Optical Sensor for Real-Time Detection of Trichlorofluoromethane
Abstract
1. Introduction
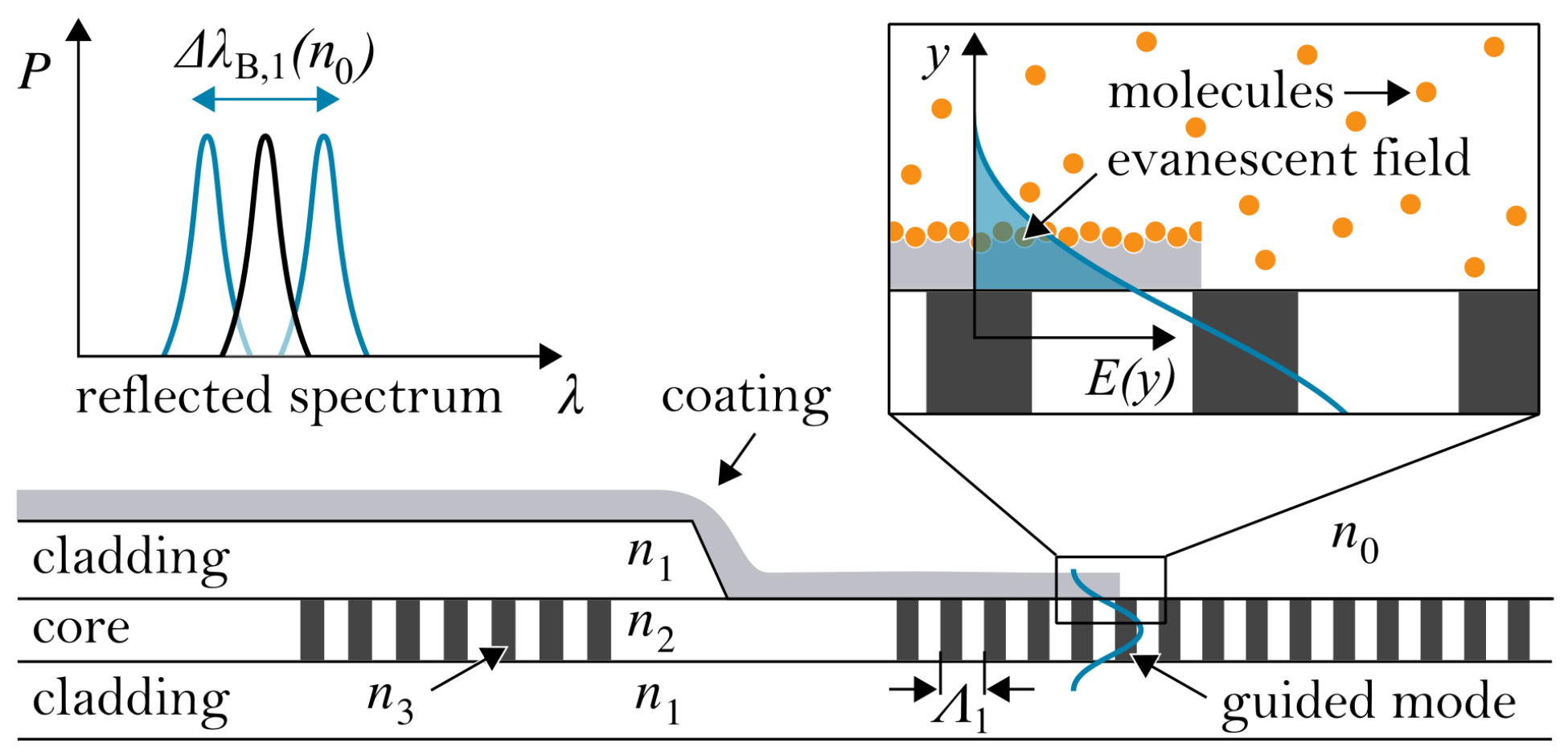
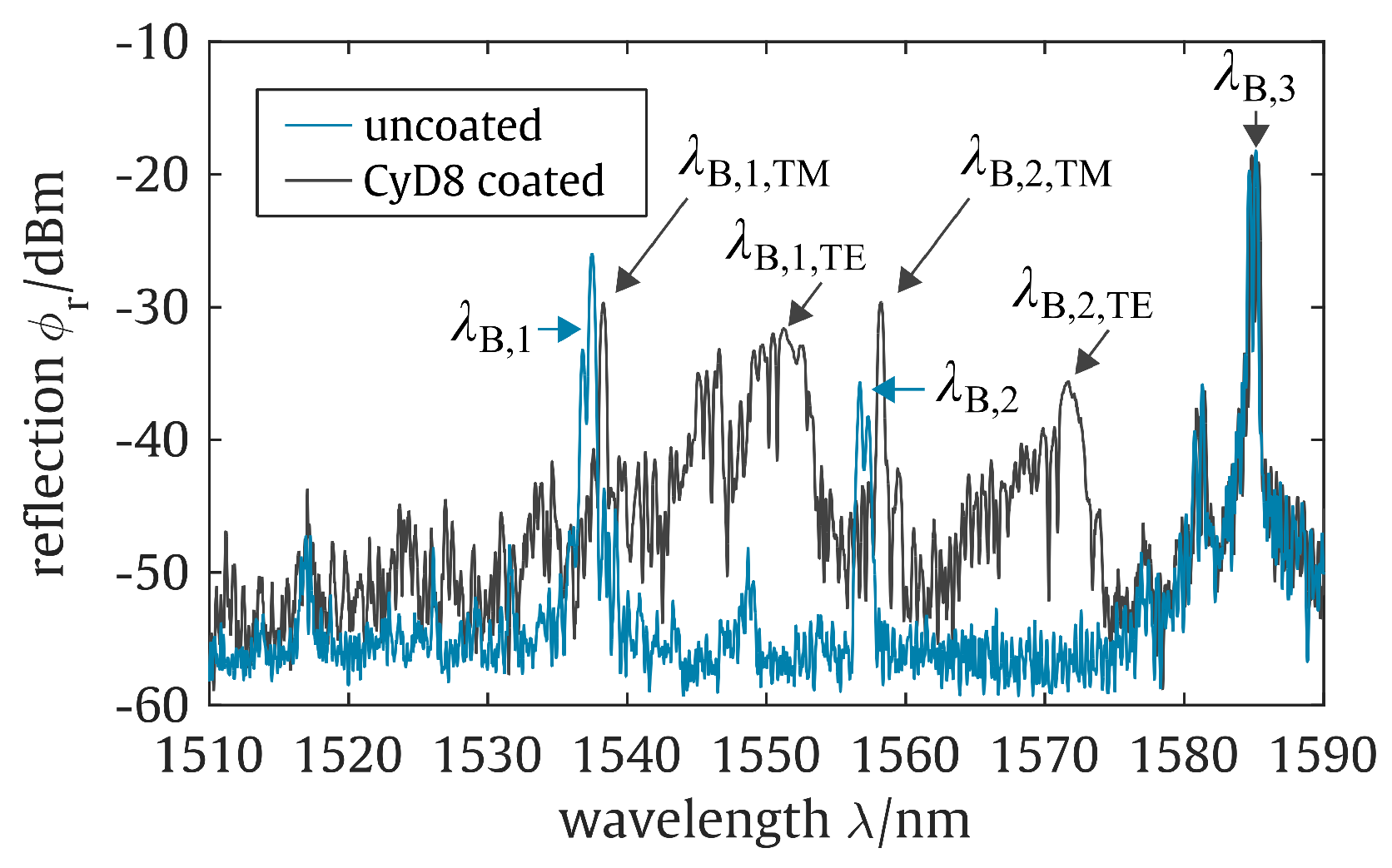
2. Planar Bragg Grating Sensor
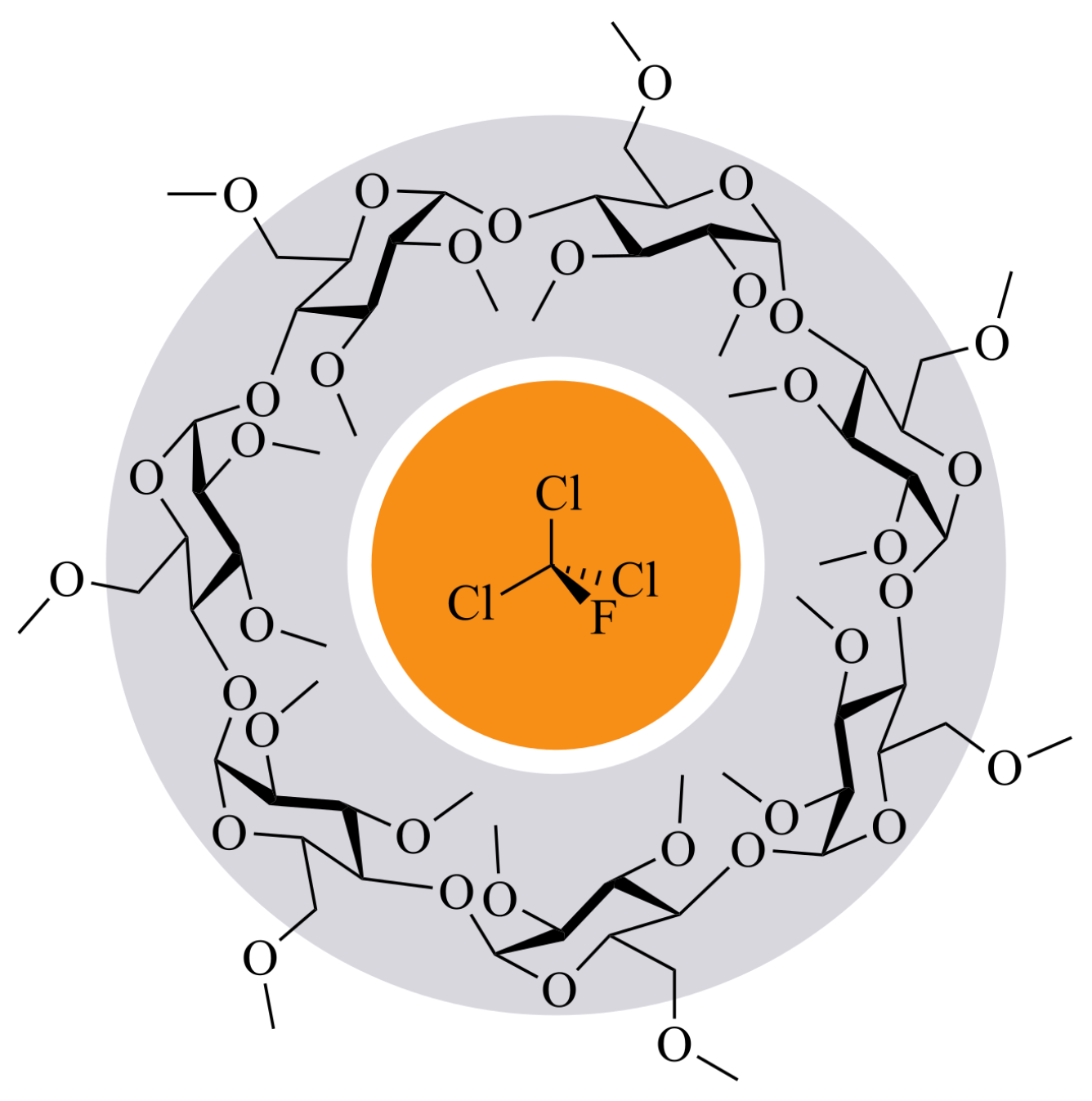
3. Substituted Cyclodextrins for Sensor Sensitization
- Per-methyl substituted cyclodextrin derivatives:
- CyD1: hexakis(2,3,6-tri-O-methyl)--cyclodextrin
- CyD2: heptakis(2,3,6-tri-O-methyl)--cyclodextrin
- CyD3: octakis(2,3,6-tri-O-methyl)--cyclodextrin
- Per-ethyl substituted cyclodextrin derivatives:
- CyD4: hexakis(2,3,6-tri-O-ethyl)--cyclodextrin
- CyD5: heptakis(2,3,6-tri-O-ethyl)--cyclodextrin
- CyD6: octakis(2,3,6-tri-O-ethyl)--cyclodextrin
- Per-allyl substituted cyclodextrin derivatives:
- CyD7: hexakis(2,3,6-tri-O-allyl)--cyclodextrin
- CyD8: heptakis(2,3,6-tri-O-allyl)--cyclodextrin
- CyD9: octakis(2,3,6-tri-O-allyl)--cyclodextrin
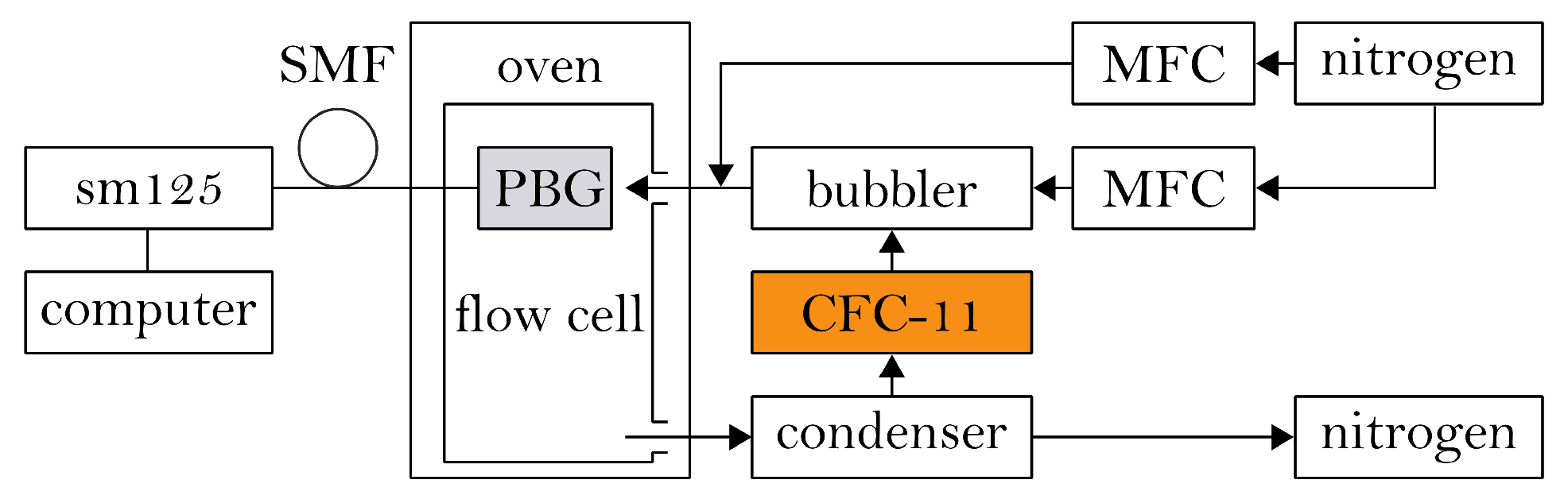
4. Experimental Setup
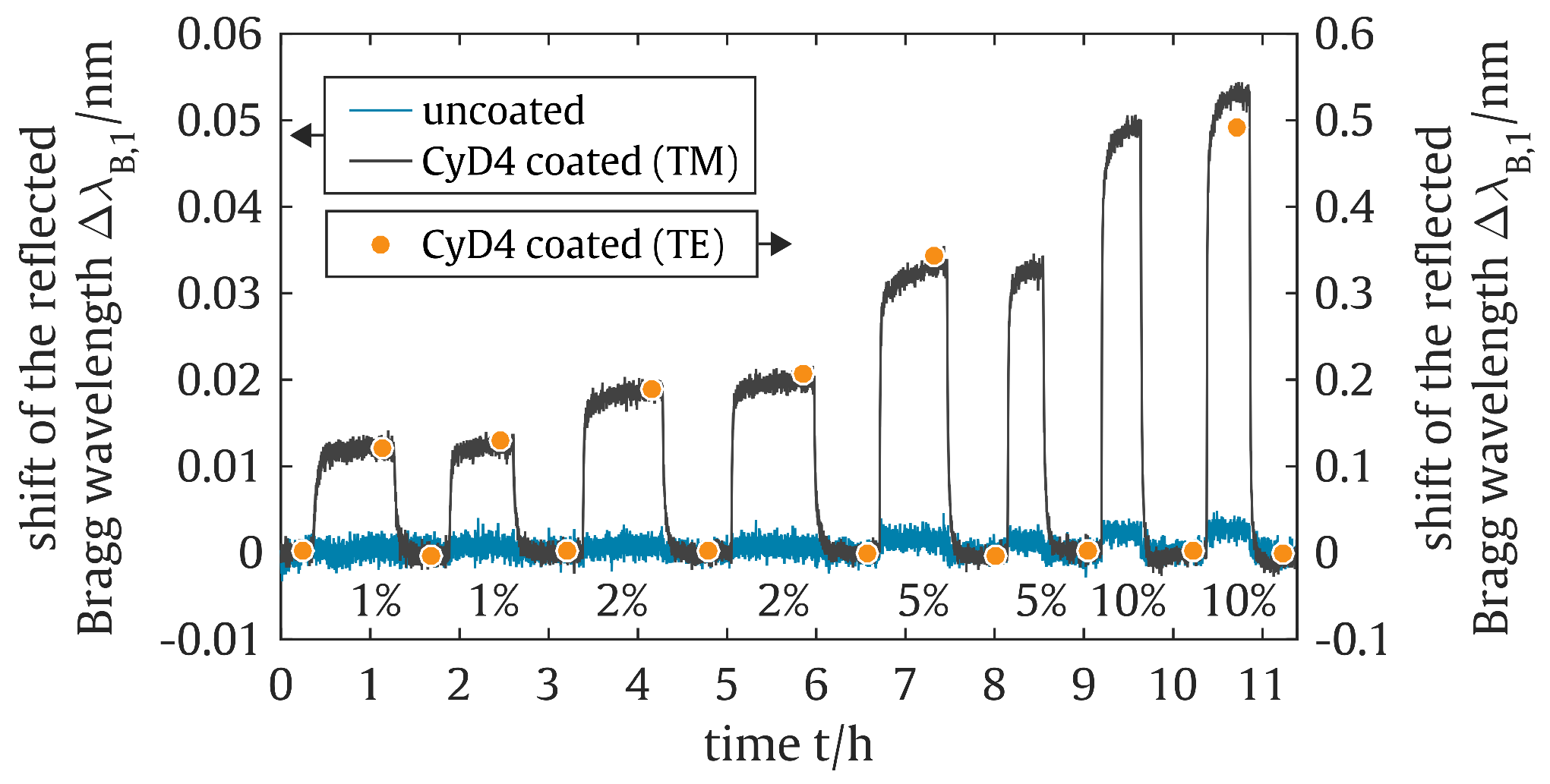
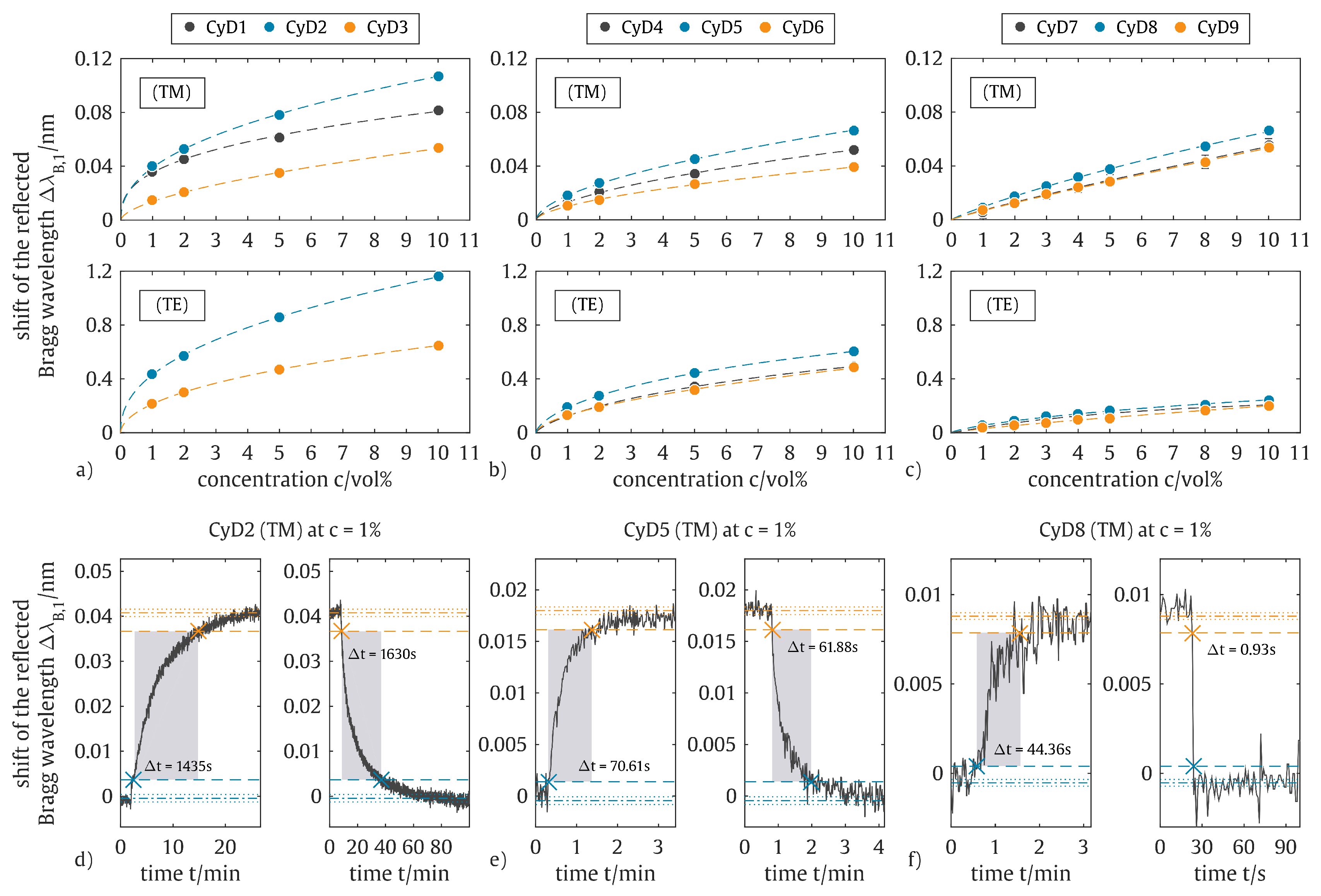
5. Results and Discussion
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Gutowsky, H.S. Halocarbons: Effects on Stratospheric Ozone; National Academy of Science: Washington, DC, USA, 1976. [Google Scholar]
- Farman, J.C.; Gardiner, B.G.; Shanklin, J.D. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 1985, 315, 207–210. [Google Scholar] [CrossRef]
- United Nations Environment Programme, Ozone Secretariat. Handbook for the Montreal Protocol on Substances That Deplete the Ozone Layer, 10th ed.; UNON: Nairobi, Kenya, 2016. [Google Scholar]
- Prather, M.; Midgley, P.; Rowland, F.S.; Stolarski, R. The ozone layer: The road not taken. Nature 1996, 381, 551–554. [Google Scholar] [CrossRef]
- Mäder, J.A.; Staehelin, J.; Peter, T.; Brunner, D.; Rieder, H.E.; Stahel, W.A. Evidence for the effectiveness of the Montreal Protocol to protect the ozone layer. Atmos. Chem. Phys. 2010, 10, 12161–12171. [Google Scholar] [CrossRef]
- Papst, I. Global Banks of Ozone Depleting Substances: A Country-Level Estimate; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Frankfurt, Germany, 2017. [Google Scholar]
- Liu, N.; Somboon, V.; Middelton, C. Illegal trade in ozone depleting substances. In Handbook of Transnational Environmental Crime; Elliott, L., Schaedla, W.H., Eds.; Edward Elgar Publishing: Cheltenham, UK; Northampton, MA, USA, 2016. [Google Scholar]
- Elliott, L. Smuggling Networks and the Black Market in Ozone Depleting Substances. In Hazardous Waste and Pollution: Detecting and Preventing Green Crimes; Wyatt, T., Ed.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Mulder, K.F. Innovation by disaster: The ozone catastrophe as experiment of forced innovation. Int. J. Environ. Sustain. Dev. 2005, 4, 88–103. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dutton, G.S.; Yu, P.; Ray, E.; Portmann, R.W.; Daniel, J.S.; Kuijpers, L.; Hall, B.D.; Mondeel, D.; Siso, C.; et al. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature 2018, 557, 413–417. [Google Scholar] [CrossRef] [PubMed]
- WMO (World Meteorological Organization). Assessment for Decision-Makers: Scientific Assessment of Ozone Depletion: 2014, Global Ozone Research and Monitoring Project; WMO: Geneva, Switzerland, 2015; Volume 56. [Google Scholar]
- Hounshell, D.A.; Smith, J.K. Science and Corporate Strategy: Du Pont R&D, 1902–1980; Studies in Economic History and Policy the United States in the Twentieth Century; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Kjeldsen, P.; Jensen, M.H. Release of CFC-11 from Disposal of Polyurethane Foam Waste. Environ. Sci. Technol. Lett. 2001, 35, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, P.; Scheutz, C. Short- and Long-Term Releases of Fluorocarbons from Disposal of Polyurethane Foam Waste. Environm. Sci. Technol. 2003, 37, 5071–5079. [Google Scholar] [CrossRef]
- Górecki, T.; Pawliszyn, J. Field-portable solid-phase microextraction/fast GC system for trace analysis. Field Anal. Chem. Technol. 1997, 1, 277–284. [Google Scholar] [CrossRef]
- Allin, S.J.; Laube, J.C.; Witrant, E.; Kaiser, J.; McKenna, E.; Dennis, P.; Mulvaney, R.; Capron, E.; Martinerie, P.; Röckmann, T.; et al. Chlorine isotope composition in chlorofluorocarbons CFC-11, CFC-12 and CFC-113 in firn, stratospheric and tropospheric air. Atmos. Chem. Phys. 2015, 15, 6867–6877. [Google Scholar] [CrossRef]
- Commercial Product by Honeywell Analytics. 301IRF Refrigerant Gas Detector. Available online: https://www.honeywellanalytics.com/ (accessed on 4 April 2018).
- Commercial Product by Fieldpiece Instruments. SRL2K7 Infrared Refrigerant Leak Detector. Available online: https://www.fieldpiece.com/ (accessed on 4 April 2018).
- Moritz, W.; Fillipov, V.; Vasiliev, A.; Terentjev, A. Silicon carbide based semiconductor sensor for the detection of fluorocarbons. Sens. Actuators B Chem. 1999, 58, 486–490. [Google Scholar] [CrossRef]
- Commercial Product by Fieldpiece Instruments. SRL8 Headed Diode Refrigerant Leak Detector. Available online: https://www.fieldpiece.com/ (accessed on 4 April 2018).
- Commercial Product by Gastec. Pyrotube No. 51. Available online: https://www.gastec.co.jp/ (accessed on 4 April 2018).
- Commercial Product by Dräger. No. 8101601. Available online: https://www.draeger-mo.com/ (accessed on 4 April 2018).
- Hill, K.O.; Fujii, Y.; Johnson, D.C.; Kawasaki, B.S. Photosensitivity in optical fiber waveguides: Application to reflection filter fabrication. Appl. Phys. Lett. 1978, 32, 647–649. [Google Scholar] [CrossRef]
- Othonos, A. Fiber Bragg gratings. Rev. Sci. Instrum. 1997, 68, 4309–4341. [Google Scholar] [CrossRef]
- Hill, K.O.; Meltz, G. Fiber Bragg grating technology fundamentals and overview. J. Lightwave Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef]
- Cusano, A.; Cutolo, A.; Albert, J. Fiber Bragg Grating Sensors: Recent Advancements, Industrial Applications and Market Exploitation; Bentham Science Publishers: Sharjah, UAE, 2011. [Google Scholar]
- Girschikofsky, M.; Rosenberger, M.; Förthner, M.; Rommel, M.; Frey, L.; Hellmann, R. Waveguide Bragg Gratings in Ormocers for Temperature Sensing. Sensors 2017, 17, 2459. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, M.; Hessler, S.; Belle, S.; Schmauss, B.; Hellmann, R. Compressive and tensile strain sensing using a polymer planar Bragg grating. Opt. Express 2014, 22, 5483–5490. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Guo, Y.; Zhao, X.; Huo, J.; Sun, T. Temperature and Strain Characteristic Analysis of Fiber Bragg Grating Sensor. In Proceedings of the 2012 International Conference on Information Technology and Software Engineering; Lu, W., Cai, G., Liu, W., Xing, W., Eds.; Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2013; Volume 210, pp. 641–647. [Google Scholar] [CrossRef]
- Rogers, A. Distributed optical-fibre sensing. Meas. Sci. Technol. 1999, 10, R75–R99. [Google Scholar] [CrossRef]
- Mihailov, S.J. Fiber Bragg Grating Sensors for Harsh Environments. Sensors 2012, 12, 1898–1918. [Google Scholar] [CrossRef]
- Sparrow, I.J.G.; Smith, P.G.R.; Emmerson, G.D.; Watts, S.P.; Riziotis, C. Planar Bragg Grating Sensors—Fabrication and Applications: A Review. J. Sens. 2009, 2009, 607647. [Google Scholar] [CrossRef]
- Holmes, C.; Gates, J.C.; Carpenter, L.G.; Rogers, H.L.; Parker, R.M.; Cooper, P.A.; Chaotan, S.; Mahamd Adikan, F.R.; Gawith, C.B.E.; Smith, P.G.R. Direct UV-written planar Bragg grating sensors. Meas. Sci. Technol. 2015, 26, 112001–112021. [Google Scholar] [CrossRef]
- Emmerson, G.D.; Watts, S.P.; Gawith, C.B.E.; Albanis, V.; Ibsen, M.; Williams, R.B.; Smith, P.G.R. Fabrication of directly UV-written channel waveguides with simultaneously defined integral Bragg gratings. Electron. Lett. 2002, 38, 1531–1532. [Google Scholar] [CrossRef]
- Scheurich, S.; Belle, S.; Hellmann, R.; So, S.; Sparrow, I.J.G.; Emmerson, G.D. Application of a silica-on-silicon planar optical waveguide Bragg grating sensor for organic liquid compound detection. Proc. SPIE 2009, 7356, 73561B. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Allylated cyclodextrins as effective affinity materials in chemical sensing of volatile aromatic hydrocarbons using an optical planar Bragg grating sensor. Anal. Chim. Acta 2013, 791, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger Analogues—Beyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, S.D.; Tao, B.Y. Applications of modified cyclodextrins. Biotechnol. Adv. 1994, 12, 325–339. [Google Scholar] [CrossRef]
- Szejtli, J. The Properties and Potential Uses of Cyclodextrin Derivatives. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 14, 25–36. [Google Scholar] [CrossRef]
- Schomburg, G.; Deege, A.; Hinrichs, H.; Hübinger, E.; Husmann, H. Preparation, purification, and analysis of alkylated cyclodextrins. J. Sep. Sci. 1992, 15, 579–584. [Google Scholar] [CrossRef]
- Ryvlin, D.; Girschikofsky, M.; Schollmeyer, D.; Hellmann, R.; Waldvogel, S.R. Methyl-Substituted α-Cyclodextrin as Affinity Material for Storage, Separation, and Detection of Trichlorofluoromethane. Glob. Chall. 2018, 2, 1800057. [Google Scholar] [CrossRef]
- Leydet, A.; Moullet, C.; Roque, J.P.; Witvrouw, M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Neyts, J.; Schols, D.; De Clercq, E. Polyanion inhibitors of HIV and other viruses. 7. Polyanionic compounds and polyzwitterionic compounds derived from cyclodextrins as inhibitors of HIV transmission. J. Med. Chem. 1998, 41, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Singh, S.; Wang, L.X. Improved preparation of perallylated cyclodextrins: facile synthesis of cyclodextrin-based polycationic and polyanionic compounds. Carbohydr. Res. 2002, 337, 217–220. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 6145-9:2009—Gas Analysis—Preparation of Calibration Gas Mixtures Using Dynamic Volumetric Methods—Part 9: Saturation Method; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- William, M.H.; David, R.L.; Thomas, J.B. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 2015–2016, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Schroeder, K.; Ecke, W.; Mueller, R.; Willsch, R.; Andreev, A. A fibre Bragg grating refractometer. Meas. Sci. Technol. 2001, 12, 757–764. [Google Scholar] [CrossRef]
- Ganziy, D.; Jespersen, O.; Rose, B.; Bang, O. An efficient and fast detection algorithm for multimode FBG sensing. In Proceedings of the 24th International Conference on Optical Fibre Sensors, Curitiba, Brazil, 28 September–2 October 2015; Volume 9634. [Google Scholar] [CrossRef]
- Lubczyk, D.; Siering, C.; Lörgen, J.; Shifrina, Z.B.; Müllen, K.; Waldvogel, S.R. Simple and sensitive online detection of triacetone triperoxide explosive. Sens. Actuators B Chem. 2010, 143, 561–566. [Google Scholar] [CrossRef]
- Pyka, I.; Ryvlin, D.; Waldvogel, S.R. Application of Rigidity-Controlled Supramolecular Affinity Materials for the Gravimetric Detection of Hazardous and Illicit Compounds. ChemPlusChem 2016, 81, 926–929. [Google Scholar] [CrossRef]
- Ryvlin, D.; Dumele, O.; Linke, A.; Fankhauser, D.; Schweizer, W.B.; Diederich, F.; Waldvogel, S.R. Systematic Investigation of Resorcin[4]arene-Based Cavitands as Affinity Materials on Quartz Crystal Microbalances. ChemPlusChem 2017, 82, 493–497. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Chen, Y.; Shah, R.N.; Shimizu, K.D. Characterization of Molecularly Imprinted Polymers with the Langmuir-Freundlich Isotherm. Anal. Chem. 2001, 73, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
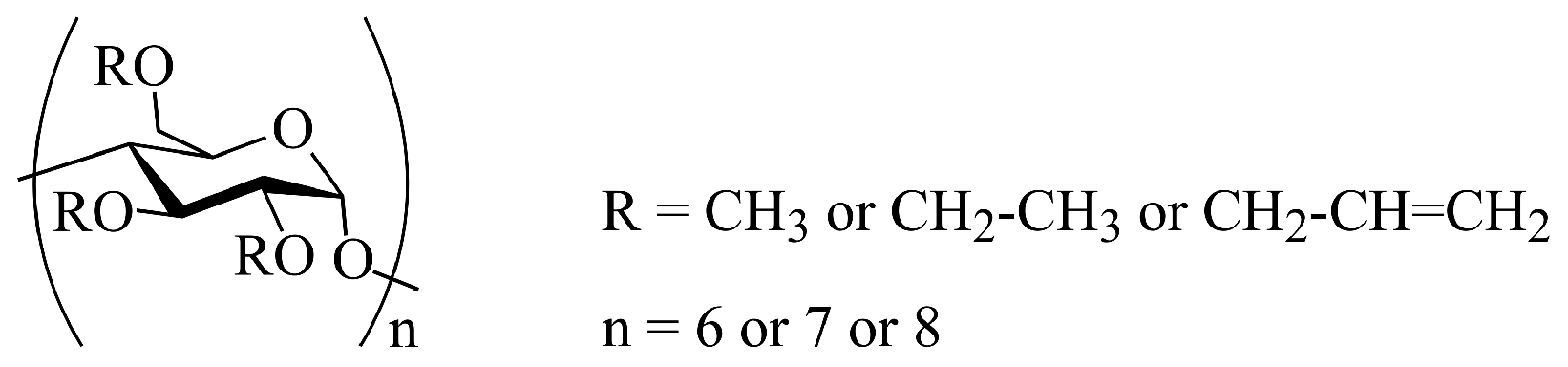
| Functionalization | LOD | Response to 1 vol% | ||
|---|---|---|---|---|
| (TM) | (TE) | Rise Time | Fall Time | |
| CyD1 | 130 ppm | — | 3092 s | 3341 s |
| CyD2 | 320 ppm | 5 ppm | 1435 s | 1630 s |
| CyD3 | 4300 ppm | 25 ppm | 1473 s | 1866 s |
| CyD4 | 4500 ppm | 220 ppm | 339 s | 429 s |
| CyD5 | 2600 ppm | 75 ppm | 71 s | 62 s |
| CyD6 | 7200 ppm | 85 ppm | 143 s | 168 s |
| CyD7 | 11,300 ppm | 1700 ppm | 24 s | 291 s |
| CyD8 | 8300 ppm | 750 ppm | 45 s | 1 s |
| CyD9 | 11,600 ppm | 2000 ppm | 350 s | 14 s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girschikofsky, M.; Ryvlin, D.; Waldvogel, S.R.; Hellmann, R. Optical Sensor for Real-Time Detection of Trichlorofluoromethane. Sensors 2019, 19, 632. https://doi.org/10.3390/s19030632
Girschikofsky M, Ryvlin D, Waldvogel SR, Hellmann R. Optical Sensor for Real-Time Detection of Trichlorofluoromethane. Sensors. 2019; 19(3):632. https://doi.org/10.3390/s19030632
Chicago/Turabian StyleGirschikofsky, Maiko, Dimitrij Ryvlin, Siegfried R. Waldvogel, and Ralf Hellmann. 2019. "Optical Sensor for Real-Time Detection of Trichlorofluoromethane" Sensors 19, no. 3: 632. https://doi.org/10.3390/s19030632
APA StyleGirschikofsky, M., Ryvlin, D., Waldvogel, S. R., & Hellmann, R. (2019). Optical Sensor for Real-Time Detection of Trichlorofluoromethane. Sensors, 19(3), 632. https://doi.org/10.3390/s19030632






