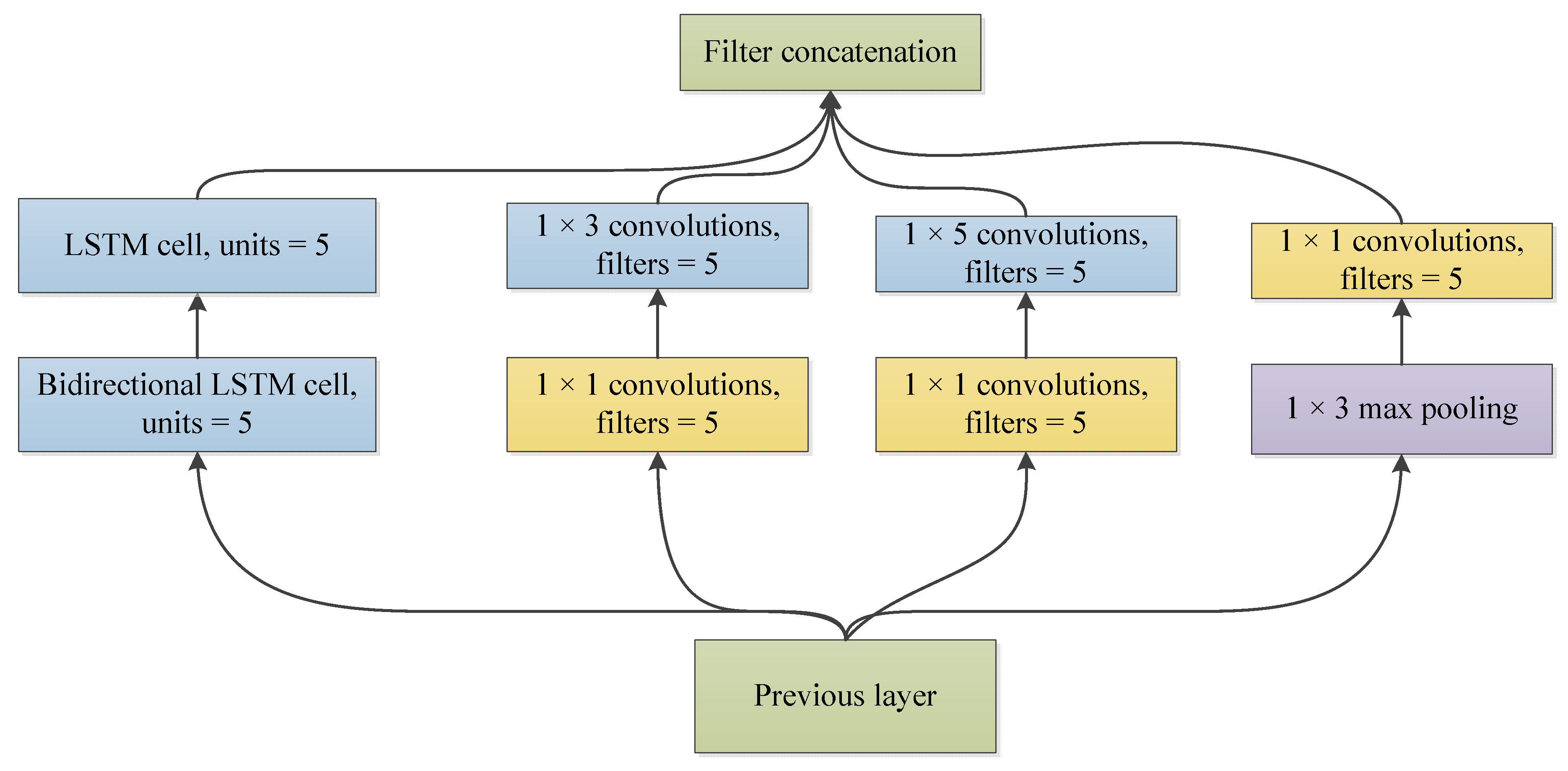
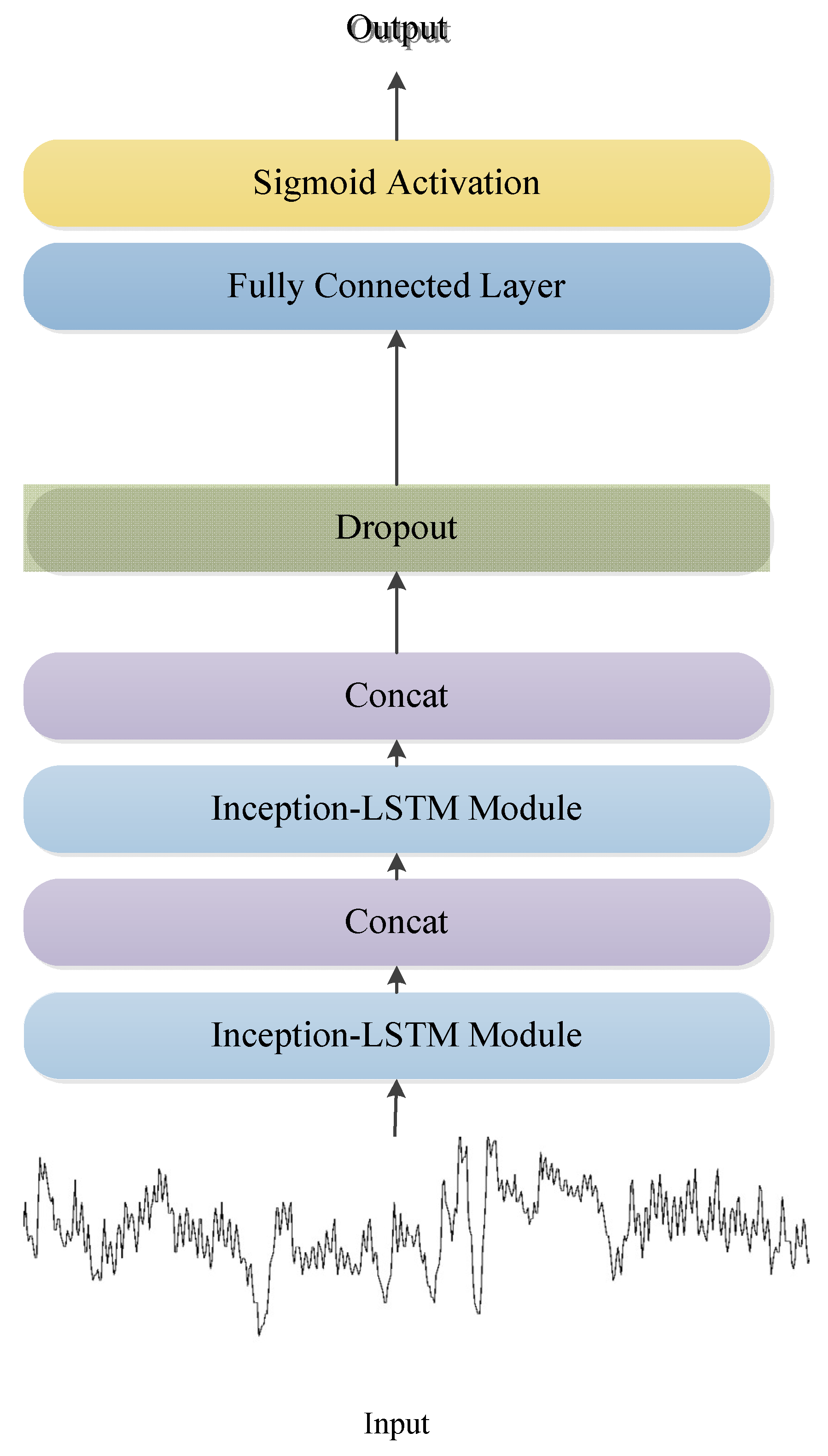
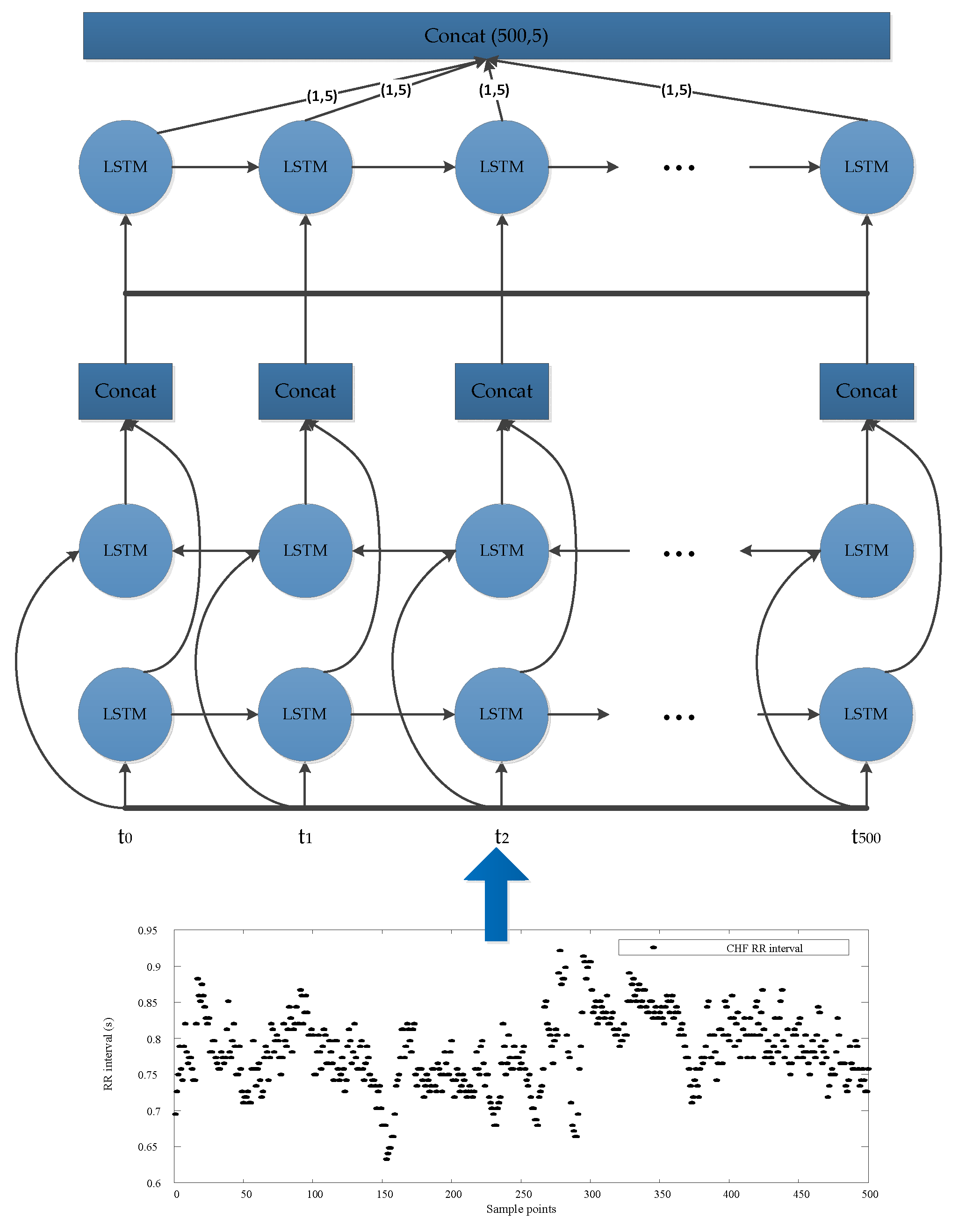
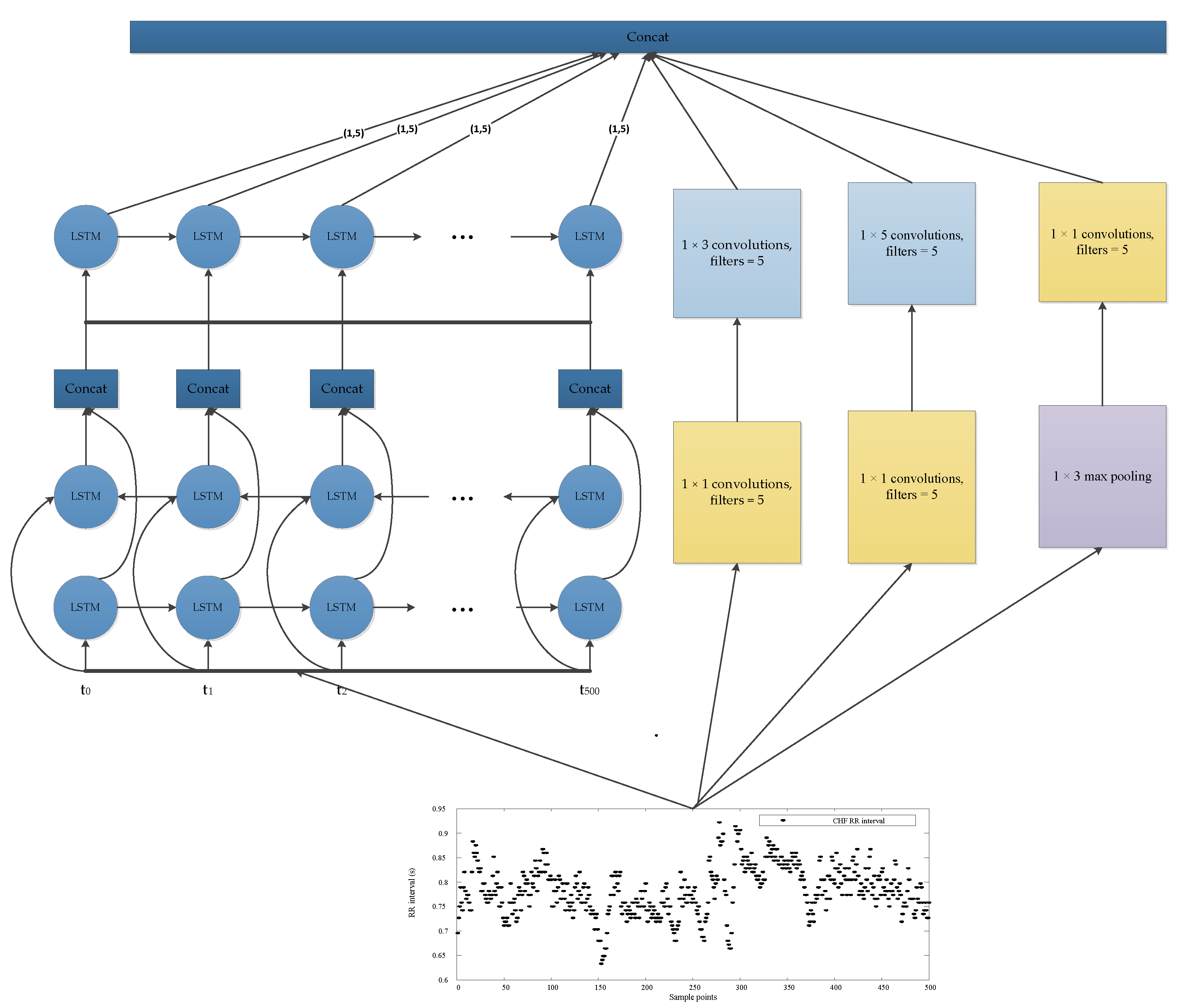
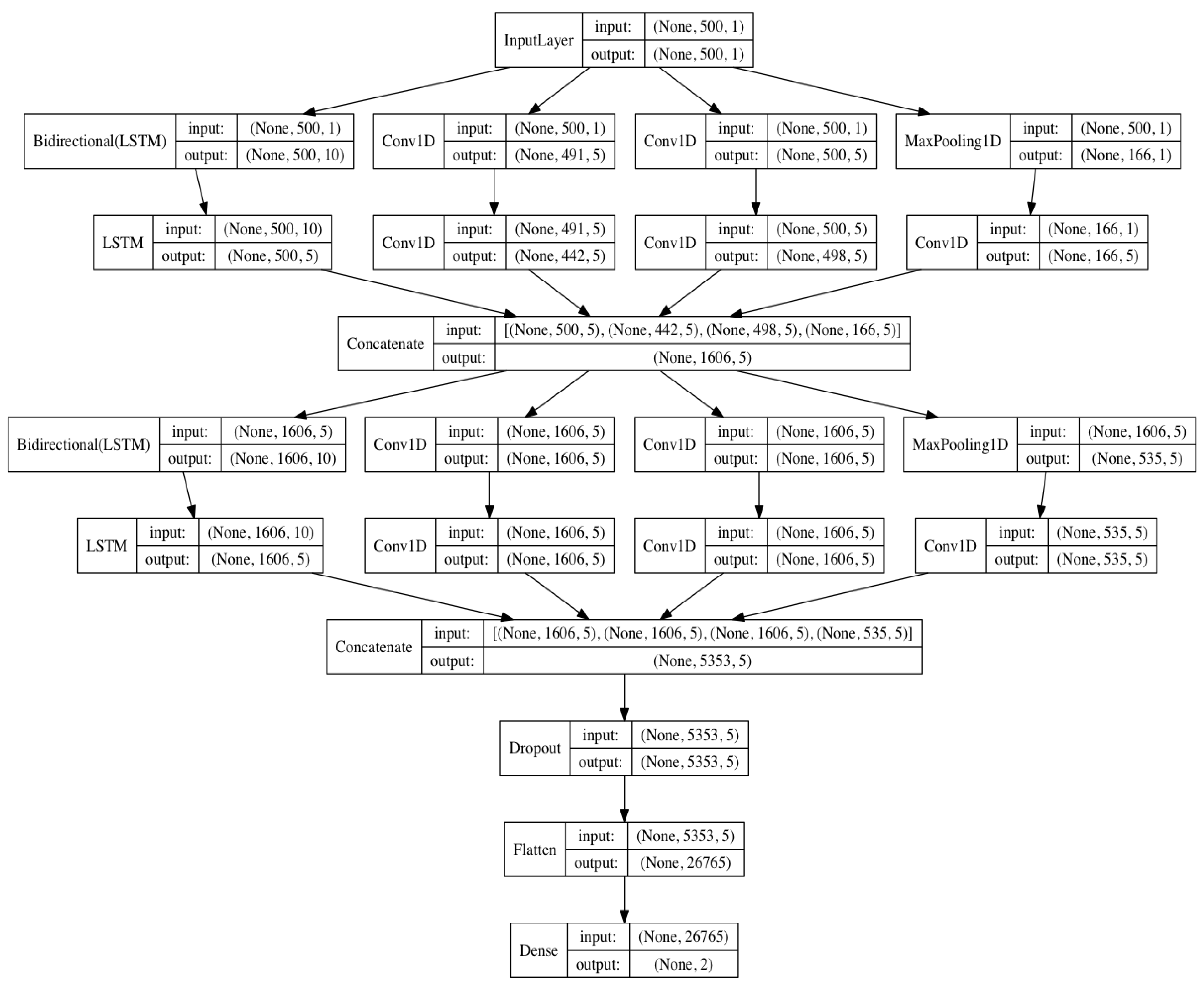
2.2. LSTM-Based Deep Convolutional Neural Network Structure
2.3. Evaluation Method
Specificity = TN/(TN + FP)
Accuracy = (TP + TN)/(TP + FP + TN + FN),
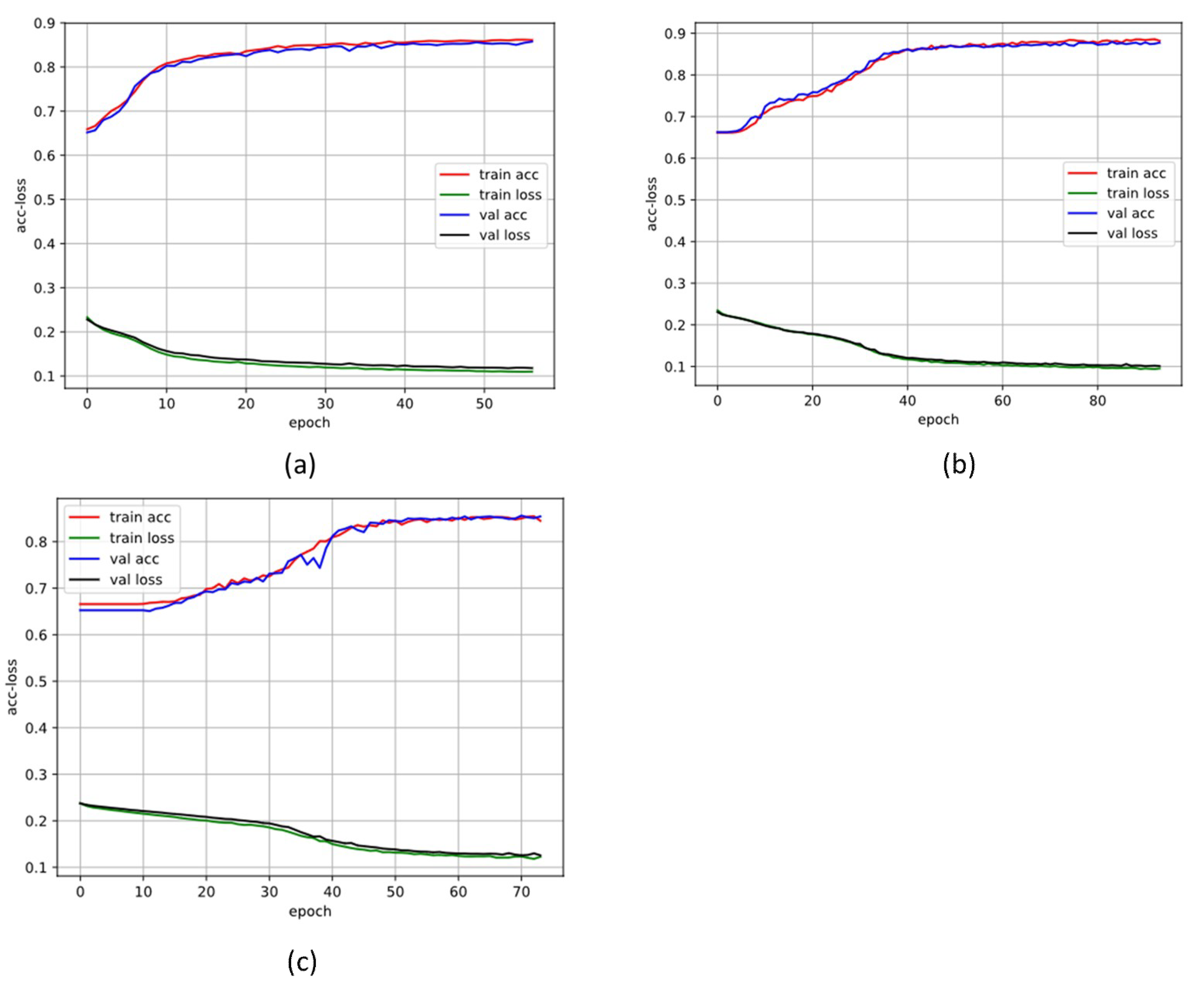
3. Results
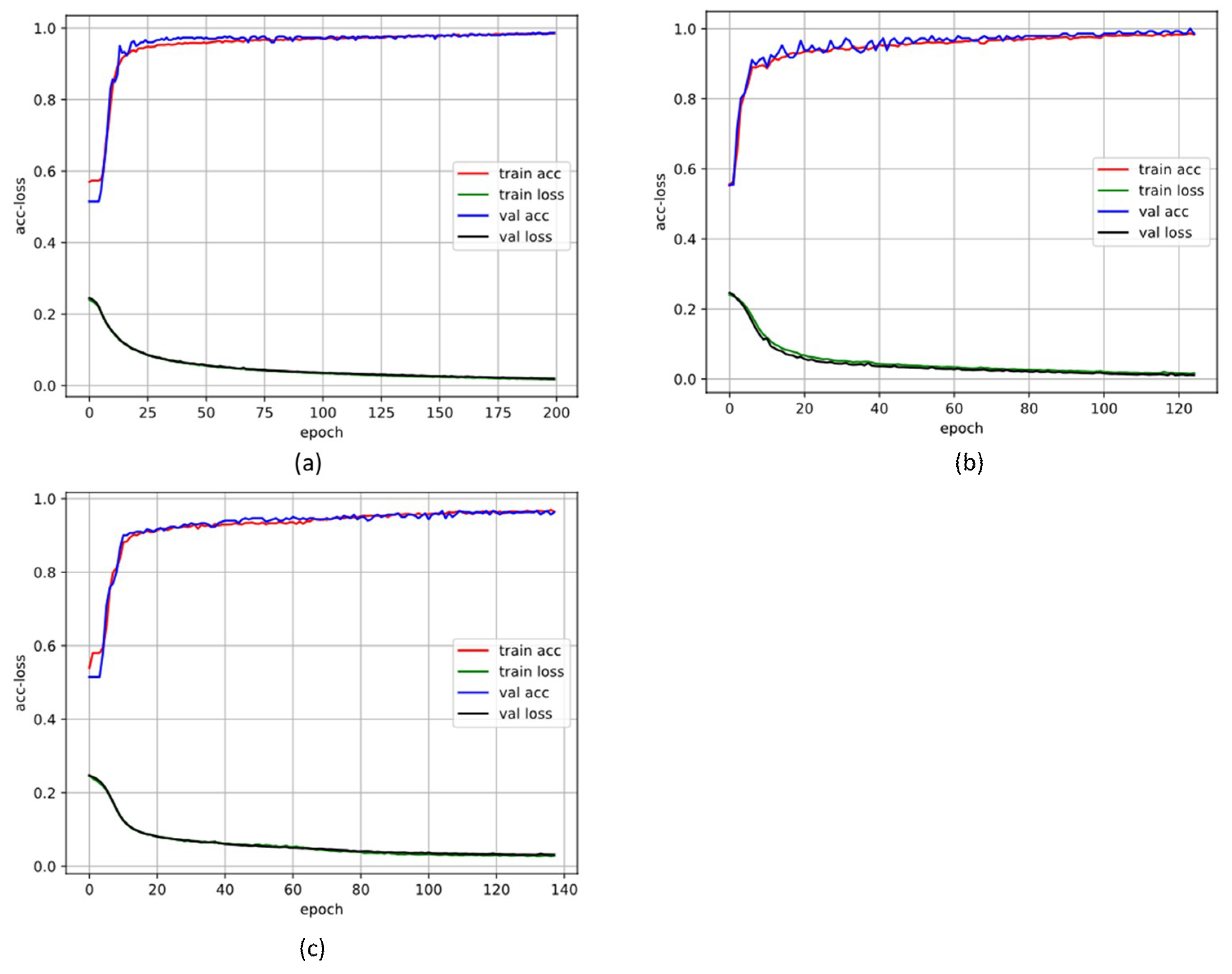
3.1. 10-Fold Cross-Validation Stage
3.2. Blind Fold Testing Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L. Understanding the epidemic of heart failure: Past, present, and future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circulation. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Rustad, J.K.; Stern, T.A.; Hebert, K.A.; Musselman, D.L. Diagnosis and treatment of depression in patients with congestive heart failure: A review of the literature. Prim. Care Companion CNS Disord. 2013, 15, PCC.13r01511. [Google Scholar] [CrossRef]
- Dhingra, R.; Pencina, M.J.; Wang, T.J.; Nam, B.-H.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Kannel, W.B.; D’Agostino, R.B.; Vasan, R.S. Electrocardiographic QRS duration and the risk of congestive heart failure: The Framingham Heart Study. Hypertension 2006, 47, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, L.G.; Cygankiewicz, I.; McNitt, S.; Vazquez, R.; Bayes-Genis, A.; Han, L.; Sur, S.; Couderc, J.P.; Berger, R.D.; de Luna, A.B.; et al. Predictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: Competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac death. Circ. Arrhythm. Electrophysiol. 2012, 5, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Batin, P.D.; Andrews, R.; Lindsay, S.J.; Brooksby, P.; Mullen, M.; Baig, W.; Flapan, A.D.; Cowley, A.; Prescott, R.J.; et al. Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 1998, 98, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Binkley, P.F.; Nunziata, E.; Haas, G.J.; Nelson, S.D.; Cody, R.J. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: Demonstration in human subjects and verification in a paced canine model of ventricular failure. J. Am. Coll. Cardiol. 1991, 18, 464–472. [Google Scholar] [CrossRef]
- Yu, S.N.; Lee, M.Y. Bispectral analysis and genetic algorithm for congestive heart failure recognition based on heart rate variability. Comput. Boil. Med. 2012, 42, 816–825. [Google Scholar] [CrossRef]
- Woo, M.A.; Stevenson, W.G.; Moser, D.K.; Middlekauff, H.R. Complex heart rate variability and serum norepinephrine levels in patients with advanced heart failure. J. Am. Coll. Cardiol. 1994, 23, 565–569. [Google Scholar] [CrossRef]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, L.; Li, K.; Wang, Q.; Liu, G.; Jiang, Q. A Novel and Effective Method for Congestive Heart Failure Detection and Quantification Using Dynamic Heart Rate Variability Measurement. PLoS ONE 2016, 11, e0165304. [Google Scholar] [CrossRef] [PubMed]
- Wenhui, C.; Guanzheng, L.; Su, S.; Qing, J.; Hung, N. A CHF detection method based on deep learning with RR intervals. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 2017, 3369–3372. [Google Scholar] [CrossRef]
- Smith, A.L.; Owen, H.; Reynolds, K.J. Heart rate variability indices for very short-term (30 beat) analysis. Part 2: Validation. J. Clin. Monit. Comput. 2013, 27, 577–585. [Google Scholar] [CrossRef]
- Lake, D.E.; Moorman, J.R. Accurate estimation of entropy in very short physiological time series: The problem of atrial fibrillation detection in implanted ventricular devices. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H319–H325. [Google Scholar] [CrossRef] [PubMed]
- Faust, O.; Shenfield, A.; Kareem, M.; San, T.R.; Fujita, H.; Acharya, U.R. Automated detection of atrial fibrillation using long short-term memory network with RR interval signals. Comput. Biol. Med. 2018, 102, 327–335. [Google Scholar] [CrossRef]
- Thakre, T.P.; Smith, M.L. Loss of lag-response curvilinearity of indices of heart rate variability in congestive heart failure. BMC Cardiovasc. Disord. 2006, 6. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Zhao, L.; Liu, F.; Zheng, D.; Liu, C.; Liu, S. Analysis of heart rate variability using fuzzy measure entropy. Comput. Boil. Med. 2013, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wei, S.; Zhang, C.; Zhang, Y.; Jiang, X.; Liu, F.; Liu, C. Determination of Sample Entropy and Fuzzy Measure Entropy Parameters for Distinguishing Congestive Heart Failure from Normal Sinus Rhythm Subjects. Entropy 2015, 17, 6270–6288. [Google Scholar] [CrossRef]
- Liu, C.; Gao, R. Multiscale Entropy Analysis of the Differential RR Interval Time Series Signal and Its Application in Detecting Congestive Heart Failure. Entropy 2017, 19, 251. [Google Scholar]
- Yoon, K.H.; Nam, Y.; Thap, T.; Jeong, C.; Ho Kim, N.; Seok Ko, J.; Noh, S.E.; Lee, J. Automatic Detection of Congestive Heart Failure and Atrial Fibrillation with Short RR Interval Time Series. J. Electr. Eng. Technol. 2017, 12, 346–355. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Baim, D.S.; Colucci, W.S.; Monrad, E.S.; Smith, H.S.; Wright, R.F.; Lanoue, A.; Gauthier, D.F.; Ransil, B.J.; Grossman, W.; Braunwald, E. Survival of patients with severe congestive heart failure treated with oral milrinone. J. Am. Coll. Cardiol. 1986, 7, 661–670. [Google Scholar] [CrossRef]
- Lee, I.; Kim, D.; Kang, S.; Lee, S. Ensemble Deep Learning for Skeleton-Based Action Recognition Using Temporal Sliding LSTM Networks. In Proceedings of the IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436. [Google Scholar] [CrossRef] [PubMed]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Yanjie, D.; Yisheng, L.; Fei-Yue, W. Travel time prediction with LSTM neural network. In Proceedings of the IEEE 19th International Conference on Intelligent Transportation Systems (ITSC), Rio de Janeiro, Brazil, 1–4 November 2016; pp. 1053–1058. [Google Scholar]
- Deng, L.; Li, J.; Huang, J.; Yao, K.; Yu, D.; Seide, F.; Seltzer, M.; Zweig, G.; He, X.; Williams, J.; et al. Recent advances in deep learning for speech research at Microsoft. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013; pp. 8604–8608. [Google Scholar]
- Potes, C.; Parvaneh, S.; Rahman, A.; Conroy, B. Ensemble of feature-based and deep learning-based classifiers for detection of abnormal heart sounds. In Proceedings of the Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 621–624. [Google Scholar]
- Hwang, B.; You, J.; Vaessen, T.; Myin-Germeys, I.; Park, C.; Zhang, B.-T. Deep ECGNet: An Optimal Deep Learning Framework for Monitoring Mental Stress Using Ultra Short-Term ECG Signals. Telemed. J. e-Health 2018, 24, 753–772. [Google Scholar] [CrossRef]
- Pourbabaee, B.; Roshtkhari, M.J.; Khorasani, K. Deep Convolutional Neural Networks and Learning ECG Features for Screening Paroxysmal Atrial Fibrillation Patients. IEEE Trans. Syst. Man Cybern. Syst. 2018, 48, 2095–2104. [Google Scholar] [CrossRef]
- Szegedy, C.; Wei, L.; Yangqing, J.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation Applied to Handwritten Zip Code Recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Iyengar, N.; Peng, C.K.; Morin, R.; Goldberger, A.L.; Lipsitz, L.A. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am. J. Physiol. 1996, 271, R1078–R1084. [Google Scholar] [CrossRef]
- Min, L.; Chen, Q.; Yan, S. Network in Network. arXiv, 2013; arXiv:1312.4400. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Kingma, D.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv, 2014; arXiv:1412.6980. [Google Scholar]
- Kumar, M.; Pachori, R.B.; Acharya, U.R. Use of Accumulated Entropies for Automated Detection of Congestive Heart Failure in Flexible Analytic Wavelet Transform Framework Based on Short-Term HRV Signals. Entropy 2017, 19, 92. [Google Scholar] [CrossRef]
| Database | Total Segments | ||
|---|---|---|---|
| N = 500 | N = 1000 | N = 2000 | |
| BIDMC congestive heart failure database (CHF) | 3214 | 1607 | 803 |
| Congestive heart failure RR interval database (CHF) | 6622 | 3311 | 1655 |
| MIT-BIH normal sinus rhythm database (NSR) | 3579 | 1739 | 869 |
| Normal sinus rhythm RR interval database (NSR) | 11,583 | 5791 | 2895 |
| Fantasia dataset (NSR) | 500 | 250 | 125 |
| Layer | Type | Depth | Segment Length | Output Shape |
|---|---|---|---|---|
| 0 | Input layer | 0 | 500 | 500 × 1 |
| 1000 | 1000 × 1 | |||
| 2000 | 2000 × 1 | |||
| 0–1 | Inception-LSTM module#1 | 2 | 500 | 1606 × 5 |
| 1000 | 3327 × 5 | |||
| 2000 | 6660 × 5 | |||
| 1–2 | Concatenate layer | |||
| 2–3 | Inception-LSTM module#2 | 2 | 500 | 5353 × 5 |
| 1000 | 11,090 × 5 | |||
| 2000 | 22,200 × 5 | |||
| 3–4 | Concatenate layer | |||
| 4–5 | Dropout | 0 | - | |
| 5–6 | fully connected | 1 | 500 | 26,765 |
| 1000 | 55,450 | |||
| 2000 | 111,000 | |||
| 6 | Sigmoid | 0 | 2 | |
| Database | BIDMC-CHF | CHF-RR | MIT-BIH NSR | NSR-RR | Fantasia |
|---|---|---|---|---|---|
| Database-1 (DB1) | √ | √ | √ | ||
| Database-2 (DB2) | √ | √ |
| Parameters | Value |
|---|---|
| Shuffled | True |
| Batch size | 128 |
| Max epochs | 100 |
| Early stopping | monitor = validation loss, patience = 5 |
| Loss function | Binary entropy |
| Optimizer | Adaptive moment estimation |
| Method | Classifier | Features | Length | Evaluation | ||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | ||||
| [38] | LS-SVM | Accumulated fuzzy entropy and accumulated permutation entropy | 500 | 98.07% | 98.33% | 98.21% |
| 1000 | 97.95% | 98.07% | 98.01% | |||
| 2000 | 97.76% | 97.67% | 97.71% | |||
| This paper | Inception module | - | 500 | 97.80% | 98.16% | 97.96% |
| 1000 | 98.67% | 96.69% | 97.84% | |||
| 2000 | 93.82% | 100.00% | 96.75% | |||
| LSTM based Inception | - | 500 | 99.45% | 98.91% | 99.14% | |
| 1000 | 97.74% | 98.72% | 98.31% | |||
| 2000 | 97.64% | 99.83% | 98.69% | |||
| Method | Classifier | Features | Length | Evaluation | ||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | ||||
| [13] | DNNs | Sparse-auto-encoder | 500 | 49.09% | 86.33% | 72.86% |
| [20] | SVM | Multiscale entropy of ΔRR | 1000 | 86.2% | 85.2% | 85.5% |
| 2000 | 84.4% | 86.8% | 85.6% | |||
| This paper | Inception module | - | 500 | 97.38% | 30.14% | 74.32% |
| 1000 | 86.38% | 58.31% | 76.56% | |||
| 2000 | 87.87% | 62.93% | 79.31% | |||
| LSTM based Inception | - | 500 | 91.21% | 74.91% | 86.42% | |
| 1000 | 92.07% | 76.47% | 87.76% | |||
| 2000 | 90.83% | 77.65% | 86.63% | |||
| Database | Blind Validation Dataset | ||||
|---|---|---|---|---|---|
| Subject Information (Age, Sex, Number) | Total Segments | ||||
| CHF | Normal | N = 500 | N = 1000 | N = 2000 | |
| Database-1 (DB1) | (54, F, #11) (63, M, #13) (61, M, #14) | (50, F, #19830) (38, F, #19140) (34, M, #19093) | 686 | 339 | 164 |
| Database-2 (DB2) | (35, unknown, #224) (66, unknown, #225) (51, unknown, #226) (64, unknown, #227) (51, unknown, #228) (58, unknown, #229) | (39, M, #049) (29, M, #050) (40, M, #051) (35, M, #054) (64, F, #001) (67, F, #003) | 2707 | 1343 | 662 |
| Dataset | Segment Length | Evaluation | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | ||
| DB1 | 500 | 99.22% | 99.72% | 99.22% |
| 1000 | 98.13% | 100.00% | 98.85% | |
| 2000 | 98.85% | 98.99% | 98.92% | |
| DB2 | 500 | 91.90% | 73.58% | 82.51% |
| 1000 | 96.85% | 75.82% | 86.68% | |
| 2000 | 94.14% | 81.25% | 87.55% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, X. Detection of Congestive Heart Failure Based on LSTM-Based Deep Network via Short-Term RR Intervals. Sensors 2019, 19, 1502. https://doi.org/10.3390/s19071502
Wang L, Zhou X. Detection of Congestive Heart Failure Based on LSTM-Based Deep Network via Short-Term RR Intervals. Sensors. 2019; 19(7):1502. https://doi.org/10.3390/s19071502
Chicago/Turabian StyleWang, Ludi, and Xiaoguang Zhou. 2019. "Detection of Congestive Heart Failure Based on LSTM-Based Deep Network via Short-Term RR Intervals" Sensors 19, no. 7: 1502. https://doi.org/10.3390/s19071502
APA StyleWang, L., & Zhou, X. (2019). Detection of Congestive Heart Failure Based on LSTM-Based Deep Network via Short-Term RR Intervals. Sensors, 19(7), 1502. https://doi.org/10.3390/s19071502





