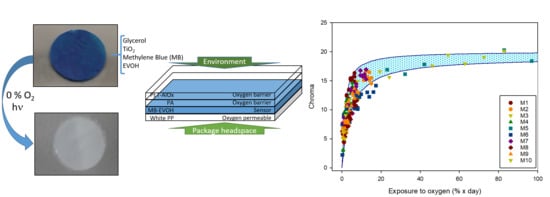
Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
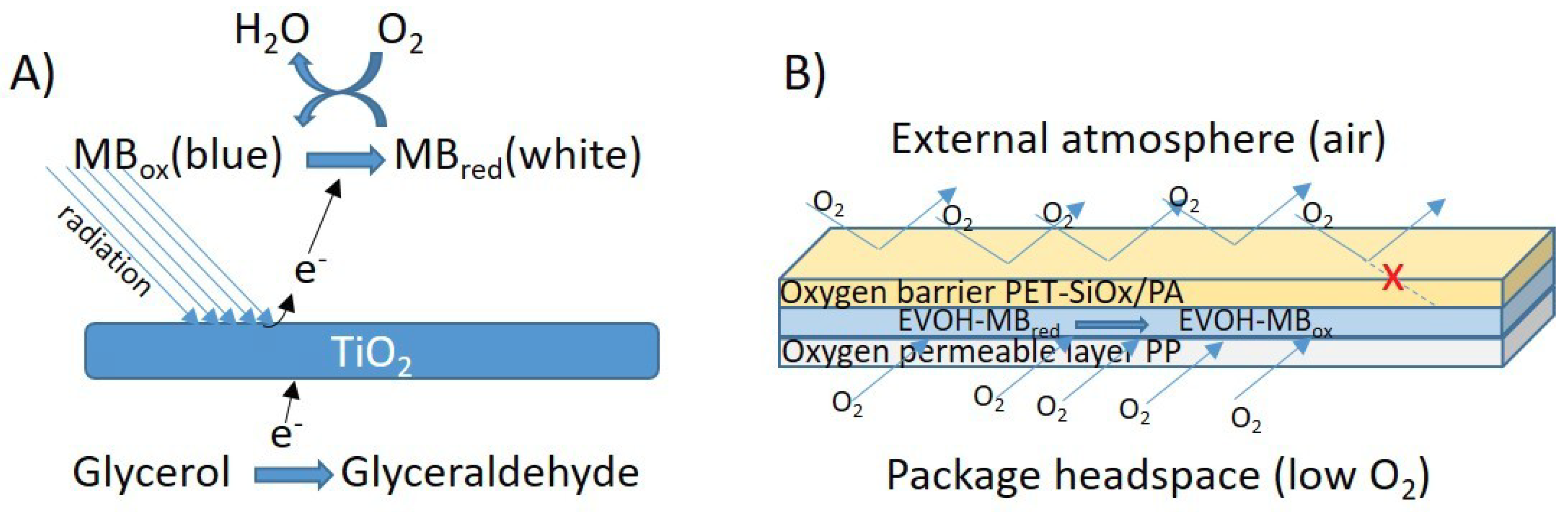
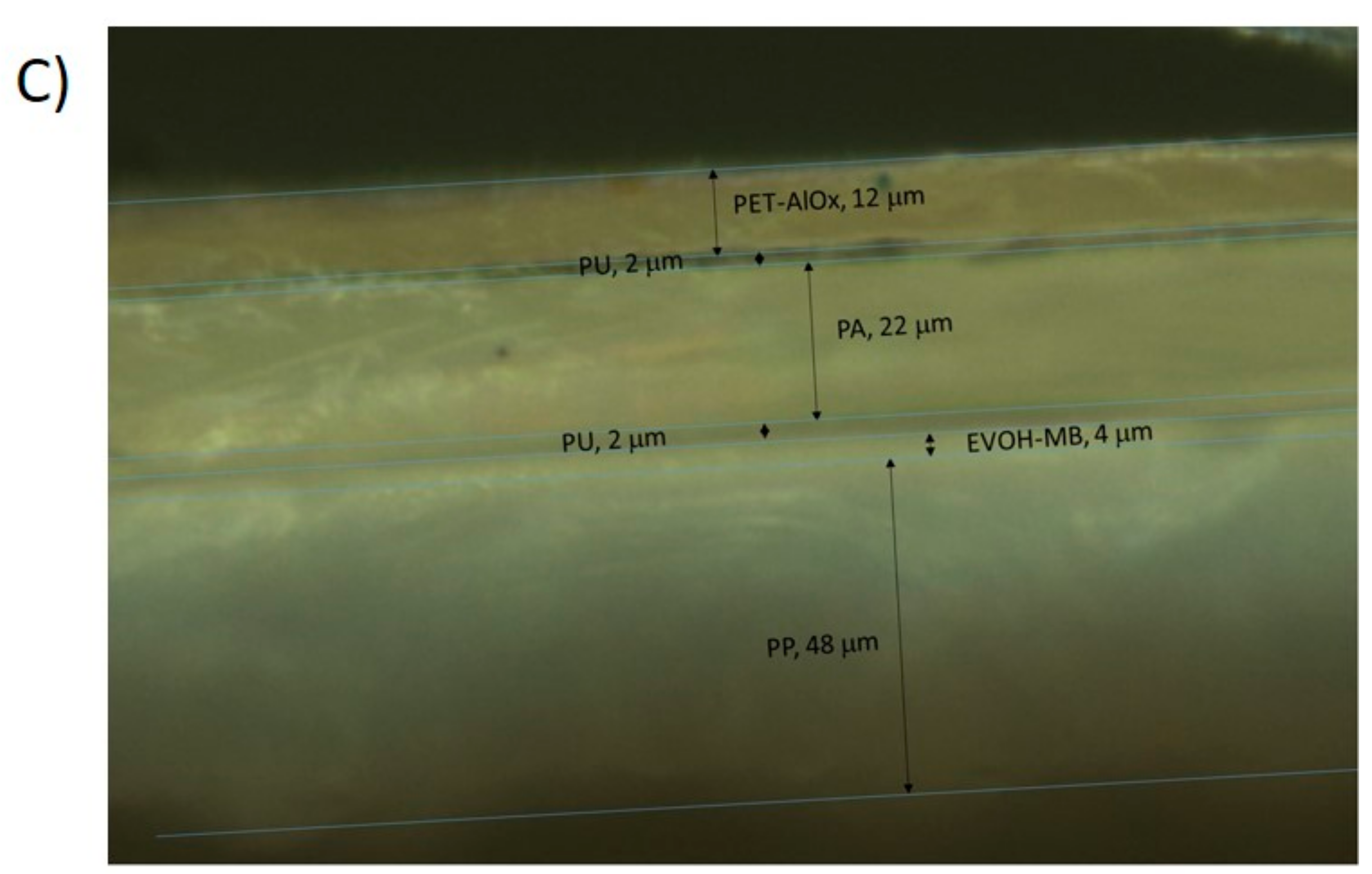
2.2.1. Sensor Preparation
2.2.2. Rheology
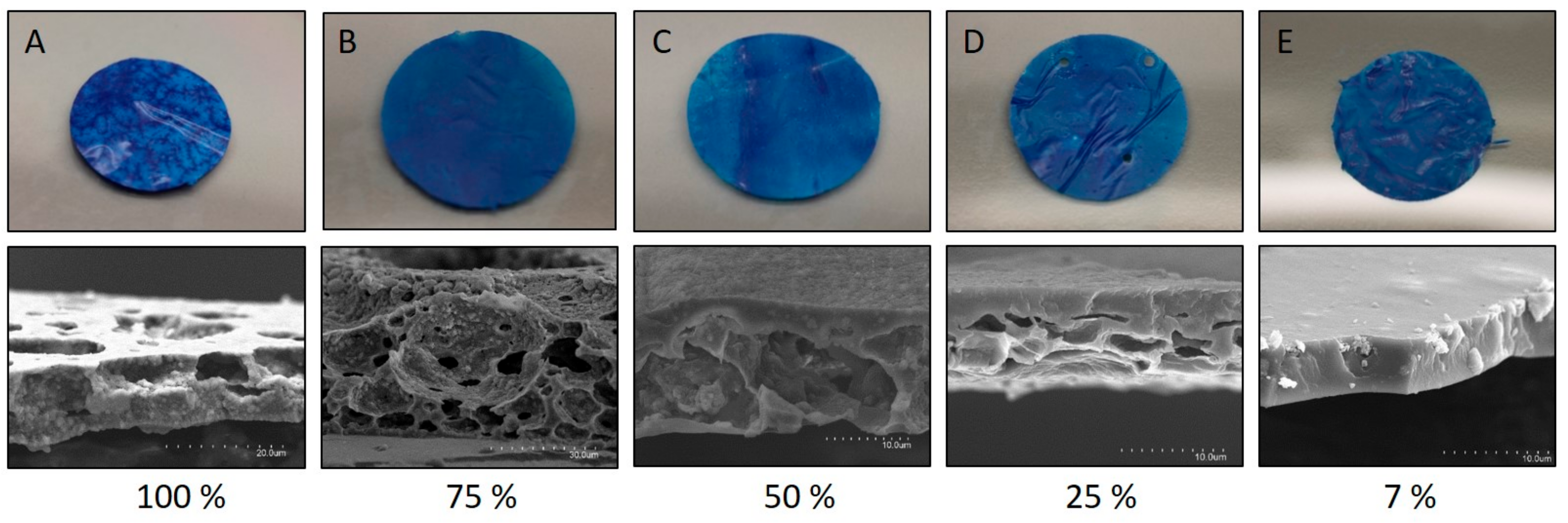
2.2.3. Morphology of the Sensor
2.2.4. Characterization of the Sensing Capacity
2.2.5. Test with a Real Food Product
2.2.6. Methylene Blue Migration
3. Results and Discussion
3.1. Rheological Analysis
3.2. Film Preparation
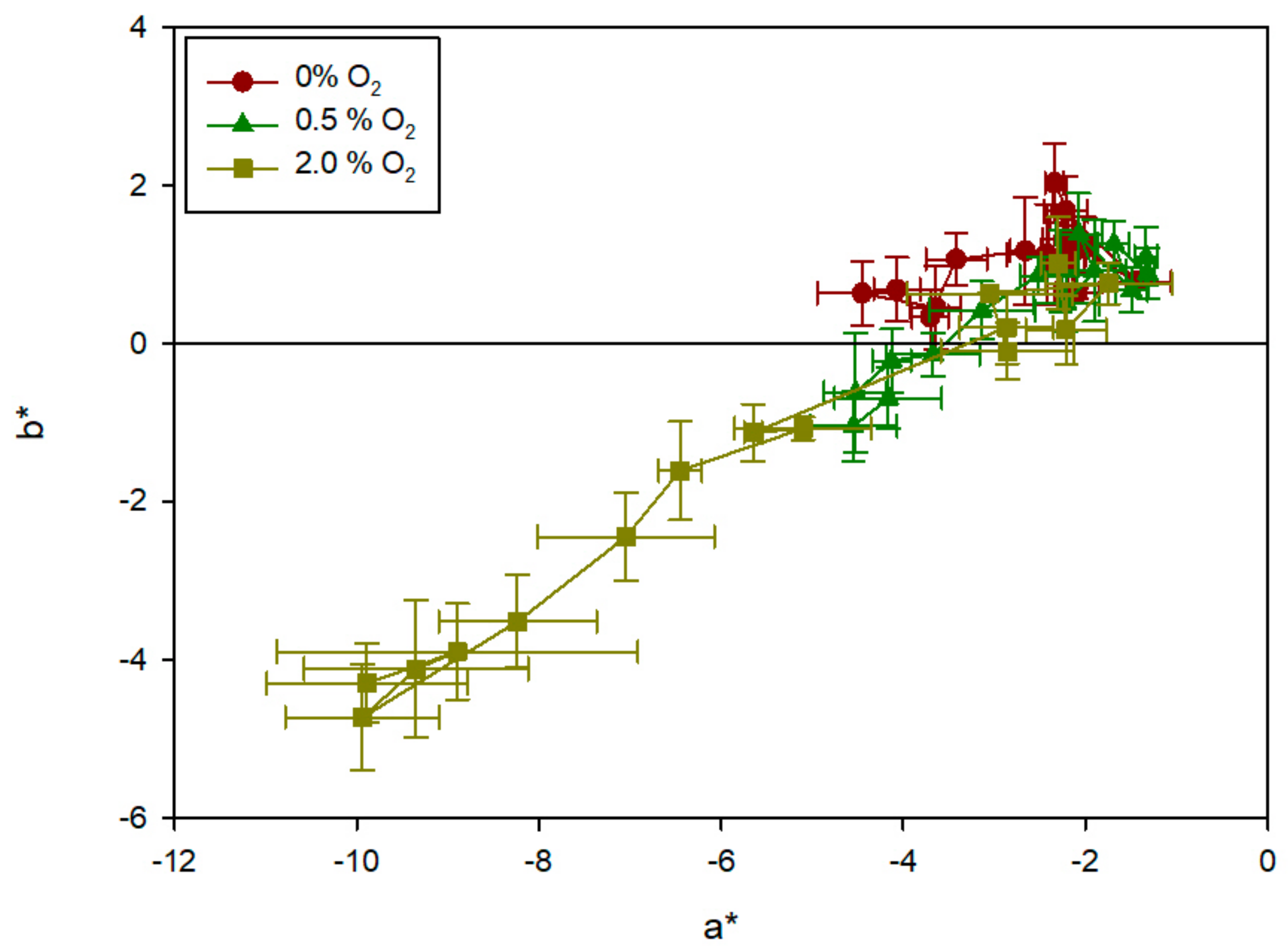
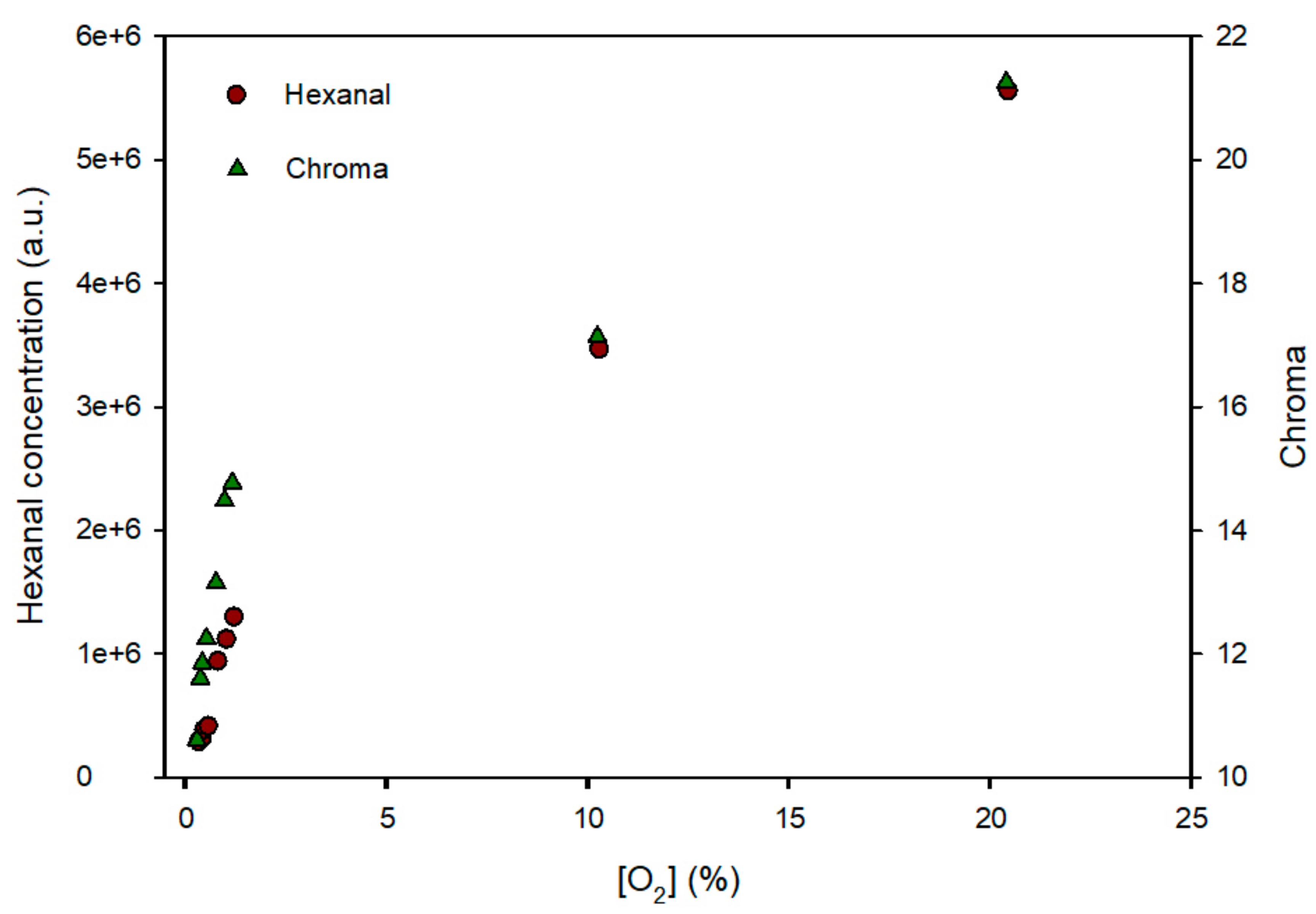
3.3. Characterization of the Sensor
3.4. Release of MB from Sensor Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanborough, T.; Fegan, N.; Powell, S.M.; Singh, T.; Tamplin, M.; Chandry, P.S. Genomic and metabolic characterization of spoilage-associated Pseudomonas species. Int. J. Food Microbiol. 2018, 268, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Wani, A.A.; Saengerlaub, S.; Langowski, H.-C. Understanding Critical Factors for the Quality and Shelf-life of MAP Fresh Meat: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 146–177. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) No 450/2009 on active and intelligent materials and articles intended to come into contact with food. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0450 (accessed on 10 September 2019).
- Mills, A. Oxygen indicators and intelligent inks for packaging food. Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhujun, Z.; Seitz, W.R. Optical sensor for oxygen based on immobilized hemoglobin. Anal. Chem. 1986, 58, 220–222. [Google Scholar] [CrossRef]
- Chung, K.E.; Lan, E.H.; Davidson, M.S.; Dunn, B.S.; Valentine, J.S.; Zink, J.I. Measurement of dissolved-oxygen in water using glass-encapsulated myoglobin. Anal. Chem. 1995, 67, 1505–1509. [Google Scholar] [CrossRef]
- Mills, A.; Lawrie, K.; Bardin, J.; Apedaile, A.; Skinner, G.A.; O’Rourke, C. An O-2 smart plastic film for packaging. Analyst 2012, 137, 106–112. [Google Scholar] [CrossRef]
- Lawrie, K.; Mills, A.; Hazafy, D. Simple inkjet-printed, UV-activated oxygen indicator. Sens. Actuators B Chem. 2013, 176, 1154–1159. [Google Scholar] [CrossRef]
- Mills, A.; Hazafy, D. A solvent-based intelligence ink for oxygen. Analyst 2008, 133, 213–218. [Google Scholar] [CrossRef]
- Lee, S.K.; Sheridan, M.; Mills, A. Novel UV-activated colorimetric oxygen indicator. Chem. Mater. 2005, 17, 2744–2751. [Google Scholar] [CrossRef]
- Chau Hai Thai, V.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef]
- Suman; Gaur, V.; Kumar, P.; Jain, V.K. Nanomaterial-based opto-electrical oxygen sensor for detecting air leakage in packed items and storage plants. J. Exp. Nanosci. 2012, 7, 608–615. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Won, K. Bioinspired molecular adhesive for water-resistant oxygen indicator films. Biotechnol. Progr. 2013, 29, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Oxygen detection using UV-activated electrospun poly(ethylene oxide) fibers encapsulated with TiO2 nanoparticles. J. Mater. Sci. 2013, 48, 5489–5498. [Google Scholar] [CrossRef]
- Marek, P.; Velasco-Veléz, J.J.; Haas, T.; Doll, T.; Sadowski, G. Time-monitoring sensor based on oxygen diffusion in an indicator/polymer matrix. Sens. Actuators B Chem. 2013, 178, 254–262. [Google Scholar] [CrossRef]
- Ollis, D.; Mills, A.; Lawrie, K. Kinetics of methylene blue (MB) photocatalyzed reduction and dark regeneration in a colorimetric oxygen sensor. Appl. Catal. B Environ. 2016, 184, 201–207. [Google Scholar] [CrossRef]
- Muriel Galet, V.; Cerisuelo, J.; Lopez Carballo, G.; Aucejo, S.; Gavara, R.; Hernández Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernandez-Munoz, P. Natural antimicrobial - containing EVOH coatings on PP and PET Films: functional and active property characterization. Packag. Technol. Sci. 2014, 27, 901–920. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Antimicrobial-releasing films and coatings for food packaging based on carvacrol and ethylene copolymers. Polym. Int. 2015, 16, 1747–1753. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Biochemical Properties of Bioplastics Made from Wheat Gliadins Cross-Linked with Cinnamaldehyde. J. Agric. Food. Chem. 2011, 59, 13212–13220. [Google Scholar] [CrossRef]
- Castellanos, D.A.; Cerisuelo, J.P.; Hernandez-Munoz, P.; Herrera, A.O.; Gavara, R. Modelling the evolution of O2 and CO2 concentrations in MAP of a fresh product: Application to tomato. J. Food Eng. 2016, 168, 84–95. [Google Scholar] [CrossRef]
- Lopez-de-Dicastillo, C.; Pezo, D.; Nerin, C.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Reducing Oxidation of Foods Through Antioxidant Active Packaging Based on Ethyl Vinyl Alcohol and Natural Flavonoids. Packag. Technol. Sci. 2012, 25, 457–466. [Google Scholar] [CrossRef]
- Ginimuge, P.R.; Jyothi, S.D. Methylene Blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar] [PubMed]
- EU. Commission Regulation (EU) No. 10/2011 of 14 January 2011 on plactic materials and articles intended to come into contact with food. Off. J. Eur. Un. 2011, 1–89. [Google Scholar]
- Massey, L.K. Permeability Properties of Plastics and Elastomers—A Guide to Packaging and Barrier Materials; Elsevier Science & Technology Books: New York, NY, USA, 2003. [Google Scholar]
- Bodart, M.; de Penaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Dai, L.; Wu, B.; Ding, T.; Zhu, J.-J.; Lin, H.; Chen, H.-L.; Shen, C.-Y.; Jiang, Y. Determination of methylene blue residues in aquatic products by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2009, 32, 4193–4199. [Google Scholar] [CrossRef]



| EVOH Solutions in PrOH/H2O | TiO2 Dispersions in 7% EVOH Solutions | ||
|---|---|---|---|
| Concentration | Time (s) | Concentration | Time (s) |
| 7% | 22.0 ± 0.5a | 0% | 22.0 ± 0.5a |
| 9% | 37.0 ± 0.5 b | 25% TiO2 | 21.8 ± 0.5a |
| 11% | 55.3 ± 0.6c | 50% TiO2 | 22.0 ± 0.5a |
| 13% | 94.7 ± 1.2d | 100% TiO2 | 21.7 ± 0.5a |
| EVOH Solutions in PrOH/H2O | TiO2 Dispersions in 7% EVOH Solutions | ||||
|---|---|---|---|---|---|
| Conc. | K (Pa·s) | n | Conc. | K (Pa·s) | n |
| 7% | 0.057 ± 0.004a | 0.996 ± 0.006a | 0% TiO2 | 0.057 ±0.004a | 0.996 ± 0.006b |
| 8% | 0.090 ± 0.052b | 0.992 ± 0.004a | 25% TiO2 | 0.088 ±0.000b | 0.967± 0.008 a |
| 9% | 0.139 ± 0.013c | 0.983 ± 0.008a | 50% TiO2 | 0.090±0.017 b | 0.968± 0.023 a |
| 11% | 0.237 ± 0.002d | 0.997 ± 0.001a | 100% TiO2 | 0.086±0.004 b | 0.974± 0.022 a |
| 13% | 0.431 ± 0.008e | 0.991 ± 0.003 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Carballo, G.; Muriel-Galet, V.; Hernández-Muñoz, P.; Gavara, R. Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging. Sensors 2019, 19, 4684. https://doi.org/10.3390/s19214684
López-Carballo G, Muriel-Galet V, Hernández-Muñoz P, Gavara R. Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging. Sensors. 2019; 19(21):4684. https://doi.org/10.3390/s19214684
Chicago/Turabian StyleLópez-Carballo, Gracia, Virginia Muriel-Galet, Pilar Hernández-Muñoz, and Rafael Gavara. 2019. "Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging" Sensors 19, no. 21: 4684. https://doi.org/10.3390/s19214684
APA StyleLópez-Carballo, G., Muriel-Galet, V., Hernández-Muñoz, P., & Gavara, R. (2019). Chromatic Sensor to Determine Oxygen Presence for Applications in Intelligent Packaging. Sensors, 19(21), 4684. https://doi.org/10.3390/s19214684