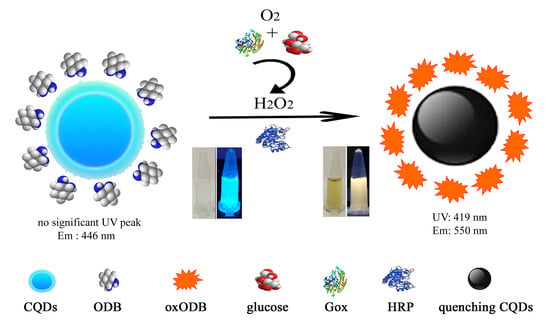
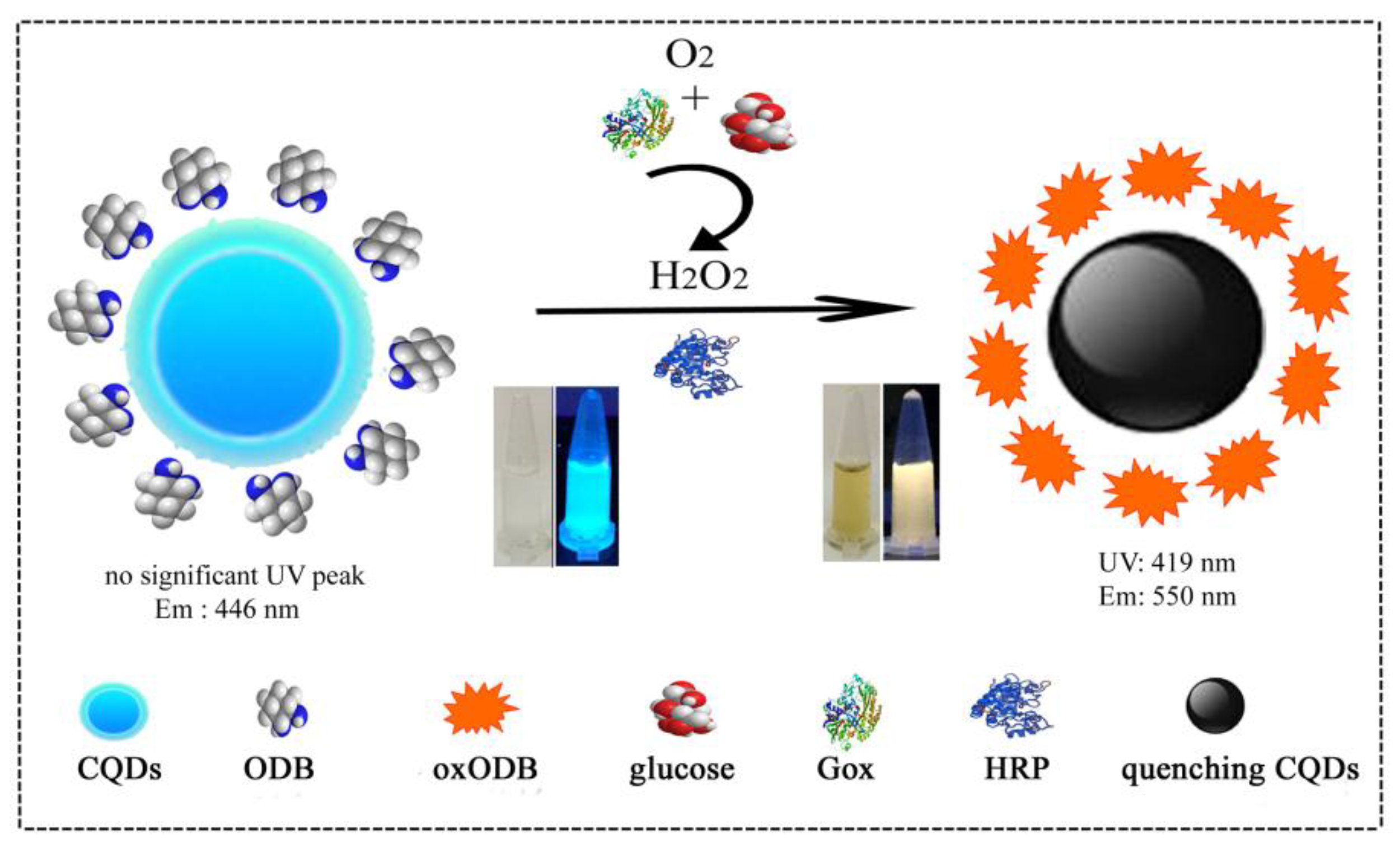
Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Synthesis of Fluorescent CQDs
2.3. Development of a Colorimetric and Ratiometric Fluorescence Dual-Mode Sensor for the Detection of Glucose
2.4. Selectivity Experiment
2.5. Determination and Spiking Recoveries of Glucose in Normal Human Serum
3. Results
3.1. The Principle of a Dual-Mode Sensor for Detecting Glucose
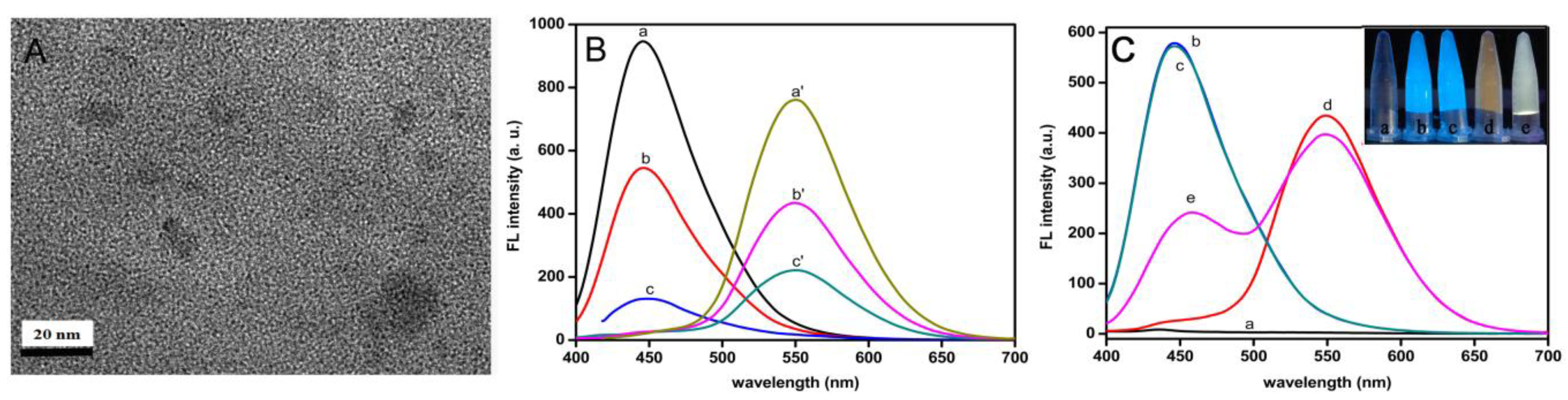
3.2. Characterization of CQDs
3.3. Optimization of Main Experimental Parameters
3.4. Detection of Glucose Using the Dual-Mode Sensor
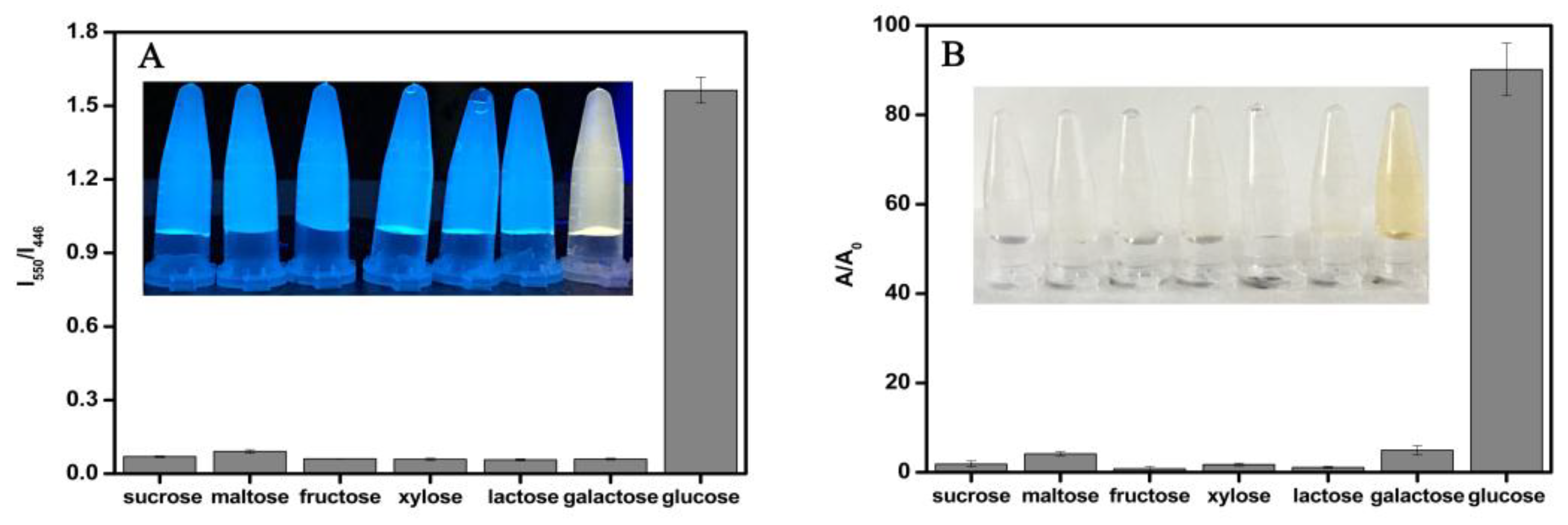
3.5. The Selectivity of the Dual-Mode Sensor
3.6. Real Samples Detection
3.7. Preparation of a Glucose Test Paper Based on the Dual-Mode Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steiner, M.S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Huang, Y.J.; Wang, T.H.; Ma, J.M. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Pandya, A.; Sutariya, P.G.; Menon, S.K. A non enzymatic glucose biosensor based on an ultrasensitive calix[4]arene functionalized boronic acid gold nanoprobe for sensing in human blood serum. Analyst 2013, 138, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Gao, W.L.; Gao, Y.S.; Wang, Q.; Umar, A. Co3O4 nanoparticles/MWCNTs composites: A potential scaffold for hydrazine and glucose electrochemical detection. RSC Adv. 2017, 7, 50087–50096. [Google Scholar] [CrossRef]
- Hui, N.; Wang, W.T.; Xu, G.Y.; Luo, X.L. Graphene oxide doped poly(3,4-ethylenedioxythiophene) modified with copper nanoparticles for high performance nonenzymatic sensing of glucose. J. Mater. Chem. B 2015, 3, 556–561. [Google Scholar] [CrossRef]
- Liu, L.L.; Ma, Q.; Li, Y.; Liu, Z.P.; Su, X.G. A novel signal-off electrochemiluminescence biosensor for the determination of glucose based on double nanoparticles. Biosens. Bioelectron. 2015, 63, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tan, X.R.; Liu, X.F.; Ou, X.; Chen, S.H.; Wei, S.P. A novel non-enzymatic electrochemiluminescence sensor for the detection of glucose based on the competitive reaction between glucose and phenoxy dextran for concanavalin A binding sites. Electrochim. Acta 2015, 180, 471–478. [Google Scholar] [CrossRef]
- Kitte, S.A.; Gao, W.Y.; Zholudov, Y.T.; Qi, L.M.; Nsabimana, A.; Liu, Z.Y.; Xu, G.B. Stainless steel electrode for sensitive luminol electrochemiluminescence detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef]
- Chaichi, M.J.; Ehsani, M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol-H2O2-gold nanoparticle chemiluminescence. Sens. Actuators B Chem. 2016, 223, 713–722. [Google Scholar] [CrossRef]
- Mao, X.X.; Lu, Y.W.; Zhang, X.D.; Huang, Y.M. β-Cyclodextrin functionalization of metal-organic framework MOF-235 with excellent chemiluminescence activity for sensitive glucose biosensing. Talanta 2018, 188, 161–167. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zhang, Y.D.; Jiang, Z.W.; Wang, D.M.; Huang, C.Z.; Li, Y.F. Fe3O4 and metal–organic framework MIL-101(Fe) composites catalyze luminol chemiluminescence for sensitively sensing hydrogen peroxide and glucose. Talanta 2018, 179, 43–50. [Google Scholar] [CrossRef]
- Bi, X.S.; Du, X.Z.; Jiang, J.J.; Huang, X. Facile and Sensitive Glucose Sandwich Assay Using in Situ Generated Raman Reporters. Anal. Chem. 2015, 87, 2016–2021. [Google Scholar] [CrossRef]
- Hu, Y.H.; Cheng, H.J.; Zhao, X.Z.; Wu, J.J.X.; Muhammad, F.; Lin, S.C.; He, J.; Zhou, L.Q.; Zhang, C.P.; Deng, Y.; et al. Surface-Enhanced Raman Scattering-Active Gold Nanoparticles with Enzyme Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef] [PubMed]
- Al-Ogaidi, I.; Gou, H.L.; Al-kazaz, A.K.A.; Aguilar, Z.P.; Melconian, A.K.; Zheng, P.; Wu, N.Q. A gold@silica core–shell nanoparticle-based surface-enhanced Raman scattering biosensor for label-free glucose detection. Anal. Chim. Acta 2014, 811, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.M.; Zhang, Y.; Li, L.; Kong, Q.K.; Zhang, L.N.; Ge, S.G.; Yu, J.H. Colorimetric and electrochemiluminecence dual mode sensing of lead ion based on integrated lab-on-paper device. ACS Appl. Mater. Interfaces 2018, 10, 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Zhao, D.; Sun, J.; Yang, X.R. A dual-mode signaling responses of AuNPs-fluorescein based probe for specific detection of thiourea. Analyst 2016, 141, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.X.; Sun, J.; Yang, X.R. Carbon quantum dots-assisted colorimetric and fluorometric dual-mode protocol for acetylcholinesterase activity and inhibitors screening based on the inner filter effect of silver nanoparticles. Analyst 2016, 141, 3280–3288. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liang, R.X.; Liu, W.; Zhao, Q.B.; Zhu, X.Y.; Yang, L.; Zou, P.; Wang, X.X.; Ding, F.; Rao, H.B. A dual-mode probe for colorimetric and fluorometric detection of cysteine based on phosphorus/nitrogen co-doped CQDs and gold nanorods. Sens. Actuators B Chem. 2018, 273, 1627–1634. [Google Scholar] [CrossRef]
- Li, N.; Na, W.D.; Liu, H.; Su, X.G. Dual mode detection of amifostine based on gold nanoparticles and sulfanilic acid functionalized graphene quantum dots. New J. Chem. 2018, 42, 12126–12133. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Rawa, K. Au@carbon quantum dot nanoconjugates as dual mode enzyme-free sensing platform for cholesterol. J. Mater. Chem. B 2017, 5, 5425–5432. [Google Scholar] [CrossRef]
- Kuo, S.Y.; Li, H.H.; Wu, P.J.; Chen, C.P.; Huang, Y.C.; Chan, Y.H. Dual Colorimetric and Fluorescent Sensor Based On Semiconducting Polymer Dots for Ratiometric Detection of Lead Ions in Living Cells. Anal. Chem. 2015, 87, 4765–4771. [Google Scholar] [CrossRef]
- Fang, A.J.; Wu, Q.Q.; Lu, Q.J.; Chen, H.Y.; Li, H.T.; Liu, M.L.; Zhang, Y.Y.; Yao, S.Z. Upconversion ratiometric fluorescence and colorimetric dual-readout assay for uric acid. Biosens. Bioelectron. 2016, 86, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Sun, C.X.; Peng, Y.; Zhang, N.N.; Si, H.H.; Liu, C.S.; Du, M. Ratiometric fluorescence sensing and colorimetric decoding methanol by a bimetallic lanthanide-organic framework. Sens. Actuators B Chem. 2018, 265, 104–109. [Google Scholar] [CrossRef]
- Feng, W.Y.; Gong, S.Y.; Zhou, E.B.; Yin, X.Y.; Feng, G.Q. Readily prepared iminocoumarin for rapid, colorimetric and ratiometric fluorescent detection of phosgene. Anal. Chim. Acta 2018, 1029, 97–103. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Zhu, Z.J.; Zhu, C.Z.; Ren, J.T.; Wang, E.K.; Dong, S.J. Multifunctional water-soluble luminescent carbon quantum dots for imaging and Hg2+ sensing. J. Mater. Chem. B 2014, 2, 6995–6999. [Google Scholar] [CrossRef]
- Shan, X.Y.; Chai, L.J.; Ma, J.J.; Qian, Z.S.; Chen, J.R.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.Y.; Zhang, B.Y.; Xu, C.; Huang, J.; Xia, Z.L. Fluorescent glucose sensing using CdTe/CQDs quantum dots–glucose oxidase complex. Anal. Methods 2016, 8, 2967–2970. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.J.; Hu, Y.; Su, Y.Y.; Lv, Y. Accelerated reducing synthesis of Ag@CQDs composite and simultaneous determination of glucose during the synthetic process. RSC Adv. 2014, 4, 3992–3997. [Google Scholar] [CrossRef]
- Ma, J.L.; Yin, B.C.; Wu, X.; Ye, B.C. Simple and Cost-Effective Glucose Detection Based on Carbon Nanodots Supported on Silver Nanoparticles. Anal. Chem. 2017, 89, 1323–1328. [Google Scholar] [CrossRef]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef]
- Xu, X.W.; Yang, X.R. Facile colorimetric detection of glucose based on an organic Fenton reaction. Anal. Methods 2011, 3, 1056–1059. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.F.; Wen, F.F.; Tan, J.; Peng, T.; Luo, B.Q.; Wang, H.G.; Yin, S.X. A flower-like MoS2-decorated MgFe2O4 nanocomposite: Mimicking peroxidase and colorimetric detection of H2O2 and glucose. Sens. Actuators B Chem. 2018, 275, 155–162. [Google Scholar] [CrossRef]
- Ponlakhet, K.; Amatatongchai, M.; Sroysee, W.; Jarujamrus, P.; Chairam, S. Development of sensitive and selective glucose colorimetric assay using glucose oxidase immobilized on magnetite-gold-folate nanoparticles. Anal. Methods 2016, 8, 8288–8298. [Google Scholar] [CrossRef]



| Sample | Added (mM) | Found (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0 | 4.48 | - | - |
| 5 | 9.76 | 105.6 | 4.3 | |
| 2 | 0 | 5.33 | - | - |
| 5 | 10.58 | 105.0 | 5.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, H.; Bai, Y.; Qin, J.; Feng, F. Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene. Sensors 2019, 19, 674. https://doi.org/10.3390/s19030674
Zhai H, Bai Y, Qin J, Feng F. Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene. Sensors. 2019; 19(3):674. https://doi.org/10.3390/s19030674
Chicago/Turabian StyleZhai, Hong, Yunfeng Bai, Jun Qin, and Feng Feng. 2019. "Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene" Sensors 19, no. 3: 674. https://doi.org/10.3390/s19030674
APA StyleZhai, H., Bai, Y., Qin, J., & Feng, F. (2019). Colorimetric and Ratiometric Fluorescence Dual-Mode Sensing of Glucose Based on Carbon Quantum Dots and Potential UV/Fluorescence of o-Diaminobenzene. Sensors, 19(3), 674. https://doi.org/10.3390/s19030674